Abstract
Wound healing is a complex overlapping biological process that involves a sequence of events coordinated by various cells, proteins, growth factors, cytokines and signaling molecules. Recent evidence indicates that forkhead box O1 (FOXO1) transcription factors play an important role in organizing these events to stimulate wound healing. The ubiquitously expressed forkhead box, class O (FOXO) transcription factors act as cell signaling molecules in various transcriptional processes that are involved in diverse cellular activities, including cell death, cell differentiation, DNA repair, apoptosis, and oxidative stress in response to stimuli, and interact with numerous proteins. Due to the activation of FOXO targeted genes, FOXOs are involved in maintaining the balance between oxidative stress and antioxidants. In humans, different isoforms of FOXO namely FOXO1, FOXO3, FOXO4 and FOXO6 are present, however only FOXO1 and FOXO3 possess biological functions such as morphogenesis, maintenance and tissue regeneration. This might make FOXOs an important therapeutic target to enhance wound healing in diabetes, and to avoid over scarring. In spite of extensive literature, little is known regarding the role of FOXO and its relationship in wound healing. This review provides a summary of FOXO proteins and their biological role in wound healing and oxidative stress.
Keywords: Diabetes, FOXO, FOXO1, FOXO3, Oxidative stress, Wound healing
Introduction
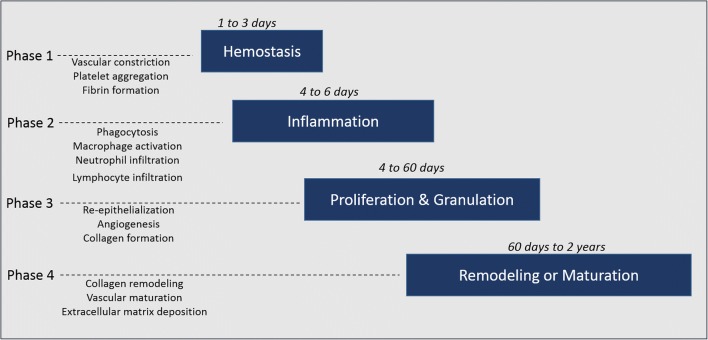
Skin plays a leading role in shielding the body against microbes, UV radiation, heat, and chemical damage. The quality and time taken for tissue repair relies on the metabolic status and cellular and immune responses at the wound site (Guo and Dipietro 2010). Wound healing or tissue repair consists of four overlapping phases: hemostasis, inflammation, proliferation, and remodeling (Fig. 1). Tissue repair mechanisms are immediately initiated following damage or disruption to skin integrity. Wound healing does not progress until hemostasis is completed, which is accompanied by vasoconstriction, platelet aggregation, fibrin deposition and blood clot formation (Shaw and Martin 2009). Throughout the inflammatory phase, several neutrophils rapidly migrate to the injured site to remove microbes, followed by the recruitment of macrophages. A major function of the inflammatory phase is to recruit inflammatory cells to the wound site. These inflammatory cells destroy any invading pathogens and remove cellular debris and damaged matrix so that the healing process can proceed. Clinical signs of inflammation, such as heat and erythema, can be observed as early as 15 min following tissue injury. The inflammatory phase is primarily controlled by the sustained production of cytokines, which in turn regulates the activation/inactivation of genes liable for cellular migration and proliferation activities. Fibroblast cells induce an angiogenic response and formation of granulation tissue (Shibata et al. 2012). Restoration of the wounded epithelium begins almost instantly after wounding. During the proliferation phase re-epithelization is initiated by the migration of epithelial cells over the newly formed granulation tissue to cover the wound site. Finally, wounded tissue is remodeled, which includes the removal of excess extracellular matrix (ECM) by enzymatic proteolytic degradation at the scar site. Scar remodeling starts to dominate as the main healing response almost three weeks after tissue injury. The thin, disorganized collagen fibers that make up an immature scar are slowly substituted with thicker collagen fibers organized in an orientation paralleling skin stresses. Collagen synthesis is downregulated by various cytokines such as interferon-γ and tumor necrosis factor-alpha (TNF-α). The role of matrix metalloproteinases (MMPs) is to degrade collagen fibers that are actively produced during the remodeling phase of wound healing (Martins et al. 2013).
Fig. 1.
Phases of wound healing. When there is tissue injury, blood vessels are disrupted resulting in bleeding. Hemostasis is the first phase of the healing process. Platelets recruit essential pro-inflammatory cytokines which modulate most of the essential steps in wound healing. Fibroblasts, keratinocytes, epithelial cells and endothelial cells proliferate and migrate towards the wound bed to deposit collagen and the extracellular matrix. Finally, matrix deposition results in wound closure and scar formation
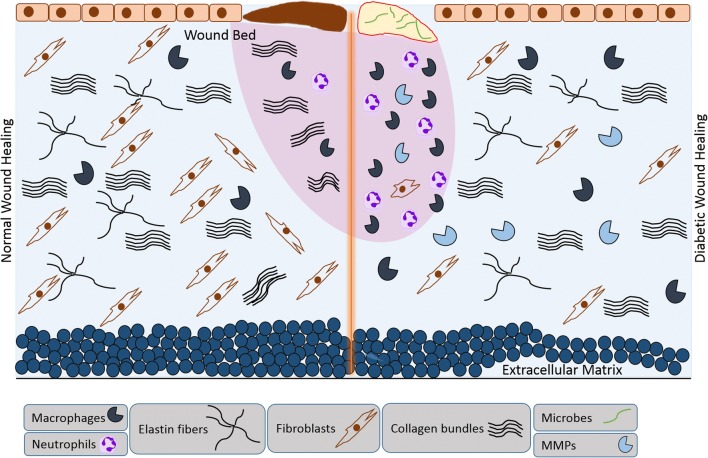
Numerous factors contribute to impaired and/or delayed healing, such as Diabetes mellitus (DM), whereby hyperglycemia results in cellular metabolic distress and elevated formation of advanced glycation end products (AGEs), and increased levels of inflammatory cytokines, MMPs and oxidative stress (Eming et al. 2014; Hameedaldeen et al. 2014). To date, several studies have focused on the molecular mechanisms linking the biological features of skin and tissue repair with age and/or metabolic-related diseases (Salathia et al. 2013; Serravallo et al. 2013). Figure 2 demonstrates the main cellular events involved in normal and delayed wound healing. The successful care and treatment of multiple diseases such as DM, cardiovascular diseases and cancer depends upon new therapeutic approaches. In this respect, forkhead box group O (FOXO) transcription factors have emerged as central targets as they can modulate various biological processes related to apoptosis, angiogenesis, cell proliferation, tumorigenesis and vascular cell longevity. In this review article, we provide a summary of FOXO proteins and their biological roles in wound healing and oxidative stress.
Fig. 2.
