Abstract
Granulocyte colony-stimulating factor (G-CSF) is commonly used in clinical practice to accelerate neutropenia recovery after chemotherapy. G-CSF is a myeloid growth factor produced by monocytes, macrophages, fibroblasts and endothelial cells. Generally, aortitis and arteritis are not a known side effect of G-CSF and is thought to be extremely rare. Here, we present a case of a 77-year-old woman who underwent adjuvant chemotherapy (combined paclitaxel and carboplatin) for ovarian cancer, and then developed acute arteritis after receiving G-CSF. She developed grade 4 neutropenia on day 7 of the third chemotherapy cycle and received six G-CSF administrations. Two days after G-CSF administration, she came down with a high-grade fever that persisted for 2 weeks. Laboratory tests revealed a white blood cell count of 8700 UI, neutrophilic sequestration of 61.5%, and C-reactive protein of 8.43 mg/dl at the highest point of her fever. Considering that we were initially treating neutropenia, we diagnosed a bacterial infection, and she was treated with a course of antibiotics. However, her blood and urinalysis cultures were negative, and antibiotics were ineffective; thus, we performed a computed tomography scan to search for the cause of her persistent fever. The computed tomography scan showed remarkable thickness of the bilateral common carotid artery and the left subclavian artery consistent with arteritis. With cessation of the antibiotics course, she was followed closely without therapy, and her condition resolved in a few days. We conclude that G-CSF induced arteritis due to our exclusion of other probable etiologies.
Keywords: Aortitis, Arteritis, G-CSF, Side effect
Introduction
Granulocyte colony-stimulating factor (G-CSF) is a myeloid growth factor that was first discovered in the 1960s and became clinically available in the 1980s. In clinical practice, it is used to mobilize peripheral blood progenitor cells from healthy donors before hematopoietic stem-cell transplantation and to accelerate neutrophil recovery in patients after induction or consolidation chemotherapy or with hematologic malignancies. D’Souza et al. have reported in detail about the adverse events associated with G-CSF. Their report presented that bone pain, headache, and fatigue were well-known common side effects [1]. In general, G-CSF is thought to be relatively safe; however, rare side effects have also been reported. For example, rheumatologic diseases and vasculitis have presented after G-CSF administration. Most of these cases were flares of arthritis from Felty’s syndrome or anti-neutrophilic cytoplasmic antibody-associated vasculitis [2–6]. Therefore, acute aortitis and arteritis after G-CSF are an extremely rare side effect with only three previously reported cases. Here, we report a case of a patient with acute arteritis after G-CSF administration, which was used for neutropenia following chemotherapy.
Case report
The patient was a 77-year-old Japanese female who received adjuvant chemotherapy for ovarian cancer. Ovarian cancer was suggested by ultrasonography, and she presented to our department with abdominal pain. Magnetic resonance imaging indicated an abdominal mass with ascites that consisted of cystic and solid components in contact with the right side of the uterus. Tumor maker levels were as follows: cancer antigen 125 was 1176.0 U/ml, cancer antigen 19–9 was 2.9 U/ml, and carcinoembryonic antigen was 1.6 ng/ml. These findings led to a preoperative diagnosis of a malignant ovarian tumor.
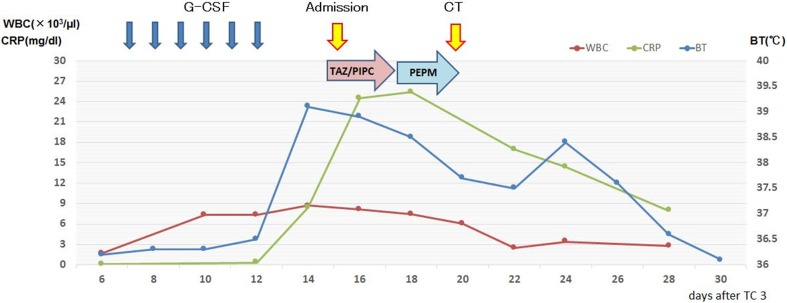
Surgical resection of the abdominal cystic mass was performed. The right ovary was enlarged, and the ascites was in the abdominal cavity. Hysterectomy, bilateral oophorectomy, pelvic lymph node dissection, and omentectomy were performed. The pathological diagnosis was serous carcinoma of the right ovary of FIGO stage IIIc (pT3cN1M0). We selected adjuvant chemotherapy of tri-weekly TC (180 mg/m2 paclitaxel on day 1 plus carboplatin AUC 5 on day 1, every 3 weeks for 6 cycles). Because grade 3 neutropenia developed after the second cycle of TC therapy, we extended the interval to 4 weeks to wait for neutrophil recovery. At the same time, we determined that therapy elicited a partial response using the Response Evaluation Criteria in Solid Tumors (v1.1). Continuously, the third cycle of TC therapy was performed, and she subsequently developed grade 4 neutropenia on day 7 of the third cycle. As a treatment, she received 75 µg G-CSF administrations subcutaneously once daily for 6 days. On day 15, she developed a high-grade fever (> 39 °C) with a chill and presented to our department (Fig. 1).