Schematic representation of the main cellular events involved in normal and delayed wound healing. Diabetic wounds are characterized by a lack of cell migration and proliferation and a paucity of granulation tissue, causing an absence of normal repair processes. High sugar levels increase the inflammatory response, including increased phagocytosis, macrophages recruitment, contributing to biofilm formation and the accumulation of necrotic debris. Altered immune cell function, endothelial cell dysfunction and impaired neovascularization results in delayed wound healing
FOXO protein family
There are 39 members of the forkhead family that have been divided into 19 subgroups, forkhead box (FOX) A to S. FOX proteins contain a highly conserved 100-residue forkhead (FKH) DNA binding domain site. Subgroup O (‘other’), or FOXO, which was first identified in Drosophila melanogaster, has received the greatest amount of attention due to their diverse roles, including their role in reactive oxygen species (ROS) detoxification (Jacobs et al. 2003; Papanicolaou et al. 2008). FOXOs are formed due to protein mutations which are forkhead-like in appearance. FOXO transcription factors consist of four different proteins namely FOXO1, FOXO3, FOXO4, and FOXO6. These proteins differ in their ability to bind to different DNA-binding domains, which offers diverse biological properties (Barthel et al. 2005). The expression of mammalian FOXO1 and FOXO3 is found in most tissues, while FOXO4 is only found in muscle, kidney, and colorectal tissue, and FOXO6 is only found in the brain and liver (Van Der Vos and Coffer 2011).
Regulation of FOXO
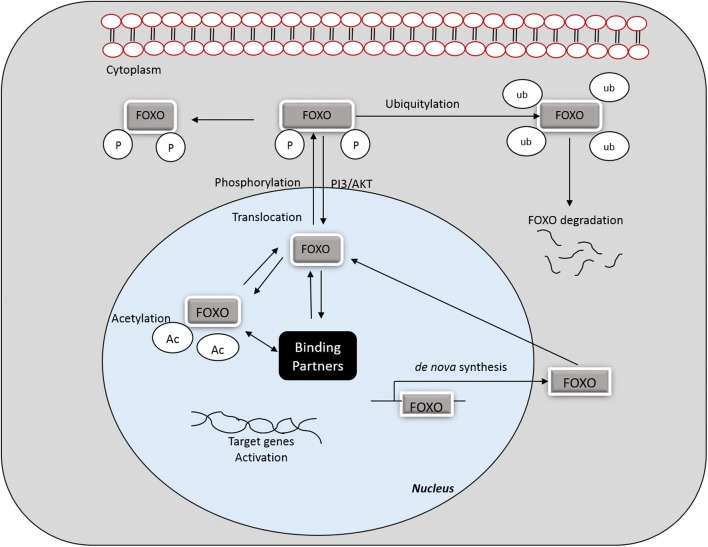
In the absence of external stimuli or suitable growth factors, FOXO proteins exhibit transcriptional activity inside the nucleus (Huang and Tindall 2007). Transcriptional function of FOXO is highly controlled by a complex array of post-translational modifications that either activate or inactivate FOXOs (Eric et al. 2013). Post-translational amendments may alter nuclear localization and DNA binding affinity. FOXOs hold four different functional motifs, including forkhead, nuclear localization, nuclear export, and transactivation domains (Eric et al. 2013). Many signaling pathways are involved in controlling FOXO protein nuclear translocation, and the treatment of cells with growth factors facilitates the entry of FOXO proteins into the cytoplasm (Huang and Tindall 2007; Essers et al. 2004). FOXOs activity is regulated by phosphroyolation, ubiquitylation and acetylation, and these processes each give rise to a distinct function (Urbanek and Klotz 2016). Figure 3 displays an overview of FOXO activation, deactivation and degradation.
Fig. 3.
Overview of FOXO activation, deactivation and degradation. FOXO transcription factor activity is controlled by various reversible and irreversible mechanisms including phosphorylation, acetylation, translocation and/or protein-protein interactions. These reactions are ubiquitin-dependent degradation or site-specific cleavage by proteases. Ub, ubiquitination; P, phosphorylation; Ac, acetylation
Phosphorylation
Huang et al., found that in both in vitro and in vivo experiments phosphorylation of FOXO1 had taken place by CDK2, mostly at serine 249 (Huang et al. 2006). This phosphorylation site falls within the CDK phosphorylation sequence [(K/R)(S/T)PX(K/R)] and is also identified in CDK2 substrates. When FOXO1 phosphorylation is mediated by CDK2, it reduces FOXO1 transcriptional activity and inhibits PTEN-meditated FOXO1 activation (Huang et al. 2006). The dual-specificity of tyrosine-phosphorylated and regulated kinase (DYRK) phosphorylates FOXO1 at a novel phosphorylation site, serine 329 (Woods et al. 2001). Insulin signaling substrate 1 and 2 regulates the activity of FOXO1 and increases that accumulation of FOXO1 in the cytosol by AKT phosphorylation (Engelman 2009; Lima et al 2012). Under hyperglycemic conditions, an oxidative stress environment induces activation of the c-Jun N-terminal kinase (JNK) signaling pathway, which phosphorylates FOXO4 at threonine 447 and threonine 451 (Wang et al. 2005). In vitro, JNK activation leads to phosphorylation at serine 184, which results in dissociation of FOXO3 from the 14–3-3 binding site in the cytoplasm, resulting in nuclear translocalization of FOXO3 (Lehtinen et al. 2006). In FOXO6, the absence of a C-terminal AKT phosphorylation site stimulates the translocation of FOXO6 from the cytosol to the nucleus. The interaction between FOXO and DNA is interrupted during AKT mediated FOXO phosphorylation, and this effect is due to inhibition of the DNA binding domain. The DNA binding domain is usually positively charged, however when FOXO is phosphorylated at AKT/SGK sites (S256 for FOXO1) it donates negative ions that inactivates the DNA binding domain (Wang et al. 2014). Table 1 lists the phosphorylation sites of various kinase proteins in FOXO1, FOXO3 and FOXO4.
Table 1.
List of phosphorylation sites of various kinase proteins in FOXO1, FOXO3 and FOXO4
| Substrates | Protein Kinase | Phosphorylation Site(s) |
|---|---|---|
| FOXO1 | AKT | T24, S256, S319 |
| MST1 | S212 | |
| ERK/p38 | S249, S287, S298, S329, S416, S418, S432, S470, T478 | |
| AMPK | T182, S544, S579, S616 | |
| CDK1/2 | S249 | |
| FOXO3 | AKT | T32, S253, S315 |
| MST1 | S209 | |
| ERK/p38 | S284, S294, S325, S425, T487 | |
| AMPK | T179, S399, S413, S555, S588, S626 | |
| IkK | S644 | |
| FOXO4 | AKT MST1 |
T28, S193, S258 S149 |
| JNK | T447, T451 | |
| ERK/p38 | S226, S237, S268, T380 | |
| AMPK | T119 |
Ubiquitylation
The Ubiquitin proteasome system plays a dual role in regulating FOXO proteins. Like many other proteins, FOXO undergoes proteasomal degradation through polyubiquitination reactions that is mediated by various enzymes such as ubiquitin E3 ligase, leading to FOXO degradation and inactivation. The use of proteasome inhibitors can prevent FOXO degradation and increase FOXO expression (Xie et al. 2012). In HepG2 cells, insulin treatment results in decreased levels of FOXO1 (Milan et al. 2015). Likewise, when chicken embryo fibroblasts are treated with platelet-derived growth factor (PDGF), reduced FOXO1 protein levels are detected. This can be overcome by using a proteasome inhibitor, namely lactacystin or PI3Kinase (Aoki et al. 2004). This proposes that FOXO1 degradation by proteasomes depends upon the activation of AKT signals. Furthermore, AKT phosphorylation is required for the polyubiquitylation of FOXO1 (Milan et al. 2015).
Monoubiquitination also plays a role in FOXO regulation and it increases FOXO nuclear localization and transcription activity. Nuclear relocalization of FOXO4 is stimulated by oxidative stress and subsequent transcription activation by the induction of monoubiquitination of FOXO4 at K199 and K211 (Calnan and Brunet 2008). An alternative mechanism includes the ROS induced formation of FOXO4 nuclear import receptor transportin-1 complex, which aids in nuclear localization (Putker et al. 2013).
Acetylation
Like phosphorylation, acetylation is involved in regulating FOXO transcriptional activity and modulates their biological role. Acetylation reduces the DNA binding activity of FOXOs, whereas deacetylation improves binding (Lalmansingh et al. 2012a, b). The effect of acetylation is highly regulated by histone acetyltransferases (HATs) and deacetylases (HDACs). Acetylation of FOXO1 at K222, K245, K248, K262, K265, K274, and K294 was reported to control its DNA binding affinity and attenuate its transcriptional activity and sensitivity to AKT phosphorylation (Daitoku et al. 2011). In stressed conditions, FOXO3 is acetylated at K242, K259, K271, K290, and K569. Elevated FOXO3 acetylation results in the over expression of pro-apoptotic genes such as Bim, TRAIL, and FasL, whereas increased deacetylated forms of FOXO3 results in the increased expression of antioxidant and cytoprotective genes. FOXO4 transcriptional activity mainly depends on the deacetylation at K186, K189, and K408 by histone deacetylases (Wang et al. 2014).
The transactivation of downstream FOXO target genes is mediated by the binding of CREB binding protein (CBP) to its regulatory gene p300 (Lalmansingh et al. 2012a, b; Tikhanovich et al. 2013). CBP induces acetylation at two major residual sites (Lys242 and Lys245) located at the C-terminal region of the DNA binding domain, resulting in the decreased binding affinity and transcriptional activity of FOXO1. Cysteinethiol disulfide-dependent complexes reduces FOXO4 induced cell cycle arrest and enhances apoptosis (Dansen et al. 2009). These complexes are formed between FOXO4 and p300/CBP acetyltransferase due to increased ROS levels. Silent information regulator 2 is a NAD-dependent deacetylase of the sirtuin family, which responds to the availability of nutrients/energy and stress stimuli in cells. The binding of STIR1 to FOXOs catalyzes its deacetylation in an NAD-dependent manner, and increases its transactivation activity by regulating its DNA binding at specific target genes (Kobayashi et al. 2005).
FOXOs interactions with protein partners
Most FOXOs associate with various protein partners to activate and/or inactivate different target genes. FOXO cellular activity mainly depends on transcriptional factors and its associated co-factors expressed in target cells. FOXOs have the ability to regulate the expression of certain target genes without directly attaching to a DNA domain site. This implies that FOXO is involved in the regulation of a subset of target genes by creating contact with other transcription factors (Ramaswamy et al. 2002). However, to regulate downstream transcription activity, FOXOs also bind to other factors. For example, FOXO3 and Runx3 interact and bind concomitantly to the promoter of Bim to promote apoptosis. This binding stimulates increased expression of Bim and initiates apoptosis in mouse embryonic fibroblasts and gastric cancer cells (Yamamura et al. 2006). FOXO proteins have also been shown to interact with β-catenin. It has been suggested that β-catenin bound to FOXO1 reduces T-cell factor activity. Thus, FOXO1 competes with T-cell factor for β-catenin and impairs β-catenin to stimulate bone formation (Stadeli et al. 2006).
Biological role of FOXO
Several studies in the last decade have confirmed the biological activity of FOXOs, and established the potential role of FOXO in regulating various cellular processes (Ho et al. 2008; Maiese 2015). The deletion of FOXOs has also given insight into their biological functions. Many researchers have observed that the deletion of FOXO1 in genetically modified mice is fatal leading to embryonic death due to incomplete vascular angiogenesis, whereas mice survived when FOXO3 or FOXO4 were deleted (Hosaka et al. 2004; Eijkelenboom and Burgering 2013; Dharaneeswaran et al. 2014). FOXO3 deletion is non-fatal, but affects lymph node proliferation and results in widespread tissue inflammation and reduced neural stem cell proliferation (Renault et al. 2009; Lam et al. 2013). FOXO4 deletion results in prolonged colitis, and FOXO6 deletion results in impaired memory consolidation (Salih et al. 2012). This highlights the significance of FOXO1, and it might be the most important FOXO factor.
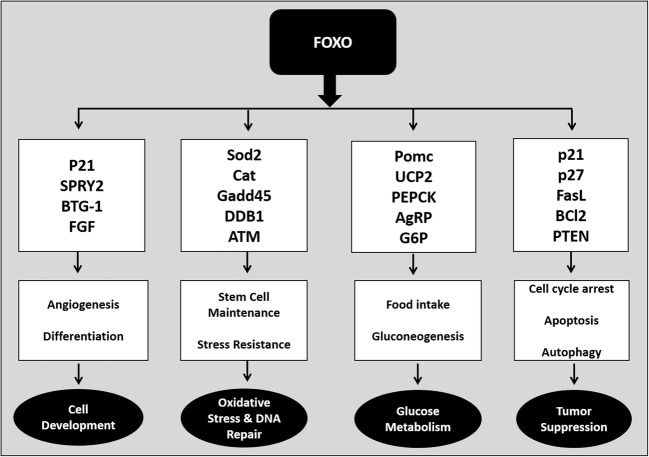
Figure 4 reveals the biological role of FOXO family proteins. The biological role of FOXO1 is well established, and it possesses numerous biological properties. The diverse functions of FOXO1 have been investigated in various in vitro and in vivo experiments in different diseased models. FOXO1 controls cell cycle progression by upregulating cyclin-dependent kinase inhibitors such as p27 and p21, and downregulating the cell cycle regulator cyclin D1 (Lee and Goldberg 2013; Zou et al. 2015). This function of FOXO1 was considered important in suppressing tumor conditions. FOXO1 regulates apoptosis and attenuates oxidative stress by upregulating various antioxidants like manganese superoxide dismutase (MnSOD), catalase, glutathione peroxidase, and glutathione-s-transferase (Dijkers et al. 2001; Storz 2011). FOXO1 protects cells against stress by regulating DNA damage response genes, growth arrest and the DNA-damage-inducible protein, GADD45 alpha (GADD45α) (Brunet et al. 2004). FOXO1 deletion can significantly increase oxidative stress. Growing evidence suggests that deletion of FOXO1 can inhibit cell proliferation in many types of tumor cells, including lung cancer cells (Siqueira et al. 2010; Ponugoti et al. 2012; Sangodkar et al. 2012).