Fig. 1.
This chart shows the course of fever
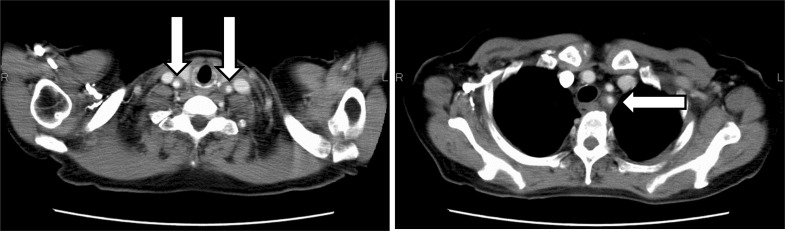
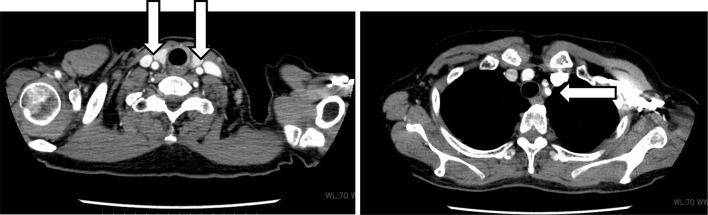
She had normal blood pressure, heart rate, and physical findings. Laboratory data revealed a white blood cell count of 8700 UI, neutrophilic sequestration of 61.5%, procalcitonin of 0.50 ng/dl, and C-reactive protein of 8.43 mg/dl. A chest X-ray and urinalysis showed normal findings. A β-d-glucan test was negative, which indicated low probability of typical fungal infection. With these laboratory data, we suspected that she had gotten a bacterial infection at the point of neutropenia. Therefore, we collected all cultivation tests (especially for blood culture, two set of bottles for aerobic and anaerobic cultivation) and empirically administered a course of intravenous antibiotics (tazobactam and piperacillin) every 8 h. At the same time, she had radiating pain from her left neck to left mandible. She could not achieve pyretolysis after antibiotic treatment for 3 days, so we changed to another antibiotic (intravenous meropenem hydrate every 12 h). After changing treatments, her general status did not improve; therefore, we performed a computed tomography (CT) scan with intravenous contrast to seek the heat source. The CT scan revealed that the walls of the bilateral common carotid artery and the left subclavian artery were remarkably thickened (Fig. 2). The erythrocyte sedimentation rate was 135 mm/h, and pANCA, cANCA, and Rheumatoid factor were within normal limits. To rule out viral infections, we performed a viral antibody test of Human T-cell Lymphotropic Virus type 1, Herpes Simplex Virus, Cytomegalovirus, Varicella-Zoster Virus, and Human Parvovirus B 19, which were all negative. On the basis of these results, we diagnosed her with arteritis; however, the source of the syndrome was unknown. Infectious and rheumatic diseases were interpreted to be negative, and only G-CSF was administrated before the fever appeared. Thus, we suspected the medication was responsible. After making her diagnosis, her general condition, including fever and thickness of arterial walls, became better with no medication. Continually, she received the same chemotherapy up to six cycles after dose reductions to prevent recurrent neutropenia that would require G-CSF. Two months later, the CT scan showed that these arterial wall thicknesses improved (Fig. 3). She has exhibited no evidence of disease for 2 years since completing the sixth chemotherapy cycle.
Fig. 2.
The CT scan revealed that the walls of the bilateral common carotid artery and the left subclavian artery were remarkably thickened
Fig. 3.
The CT scan showed that the wall thickness of the bilateral common carotid artery and the left subclavian artery improved 2 months later from onset
Discussion
We will consider several angles of this case. First, we will present potential causes of arteritis and the side effects of G-CSF. Next, we will consider this case from the viewpoint of a causal connection.
There are several factors that can cause aortitis and arteritis, including infectious, inflammatory, and isolated aortitis. The most common causes of these are large-vessel vasculitides, giant cell arteritis, and Takayasu arteritis. Based on several clinical studies, it was unlikely that this case was from these causes of aortitis. Conversely, idiopathic aortitis, which includes isolated aortitis, are generally subclinical in nature and are diagnosed incidentally during histological review after aortic aneurysm surgery [7]. Rojo-Leyva et al. reported 52 cases of idiopathic aortitis by retrospectively working up charts and pathology reviews of surgical specimens [8]. They indicated that 96% (35/36) of idiopathic aortitis cases were without systemic disease and had aortic aneurysms. However, this case did not fit within the classifications of their paper because there were no other systemic diseases or aortic aneurysms.