Fig. 4.
Biological role of FOXO family members, target gene activation and cellular functions. Various signaling pathways are involved in phosphorylation and activation/inactivation of FOXO. Once FOXO is stimulated it translocates to the nucleus which leads to the regulation of various downstream genes related to multiple cellular functions such as cell development, cell differentiation, survival, glucose metabolism, oxidative stress and tumor suppression. Note that this figure does not include all FOXO target genes. p21, cyclin-dependent kinase inhibitor 1A; SPRY2, sprout homolog 2, BTG-1, B-cell translocation gene 1; FGF, fibroblast growth factor; SOD2, superoxide dismustase 2; CAT, catalase; Gadd45, growth arrest and DNA damage inducible protein 45, DDB1, DNA damage-binding protein 1, ATM, ATM serine/threonine kinase, POMC, pro-opiomelanocortin, UCP2, mitochondrial uncoupling protein 2; PEPCK, phosphoenolpyruvate carboxykinase; G6P, glucose-6-phosphatase; p27, cyclin-dependent kinase inhibitor 1B; FasL, Fas ligand; Bcl2, B-cell lymphoma 2 protein; PTEN, phosphatase and tensin homolog
Once FOXO1 is phosphorylated, it translocates to the cytoplasm and thereby its ability to bind to target regulatory elements is reduced (Kortylewski et al. 2003). In vivo studies using mice suggests that FOXO1 aggravates myotube fusion and myogenesis, and inhibits muscle cell differentiation (Gross et al. 2009; Hakuno et al. 2011). Lin and coworkers showed that in SIRT3 transgenic mice, FOXO1 deletion results in skeletal muscle mass reduction accompanied with impaired skeletal muscle function (Lin et al. 2014). Table 2 lists FOXO family members and their involvement in various biological functions.
Table 2.
List of FOXO family members and their involvement in various biological functions
| FOXO members | Alternative names | Expression site | Activated targeted genes | Biological functions | References |
|---|---|---|---|---|---|
| FOXO1 | FKHR | All tissues, predominant in muscle, liver, pancreas | IL-1B, Il-6, PGC1, G2-M cell cycle, SOD, MnSOD, GpX | Cell proliferation, Inflammation, Muscular atrophy, Apoptosis, Oxidative stress | Hsu et al. 2010; Van Der Vos and Coffer 2011; Brown et al. 2011 |
| FOXO3 | FKHRL1 | All tissues, predominant in muscle, liver, pancreas | PGC1a, PEPCK, UCP2, neurogenin 3, Bim, PUMA, PTEN, TRAIL, Catalase, MnSOD, GADD45, ATM, | Metabolism, Cell differentiation, Apoptosis, Oxidative stress | Van Der Vos and Coffer 2011; Alikhani et al. 2010; Ponugoti et al. 2013 |
| FOXO4 | AFX1 | Kidney, colorectal tissue | P15, P27, G1-S & G2-M cell cycle, Bim, PUMA, PTEN, TRAIL | Cell proliferation, Apoptosis | Rached et al. 2010; Alikhani et al. 2010 |
| FOXO6 | – | Brain, liver | Plxna4, PGC-1 | Gluconeogenesis, Neuronal cell development | Kim et al. 2011; Paap et al. 2016 |
Role of FOXO in diabetic complications
DM is a metabolic disease that can affect any tissue, organ and mechanism of the body. Prolonged hyperglycemia, and a deficiency in insulin production or insulin resistance leads to various health problems such as diabetic retinopathy and delayed healing (Asmat et al. 2016). Increased oxidative stress and ROS are major players in the development of diabetic complications. Several lines of evidence proposes that FOXO protein deletion or inactivation may promote cytoprotection during diabetes, and enhanced insulin secretion (OS et al. 2015). Diabetic complications can be associated with altered FOXO1 expression and its activity. FOXO1 plays a major role in regulating insulin response, and the liver is one of its critical sites of action. In the liver, FOXO1 expression leads to a unique mechanism of excessive glucose production, and increased lipid synthesis and secretion. FOXO1 is also required to maintain beta cell differentiation and regeneration in the pancreas. In endothelial cells, FOXOs intensely stimulate atherosclerosis by suppressing nitric oxide generation and increasing inflammatory responses.
Under fasting conditions, the lower production of insulin stimulates the activation of glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinases (PEPCK), which results in gluconeogenesis (a metabolic reaction in which glucose is generated from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids). This metabolic reaction depends mostly on the crosstalk between AKT and FOXO1. Other than AKT induced FOXO1 phosphorylation, the activity of FOXO1 is also regulated by various processes such as the balance between acetylation and deacetylation, and deacetylation of FOXO1 by SIRT1. During physiological stress, increased levels of free radicals activates FOXO1 and overcomes the nuclear exclusion effect of AKT. It also promotes nuclear translocation and expression of FOXO1 target genes (G6Pase & PEPCK) involved in gluconeogenesis. SIRT1 controls nuclear shuttling and transcriptional activity of forkhead transcription factors. SIRT1 regulates FOXO1 activity either in a positive or a negative way, depending on the target gene or target cell type (Giannakou and Partridge 2004).
In DM, the increase in the expression and activity of FOXO1 directly induces apoptosis in pericytes and microvascular endothelial cells, thus resulting in diabetic retinopathy (Behl et al. 2009; Wang et al. 2014). In vivo experiments carried out in diabetic mice resulted in increased levels of FOXO1 mRNA levels and increased nuclear translocation (Wang et al. 2014). FOXO1 nuclear translocation is mediated by TNF-α, and in control experiments the deletion of FOXO1 by siRNA lowers the risk of retinal microvascular endothelial cell damage (Behl et al. 2009). Hyperglycemia-induced FOXO plays a significant role in the production of pro-inflammatory cytokines, further prolonging the inflammatory phase (Ponugoti et al. 2012). In in vitro diabetic experiments, the profiling of mRNA proposed that under hyperglycemic conditions FOXO1 induces the expression of CCL2 and CCL5, which activates endothelial cells; FOXO1 also increases mRNA levels of BCL2 and CASP3, which induces apoptosis. It also enhances mRNA expression of ITGA5 and ITGAV-M that regulates angiogenesis (Wolfgang and Fernandex-Marcos 2017). In diabetic retinopathy, in vitro elevation of TNF-α and AGEs activates FOXO1 transcription factor, thereby inducing apoptosis in pericytes (Alikhani et al. 2010).
ROS is produced in response to normal cellular metabolism, and is necessary in low quantities in physiological cellular processes; however, at higher concentrations it damages cellular structures such as lipids, nucleic acids, carbohydrates, and proteins, and modifies their functions (Birben et al. 2012). Oxidative stress is defined as an imbalance between oxidants and antioxidants. ROS is produced from molecular oxygen i.e., free radicals (molecules containing one or more unpaired electrons) and non-radicals (Birben et al. 2012). Some of the major free radicals produced that are of physiological significance include superoxide anions (O2-.), hydrogen peroxide (H2O2), hydroxyl radicals (•OH), nitric oxide (NO) and peroxynitrite (ONOO−). Typically, antioxidants are effective in impeding the harmful effects of oxidants; however, under certain conditions these systems can be inundated. Oxidative stress has been implicated in and contributes to numerous pathological conditions, including diabetes, non-healing ulcers, and delayed wound healing (Chong et al. 2005; Chong and Maiese 2007; Schafer and Werner 2008).
In normal wounds, lower levels of reactive species or free radicals are immediately scavenged by endogenous antioxidants present at the wound site (Slomka et al. 2008). In vitro studies using neuronal cells suggest that FOXO3, in conjunction with JNK, results in the modulation of apoptotic ligands, which in turn activates the Fas-mediated death pathway leading to apoptosis. In normal physiological environments, FOXO1 increases mRNA levels of antioxidants, thereby attenuating apoptosis (Maiese et al. 2007). In vivo studies have shown that pancreatic β-cell damage can be protected through attenuation of oxidative stress by FOXO1 (Chong and Maiese 2007; Anastasiou et al. 2011). In normal healing, oxidative stress is reduced by FOXO1 that induces keratinocyte migration and prevents apoptosis (Anastasiou et al. 2011). In contradiction, higher levels of oxidative stress present in diabetic conditions induces cell death by the FOXO1 signaling pathway (Slomka et al. 2008). FOXOs detoxify superoxide radicals by increasing the levels of MnSOD in mitochondria. Like FOXO1, FOXO3 holds a pivotal role in protecting cells from death by apoptosis (Maiese et al. 2009).
In vivo experiments demonstrate that FOXO3 elevates antioxidant levels such as MnSOD, catalase, and peroxiredoxin III, thereby protecting cells from oxidative damage (Lu et al. 2013). Among the FOXO isoforms, FOXO3 is predominantly expressed in neural stem cells and progenitor cells. FOXO3 regulated genes are mostly related to antioxidants that prevents neuron stem cells from oxidative stress (Anastasiou et al. 2011). The relationship between FOXO activation and cell death were first recognized when it was discovered that phosphorylation and inhibition of FOXO protein mediated by AKT (regulator protein) resulted in cell survival (Tang et al. 1999).
Role of FOXO in wound healing
Transcription factors are important in coordinating events that are needed for wound healing. Restoration of damaged tissue requires stimulatory and inhibitory mediators. FOXO transcription factors (homeostatic factors) are involved in regulating wound healing and tissue regeneration, however their exact function is not fully understood (Roupe et al. 2014; Shaklai et al. 2015; Zhang et al. 2015). Mori et al., reported that in a murine skin injury model there is elevated mRNA expression and nuclear localization of FOXO1 and FOXO3. Initially, elevated levels of FOXO1 was observed predominantly in basal keratinocytes, however, a week later elevated FOXO1 levels were seen in endothelial cells, macrophages and fibroblasts (Mori et al. 2014).
Ponugoti et al., hypothesized that FOXO1 has a damaging effect on wound healing because of its ability to induce apoptosis in a biological system. Contrary to the actual hypothesis, they found that FOXO1 is required for keratinocyte mobilization and migration, and resulted in the upregulation of transforming growth factor-beta (TGF-β1) and its downstream targets such as integrin’s and MMPs. Furthermore, they also found that upregulation of FOXO1 in keratinocytes in-turn reduces oxidative stress levels in order to maintain cell proliferation and migration. There is also prevention of cell death and increased nuclear localization of FOXO1 (Ponugoti et al. 2013). Similarly, Xu et al., found that the deletion of FOXO1 in oral mucosa resulted in reduced TGF-β1 expression in keratinocytes, with reduced mucosal epithelial cell migration and impaired re-epithelialization. Therefore, in normal wounds, FOXO1 functions as an important transcription factor for wound healing (Xu et al. 2015). Figure 5 displays the possible mechanism of action of FOXO1 in normal and delayed wound healing.
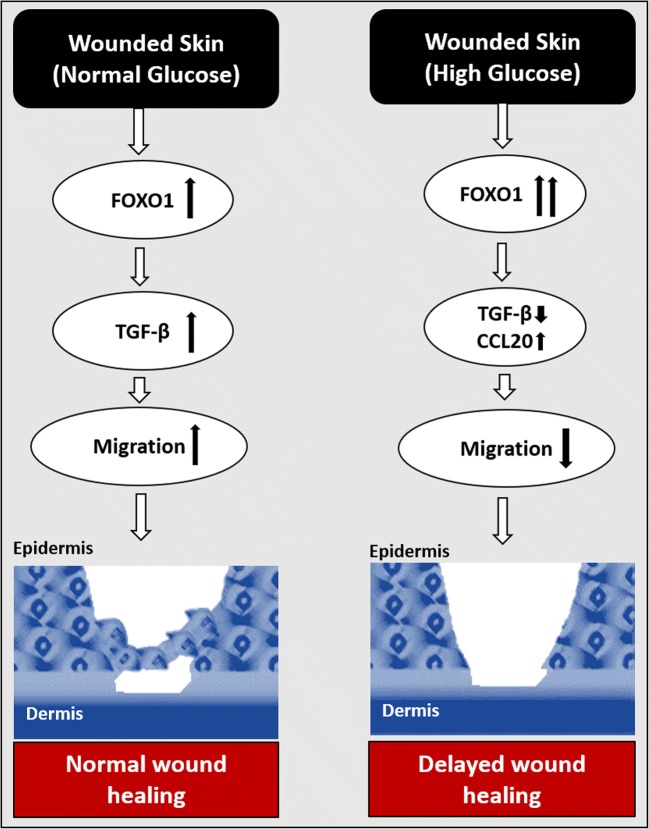
Fig. 5.