Second, we will consider the relationship between G-CSF side effects and arteritis development. G-CSF is commonly used in typical clinical situations and is thought to be relatively safe. D’Souza et al. reported that common side effects of G-CSF were bone pain, headache, and fatigue. As to rheumatologic diseases and vasculitis following G-CSF administration, arthritis flares by Felty’s syndrome have been reported [1–6]. Acute aortitis and arteritis after G-CSF administration are a rare side effect; three cases have been previously reported [9–11] (Table 1). In all previous cases, pathological diagnoses were not made, rather only imaging was used to make diagnoses. The primary aortitis lesion was in the abdominal aorta, and steroid administration was needed. One of these cases had a left iliac aneurysm.
Table 1.
Previous cases of aortitis after G-CSF administration
| Darie et al. | Adiga et al. | Miller et al. | This case | |
|---|---|---|---|---|
| Age/sex | 55/F | 54/M | 52/M | 77/F |
| Underlying disease | None | Lung.ca (SCC) | None | Ova.ca |
| G-CSF therapy (days) | 5 | 8 | 4 | 6 |
| Time to onset | 2 days | 8 days | 6 months | 8 days |
| Symptoms | Fever, epigastric pain | Fever, epigastric pain | Fever, epigastric pain | Fever, neck pain |
| ESR | 108 | 113 | 39 | 135 |
| Complications | None | None | Aneurysm | None |
The causal relationship between G-CSF administration and aortitis development remains unknown. However, the fact that G-CSF affects lymphocyte function and lymphocyte-derived cytokine production is commonly known [11, 12]. There has not been a report about the relevance of these immune activities and arteritis. Thus, future research into the mechanisms underlying this phenomenon is required.
Acknowledgements
We thank James P. Mahaffey, PhD, from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
There is no editorial or financial conflict of interest among authors.
Conflict of interest
All authors have no conflict of interest.
Consent for publication
The case report approval was obtained from the Hospital Research Ethics Board.
Informed consent
Informed consent was obtained from individual participants included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Souza A, Jaiyesimi I, Trainor L, et al. Granulocyte colony-stimulating factor administration: adverse events. Transfus Med Rev. 2008;22(4):280–290. doi: 10.1016/j.tmrv.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 2.McMullin MF, Finch MB. Felty’s syndrome treated with rhG-CSF associated with flare of arthritis and skin rash. Clin Rheumatol. 1995;14(2):204–208. doi: 10.1007/BF02214945. [DOI] [PubMed] [Google Scholar]
- 3.Hayat SQ, Hearth-Holmes M, Wolf RE. Flare of arthritis with successful treatment of Felty’s syndrome with granulocyte colony stimulating factor (GCSF) Clin Rheumatol. 1995;14(2):211–212. doi: 10.1007/BF02214946. [DOI] [PubMed] [Google Scholar]
- 4.Iking-Konert C, Ostendorf B, Foede M, et al. Granulocyte colony-stimulating factor induces disease flare in patients with antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2004;31(8):1655–1658. [PubMed] [Google Scholar]
- 5.Farhey YD, Herman JH. Vasculitis complicating granulocyte colony stimulating factor treatment of leukopenia and infection in Felty’s syndrome. J Rheumatol. 1995;22(6):1179–1182. [PubMed] [Google Scholar]
- 6.Vidarsson B, Geirsson AJ, Onundarson PT. Reactivation of rheumatoid arthritis and development of leukocytoclastic vasculitis in a patient receiving granulocyte colony-stimulating factor for Felty’s syndrome. Am J Med. 1995;98(6):589–591. doi: 10.1016/S0002-9343(99)80019-9. [DOI] [PubMed] [Google Scholar]
- 7.Gornik HL, Creager MA, Aortitis Circulation. 2008;117(23):3039–3051. doi: 10.1161/CIRCULATIONAHA.107.760686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojo-Leyva F, Ratliff NB, Cosgrove DM, et al. Study of 52 patients with idiopathic aortitis from a cohort of 1,204 surgical cases. Arthritis Rheum. 2000;43(4):901–907. doi: 10.1002/1529-0131(200004)43:4<901::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Darie C, Boutalba S, Fichter P, et al. Aortitis after G-CSF injections. Rev Med Interne. 2004;25(3):225–229. doi: 10.1016/j.revmed.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Adiga GU, Elkadi D, Malik SK, et al. Abdominal aortitis after use of granulocyte colony-stimulating factor. Clin Drug Investig. 2009;29(12):821–825. doi: 10.2165/11530790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Miller EB, Grosu R, Landau Z. Isolated abdominal aortitis following administration of granulocyte colony stimulating factor (G-CSF) Clin Rheumatol. 2016;35(6):1655–1657. doi: 10.1007/s10067-016-3253-6. [DOI] [PubMed] [Google Scholar]
- 12.Sloand EM, Kim S, Maciejewski JP, et al. Pharmacologic doses of granulocyte colony-stimulating factor affect cytokine production by lymphocytes in vitro and in vivo. Blood. 2000;95(7):2269–2274. [PubMed] [Google Scholar]