Possible mechanism of action of FOXO1 in normal and delayed wound healing. Under normoglycaemic conditions, FOXO1 significantly increases TGF-β, which actively promotes wound healing. This also leads to increased fibroblast and keratinocyte migration towards the wound bed, resulting in increased re-epithelialization and matrix deposition. However, in pathological conditions such as diabetes, hyperglycaemia stimulates FOXO1 to increase the production of inflammatory cytokines (CCL20), and the synthesis of TGFβ is blocked/interrupted. The increased expression of CCL20 and lower expression of TGF-β affects keratinocyte migration and matrix deposition. Thus, affecting the healing process and leading to delayed wound healing
Several lines of evidence suggests that FOXO1 and FOXO4 play several key roles during tissue repair. In normal wounds, typical levels of FOXO1 and FOXO4 are expressed in the epidermis, whereas in diabetic impaired wounds enhanced activation of FOXO1 and FOXO4 are observed, which induces apoptosis and delays the wound healing process (Roupe et al. 2010; Siqueira et al. 2010). Other studies have recently reported that scalp wound healing is impaired in FOXO1± genetic mice with reduced re-epithelialization (Ponugoti et al. 2013). In diabetic mice, the conditional deletion of FOXO1 has a positive effect, with enhanced wound closure as compared with normal wounds (Zhang et al. 2015; Xu et al. 2015). In order to determine the differential effect of FOXO1 under hyperglycemic conditions, mRNA profiling was carried out in diabetic mice. It was established that FOXO1 upregulates genes such as chemokine ligand 20 (CCL20), serpin peptidase inhibitor clade B2 (SERPINB2), and interleukin-36γ (IL-36γ). Furthermore, in DM, unfavorable conditions such as hyperglycemia, AGEs and increased expression of TNF-α stimulates FOXO1 binding to the promoter site of these genes to increase their expression (Zhang et al. 2015). Elevated levels of CCL20, SERPINB2, and IL-36γ inhibits keratinocyte migration and reduces re-epithelization, thereby a delay in healing is observed (Xu et al. 2015). Additionally, hyperglycemia induced AGE formation and increased levels of TNF-α disrupts FOXO1 interaction with TGF-β1, thus preventing FOXO1 from inducing TGF-β1 transcription. These studies reveal the negative side of TGF-β1 in the healing process, resulting in impaired healing (Zhang et al. 2015).
Moreover, increased nuclear translocalization of FOXO1 was observed in diabetic keratinocytes as compared to normal control keratinocytes (Ponugoti et al. 2013). Contradictory to this, other research groups found that in human and murine wound models, reduced levels of FOXO1 and FOXO3 gene expression were observed (Roupe et al. 2014). Another in vivo experiment demonstrated that FOXO deletion resulted in impaired TGF-β1-dependent keratinocyte migration and elevated apoptosis at the wound site, resulting in impaired healing. In FOXO1a± mice, partial deletion or inhibition of FOXO1 increased fibroblast growth factor 2 (FGF-2) expression, as well as adiponectin and Notch1 at the site of injury. On the other hand, reduced collagen fiber organization, and lower gene expression of type 1 collagen α1, and reduced levels of collagen type I and III were observed at the wound site as compared to wild-type mice (Mori et al. 2014).
In human keloid scars, an alteration in FOXO1 activity has been identified, which gives rise to elevated levels of inflammatory and fibroblast cells, accompanied with an overgrowth of fibrotic tissue (Shaklai et al. 2015). Siqueria et al., found that as compared to non-diabetic mice, diabetic mice displayed higher levels of FOXO1 in the wound bed, and it was associated with elevated levels of TNF-α which induces inflammation, thereby increasing apoptosis in fibroblasts (Siqueira et al. 2010). Many researchers are trying to establish the cause for a switch between wound acceleration and impairment, which may be related to hyperglycemia-regulated FOXO1 (Shaklai et al. 2015).
It has been hypothesized that FOXO1 may have a negative effect on bone healing in DM. Several studies propose that the activation of FOXO1 might increase inflammatory cytokines and apoptosis, which damages cartilage in diabetic fracture conditions. In vitro, FOXO1 mediates TNF-α induced expression of pro-osteoclastogenic factors in chondrocytes (TNF-α, RANKL, M-CSF, IL-1β, and IL-6) and the chemokine CCL4, which is linked to a burst of osteoclast activity and accelerated loss of cartilage in diabetic fractures (Alblowi et al. 2009). An in vitro study using chondrogenic cells showed that increased levels of FOXO1 stimulates TNF-α induced apoptosis, thereby upregulating pro-apoptotic genes and cell death (Kayal et al. 2010).
Concluding remarks and future perspectives
Wound healing is a complex overlapping process that relies on various molecules and signaling pathways. FOXO activity is highly controlled by acetylation, phosphorylation and ubiquitylation, and once FOXO proteins are activated, they translocate to the nucleus and regulate the transcription of other genes. FOXO transcription factors are emerging as master signaling molecules that influence many physiological and pathological processes of wound healing. FOXO transcription proteins control numerous biological functions such as inflammation, oxidative stress, apoptosis, cell proliferation, migration, stress resistance, and metabolism through regulation of multiple transcriptional targets. The control and balance in FOXO augments wound healing, and decreases oxidative stress and apoptosis. It would appear as if the transcription factors of FOXO serve as molecular controls, determining cellular fate in response to oxidative stress by either promoting cell survival, through the upregulation of antioxidants, or promoting cellular death through the upregulation of pro-apoptotic genes. However, this exact mechanism remains unclear and further research is necessitated, and a further understanding of FOXOs role will provide the necessary insight to both basic and clinical science.
Hyperglycemia-induced free radicals and consequent elevated oxidative stress are chief contributors to the development and advancement of DM and associated complications. Diabetic wounds have decreased cellular proliferation and migration, and elevated levels of oxidative stress and apoptosis. It has been identified that FOXO transcription factors control the fate of many cells and plays a key role in diabetes-induced oxidative stress resistance and apoptosis. Targeting FOXO1 could be a potential treatment for patients with diabetes, which results in hyperglycemia-induced nuclear translocation of FOXO1, lower mRNA levels of FOXO1 target genes, and decreased inflammatory cells in the wound site. To date, FOXOs role in the healing of wounds is not fully established. On the positive side, increased levels of FOXOs following skin injury is essential to maintain a normal healing process by attenuating oxidative stress at the wound site. On the negative side, in pathological conditions like DM, an alteration in FOXO1 levels results in elevated levels of oxidative stress that hasten the wound and stimulate fibrotic tissue over growth that leads to keloid scars. Further clarification of the role and control of FOXO in diabetic wound healing may present new treatment targets.
Acknowledgements
This work was supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant No 98337); the University of Johannesburg; the CSIR National Laser Centre Laser Rental Pool Program; and the National Research Foundation of South Africa. Sathish Sundar Dhilip Kumar is supported by funding from the Claude Leon Foundation, South Africa. Funding sources had no involvement in study design, data collection, analysis and interpretation, writing of the report, and in the decision to submit the article for publication.
Compliance with ethical standards
Conflict of interests
The authors confirm that this article content has no conflict of interest.
References
- Alblowi J, Kayal RA, Siqueria M. High levels of tumor necrosis factor-α contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009;175:1574–1585. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikhani M, Roy S, Graves DT. FOXO1plays an essential role in apoptosis of retinal pericytes. Mol Vis. 2010;16:408–415. [PMC free article] [PubMed] [Google Scholar]
- Anastasiou D, Poulogiannis G, Asara JM. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki M, Jiang H, Vogt PK. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and AKT oncoproteins. PNAS. 2004;101:13613–13617. doi: 10.1073/pnas.0405454101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-a concise review. Saudi Pharm J. 2016;24:547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel A, Schmoll D, Unterman TG. FOXO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Behl Y, Krothapalli P, Dessta T, Roy S, Graves DT. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes. 2009;58:917–925. doi: 10.2337/db08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birben E, Murat US, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. WAO Journal. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Wang H, Suttles J, Graves DT, Martin M. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates toll-like receptor 4-mediated inflammatory response via FoxO1. The J Biol Chem. 2011;286(52):44295–44305. doi: 10.1074/jbc.M111.258053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27(16):2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22:1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitoku H, Sakamaki JI, Fukamizu A. Regulation of FOXO transcription factors by acetylation and protein-protein interactions. Biochim Biophy Acta. 2011;1813:1954–1960. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Dansen TB, Smits LMM, van Triest MH. Redoxsensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol. 2009;5(9):664–672. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- Dharaneeswaran H, Ruhul A, Yuan L, et al. FOXO1-mediated activation of AKT plays a critical role in vascular homeostasis. Circ Res. 2014;115(2):238–251. doi: 10.1161/CIRCRESAHA.115.303227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2001;10:1201–1204. doi: 10.1016/S0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms. Signaling and translation. Sci Transl Med. 2014;6:265–274. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signaling in cancer: opportunities, challenges and limitations. Nat Reviews Can. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Eric WF, Brosens JJ, Gomes AR, Koo CY. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rew Can. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004;14:408–412. doi: 10.1016/j.tcb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208–214. doi: 10.1007/s11892-009-0034-5. [DOI] [PubMed] [Google Scholar]
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakuno F, Yamauchi Y, Kaneko G, Yoneyama Y. Constitutive expression of insulin receptor substrate (IRS)-1 inhibits myogenic differentiation through nuclear exclusion of FOXO1 in L6 myoblasts. PLoS One. 2011;6:e25655. doi: 10.1371/journal.pone.0025655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameedaldeen A, Liu J, Batres A, Graves GS, Graves DT. FOXO1, TGF-beta regulation and wound healing. Int J Mol Sci. 2014;15:16257–16269. doi: 10.3390/ijms150916257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KK, Myatt SS, Lam EW. Many forks in the path: cycling with FOXO. Oncogene. 2008;27:2300–2311. doi: 10.1038/onc.2008.23. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Zhai P, Yamamoto T, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122(21):2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phophorolation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- Huang H, Tindall DJ. Dynamic FOXO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- Jacobs FM, van der Heide LP, Wijchers JP, Burbach M, Hoekman MF, Smidt S. FOXO6, a novel member of the FOXO class of transcription factors with distinct shuttling dynamic. J Biol Chem. 2003;278:35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- Kayal RA, Siqueira M, Alblowi J, et al. TNF-a mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J Bone and Min Res. 2010;25:1604–1615. doi: 10.1002/jbmr.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Perdomo G, Zhang T. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60(11):2763–2774. doi: 10.2337/db11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Furukawa-Hibi Y, Chen C. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16(2):237–243. [PubMed] [Google Scholar]
- Kortylewski M, Feld F, Kruger KD, Bahrenberg G, Roth RA, Joost HG, Heinrich PC, Behrmann I, Barthel A. Akt modulates STAT3-mediated gene expression through a FKHR (FOXO1a)-dependent mechanism. JBiol Chem. 2003;278:5242–5249. doi: 10.1074/jbc.M205403200. [DOI] [PubMed] [Google Scholar]
- Lalmansingh AS, Karmakar S, Jin Y. Multiple modes of chromatin remodeling by Forkhead box proteins. Biochim Biophys Acta. 2012;181:707–715. doi: 10.1016/j.bbagrm.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Lalmansingh AS, Karmakar S, Jin Y, Nagaich AK. Multiple modes of chromatin remodeling by Forkhead box proteins. Biochim Biophys Acta: Gene Regul Mech. 2012;1819(7):707–715. doi: 10.1016/j.bbagrm.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Lam EW, Brosens JJ, Gomes AR. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- Lee D, Goldberg AL. SIRT1 protein, by blocking the activities of transcription factors FOXO1 and FOXO3, inhibits muscle atrophy and promotes muscle growth. J Biol Chem. 2013;288:30515–30526. doi: 10.1074/jbc.M113.489716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker E. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Lima MH, Caricilli AM, deAbreu LL, Araujo EP, Pelegrinelli FF, Thirone AC, Tsukumo DM, Pessoa AF, dos Santos MF, de Moraes MA, Carvalheira JB, Velloso LA, Saad MJ. Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS One. 2012;7:e36974. doi: 10.1371/journal.pone.0036974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Chen L, Abdel Khalek W, Ward JL, Yang H, Chabi B, Wrutniak-Cabello C, Tong Q. Regulation of skeletal muscle oxidative capacity and muscle mass by SIRT3. PLoS One. 2014;9:e85636. doi: 10.1371/journal.pone.0085636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Zhai Y, Cheng Q. The Akt-FOXO3a-manganese superoxide dismutase pathway is involved in the regulation of oxidative stress in diabetic nephropathy. Exp Physiol. 2013;98:934–945. doi: 10.1113/expphysiol.2012.068361. [DOI] [PubMed] [Google Scholar]
- Rached M-T, Kode A, Xu L, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11(2):147–160. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015) FOXO proteins in the nervous system. Anal Cell Pathol 56 [DOI] [PMC free article] [PubMed]
- Maiese K, Hou J, Chong ZZ, Shang YC. Erythropoietin, Forkhead proteins and oxidative injury: biomarkers and biology. Sci World J. 2009;9:1072–1104. doi: 10.1100/tsw.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins VL, Caley M, O’Toole EA. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013;351:255–268. doi: 10.1007/s00441-012-1410-z. [DOI] [PubMed] [Google Scholar]
- Milan G, Romanello V, Pescatore F, Armani A, Paik JH. Regulation of autophagy and the ubiquitin-proteasome system by the FOXO transcriptional network during muscle atrophy. Nat Commun. 2015;6:6670–6683. doi: 10.1038/ncomms7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R, Tanaka K, Kerckhove M, Kashiyama MO. Reduced FOXO1 expression accelerates skin wound healing and attenuates scarring. Am J Pathol. 2014;184:2465–2479. doi: 10.1016/j.ajpath.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OS I, Zhang W, Wasserman DH, Liew CW, Liu J, Paik J. FOXO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nature Comm. 2015;6:70–79. doi: 10.1038/ncomms8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paap RH, Oosterbroek S, Wagemans CM, Oerthel LV, et al. FOXO6 affects Plxna4 mediated neuronal migration during mouse cortical development. PNAS. 2016;113(45):23–32. doi: 10.1073/pnas.1609111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Izumiya Y, Walsh K. Forkhead transcription factor and cardiovascular biology. Circ Res. 2008;102:16–31. doi: 10.1161/CIRCRESAHA.107.164186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponugoti B, Dong G, Graves DT. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res. 2012;51:93–97. doi: 10.1155/2012/939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponugoti B, Xu F, Zhang C, Tian C, Pacios S, Graves DT. FOXO1 promotes wound healing through the up-regulation of TGF-beta1 and prevention of oxidative stress. J Cell Biol. 2013;203:327–343. doi: 10.1083/jcb.201305074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putker M, Madl T, Vos HR. Redox-dependent control of FOXO/DAF-16 by transportin-1. Mol Cell. 2013;49(4):730–742. doi: 10.1016/j.molcel.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2(1):81–91. doi: 10.1016/S1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- Renault VM, Rafalski VA, Morgan AA. FOXO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roupe KM, Alberius P, Schmidtchen A, Sorensen OE. Gene expression demonstrates increased resilience toward harmful inflammatory stimuli in the proliferating epidermis of human skin wounds. Exp Dermatol. 2010;9:e329–e332. doi: 10.1111/j.1600-0625.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- Roupe S, Veerla A, Olson J, Stone EL, Sorensen OE, Hedrick SM, Nizet V. Transcription factor binding site analysis identifies FOXO transcription factors as regulators of the cutaneous wound healing process. PLoS One. 2014;9:e89274. doi: 10.1371/journal.pone.0089274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathia NS, Shi J, Zhang J, Glynne RJ. An in vivo screen of secreted proteins identifies adiponectin as a regulator of murine cutaneous wound healing. J Invest Dermatol. 2013;133:812–821. doi: 10.1038/jid.2012.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih DA, Rashid AJ, Colas D. FOXO6 regulates memory consolidation and synaptic function. Gen Devel. 2012;26:2780–2801. doi: 10.1101/gad.208926.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangodkar J, Dhawan NS, Melville H, Singh VJ, Yuan E, Rana H. Targeting the FOXO1/KLF6 axis regulates EGFR signaling and treatment response. J Clin Invest. 2012;122:2637–2651. doi: 10.1172/JCI62058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Werner S. Oxidative stress in normal and impaired wound repair, Pharmacol. Res. 2008;58(2):165–171. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Serravallo M, Jagdeo J, Glick SA, Siegel DM, Brody NL. Sirtuins in dermatology: applications for future research and therapeutics. Arch Dermatol Res. 2013;305:269–282. doi: 10.1007/s00403-013-1320-2. [DOI] [PubMed] [Google Scholar]
- Shaklai G, Shefer N, Stern K. Glucose-dependent FOXO1 switch in healing wounds: a shred of hope for diabetic ulcers? Diabetes. 2015;64:6–8. doi: 10.2337/db14-1440. [DOI] [PubMed] [Google Scholar]
- Shaw TS, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Tada Y, Asano Y, Hau CS, Kato T, Saeki H, Yamauchi T, Kubota N, Kadowaki T, Sato S. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J Immunol. 2012;189:3231–3241. doi: 10.4049/jimmunol.1101739. [DOI] [PubMed] [Google Scholar]
- Siqueira MF, Li J, Chehab L, Desta T, Chino T, Krothpali N, Behl Y, Alikhani M, Yang J, Braasch C, Graves DT. Impaired wound healing in mouse models of diabetes is mediated by TNF-alpha dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1) Diabetologia. 2010;53:378–388. doi: 10.1007/s00125-009-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomka M, Zieminska E, Lazarewicz J. Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp. 2008;68:1–9. doi: 10.55782/ane-2008-1666. [DOI] [PubMed] [Google Scholar]
- Stadeli R, Hoffmans R, Basler K. Transcription under the ¨ control of nuclear arm/훽-catenin. Curr Biol. 2006;16(10):378–385. doi: 10.1016/j.cub.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011;14:593–605. doi: 10.1089/ars.2010.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- Tikhanovich I, Cox J, Weinman SA. Forkhead box class O transcription factors in liver function and disease. J Gastroenterol Hepatol. 2013;28:125–131. doi: 10.1111/jgh.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek P, Klotz LO (2016) Posttranscriptional regulation of FOXO expression: microRNAs and beyond. Br J Pharmacol. 10.1111/bph.13471 [DOI] [PMC free article] [PubMed]
- Van Der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Antioxid Redox Signal. 2011;14:579–592. doi: 10.1089/ars.2010.3419. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Y, Graves DT (2014) FOXO transcription factors: their clinical significance and regulation. BioMed res. Int. 1-13 [DOI] [PMC free article] [PubMed]
- Wolfgang L, Fernandex-Marcos PJ. FOXO transcription factors at the interface of metabolism and cancer. Int J Can. 2017;15:2379–2391. doi: 10.1002/ijc.30840. [DOI] [PubMed] [Google Scholar]
- Woods YL, Rena G, Morrice N, Barthel A, Becker W, Guo S, Unterman TG, Cohen P. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro a novel in vivo phosphorylation site. Biochem J. 2001;355:597–607. doi: 10.1042/bj3550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Chen J, Yuan Z. Post-translational regulation of FOXO. Acta Biochim Biophys Sin. 2012;44(11):897–901. doi: 10.1093/abbs/gms067. [DOI] [PubMed] [Google Scholar]
- Xu F, Othman B, Lim J, Batres A, Ponugoti B, Zhang C, Yi L, Liu J, Tian C. FOXO1 inhibits diabetic mucosal wound healing but enhances healing of normoglycemic wounds. Diabetes. 2015;64:243–256. doi: 10.2337/db14-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y, Wei LL, Inoue KI, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem. 2006;281(8):5267–5276. doi: 10.1074/jbc.M512151200. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ponugoti B, Tian C, Xu F, Tarapore R, Batres A, Alsadun S, Lim J, Dong G, Graves DT. FOXO1 differentially regulates both normal and diabetic wound healing. J Cell Biol. 2015;209:289–303. doi: 10.1083/jcb.201409032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou ZQ, Xu J, Li L, Han YS. Down-regulation of SENCR promotes smooth muscle cells proliferation and migration in db/db mice through up-regulation of FOXO1 and TRPC6. Biomed Pharmacother. 2015;74:35–41. doi: 10.1016/j.biopha.2015.06.009. [DOI] [PubMed] [Google Scholar]