Abstract
Notch signaling has been reported to correlate with tumor progression and metastasis in several types of cancer. In cholangiocarcinoma (CCA), it has recently been shown that NOTCH1 is overexpressed in both nucleus and cytoplasm of CCA cells; however, the complete understanding of Notch signaling in CCA is still lacking. Here, we aimed to understand the functions of NOTCH1 in CCA cells and the molecular mechanisms that underlie those functions. We used retroviral vectors to overexpress active forms of NOTCH1, the NOTCH1 intracellular domain (N1ICD) and N1ICD that lacks the RBP-J-associated module (RAM), in human CCA cell lines RMCCA-1 and HuCCA-1. Our results showed that activation of Notch signaling by both N1ICD variants enhanced CCA cell proliferation and survival via upregulation of pro-survival protein Mcl-1 and Bcl-xL. Moreover, our LC-MS/MS proteomic studies demonstrated that NOTCH1 may cooperate with 14-3-3 theta to promote CCA cell survival. Knockdown of 14-3-3 theta in RMCCA-1 cells overexpressing N1ICD, diminished pro-survival effects of N1ICD under gemcitabine treatment. In conclusion, these data demonstrated that NOTCH1 plays a role in CCA cell proliferation and survival via the regulation of 14-3-3 theta in a RAM-independent fashion.
Keywords: Cholangiocarcinoma, Notch, Proteomics, 14-3-3 theta
Introduction
Notch signaling regulates a wide variety of cellular processes, including stem cell self-renewal, cell fate specification, cellular differentiation, proliferation, and apoptosis (Greenwald 1998, 2005). In mammals, there are four transmembrane Notch receptors (Notch1–4) and five transmembrane ligands (Delta-likes Dll1, Dll3, Dll4, Jagged1, and Jagged2). Activation of the Notch pathway occurs after a Notch ligand binds the extracellular domain of a Notch receptor, leading to proteolytic cleavages and a release of the intracellular domain (NICD). The NICD is then translocated to the nucleus, where it forms a complex with CSL (CBF-1/RBP-J, Suppressor of Hairless, LAG-1) and Mastermind-Like (MAML) and activates the transcription of Notch target genes including basic-helix-loop helix HES and HEY transcriptional repressors (Nakagawa et al. 2000; Davis and Turner 2001; Kovall and Blacklow 2010).
A role for Notch signaling has been reported in both solid tumors and hematological malignancies (Ranganathan et al. 2011). Aberrant Notch signaling has been associated with several human cancers, including acute T cell lymphoblastic leukemia (T-ALL), breast, cervical, lung cancers, and cholangiocarcinoma (CCA) (Stylianou et al. 2006; Grabher et al. 2006; Zender et al. 2013). It has been demonstrated that overactivation of NOTCH1 resulted in tumor formation in a hepatocyte-specific Notch-transgenic mouse, thereby leading to the development of intrahepatic CCA formation. Inhibition of Notch activity in CCA blocked tumor cell proliferation and induced apoptosis (Zender et al. 2013). Additionally, overactivation of Notch signaling resulted in the upregulation of anti-apoptotic proteins and protected T-cells from diverse apoptotic stimuli (Palaga et al. 2013). Notch activity inhibits changes in Bax conformation and dynamics (Sade et al. 2004). Perumalsamy and colleagues using site-directed mutagenesis identified AktS473 as a key downstream target in the anti-apoptotic pathway activated by NICD. Notch signaling has also been implicated in an autocrine signaling loop that activates Akt in breast epithelial cells, leading to suppression of apoptosis (Meurette et al. 2009).
In addition to RBP-J, recent reports demonstrated that NICD can also interact with other proteins in the cytoplasm and the nucleus, implicating that Notch signaling could possibly use an alternate route to exert some of its effects in an RBP-J-independent or “non-canonical” pathway. Deletion of the RBP-J-associated module (RAM) domain, which mediates NICD-RBP-J interactions, did not abrogate NICD function in leukemic T cells (Aster et al. 2000; Kurooka and Honjo 2000). Furthermore, the RAM-deleted NICD inhibits Bax-induced cellular damage in Hela cells (Perumalsamy et al. 2010). However, it is unknown whether non-canonical Notch activation promotes cell proliferation, migration, and apoptosis of CCA cells. Therefore, in this study we investigated the role of Notch signaling using the N1ICD constructs with or without the RAM domain to assess Notch functions in both RBP-J-dependent and -independent pathways in CCA cells. In addition, to elucidate the molecular mechanisms we also analyzed differentially expressed proteins using proteomic analysis by LC-MS/MS and identified proteins in the 14-3-3 family as candidates for Notch targets, especially 14-3-3 theta. Expression and functional validation confirmed that 14-3-3 theta is regulated by RBP-J-independent Notch signaling in promoting CCA cell proliferation and survival under chemotherapeutic treatment, suggesting that Notch and its downstream effectors may represent interesting candidates for cancer targeting and novel therapeutic strategies.
Materials and methods
Cell lines and reagents
All cell cultures were maintained at 37 °C in a mixture of 5% CO2 and 95% humidified air. The human cholangiocarcinoma cell lines RMCCA-1 and HuCCA-1 were obtained from the Tohtong and Sirisinha laboratories (Sirisinha et al. 1991; Rattanasinganchan et al. 2006). Both cancer cell lines were maintained in 1X High Glucose DMEM (Invitrogen) with 10% fetal bovine serum and 1X Pen-Strep (Invitrogen).
cDNA constructs and retroviral transduction
The retroviral vectors, MSCV-IRES-GFP (MIG), that contain cDNAs encoding NOTCH1 intracellular domain (N1ICD) and its variants lacking the RBPJ-associated module (ΔRAM), were obtained from the Warren Pear laboratory and previously described (Aster et al. 2011). HEK 293 T cells were transfected with the pCL-Ampho and MIG plasmids using the calcium phosphate transfection method. Recombinant retroviruses were collected 24 h after transfection for cholangiocarcinoma cell transduction. Successful expression of the transgenes was confirmed by GFP signals. To obtain GFP-positive cell populations, fluorescence-activated cell sorting (FACS) was performed using the FACS Aria II (Becton Dickinson).
Cell viability and migration assays
Cells were seeded in 96-well plates at a density of 3000 cells/well. The MTT assay was performed at 48 h and measured at 540 nm for cell viability. To determine cell migration, cells were seeded at 1.0 × 105 cells per well in 24-well plates. After 24 h when cells are confluent, the cell monolayer was scratched with a pipette tip across the diameter of each well. The medium and dislodged cells were removed, complete medium with compounds was then added to the plates, and cells were incubated at 37 °C. Cell migration to the scratch area was photographed at 6-, 12-, and 24-h time points. The rate of cell migration was assessed using the TScratch program as described (Gebäck et al. 2009).
Proteomic analysis by tandem mass spectrometry (GeLC-MS/MS)
Protein samples from cells were subjected to proteomic identification by SDS-PAGE followed by GeLC-MS/MS as previously described (Paemanee et al. 2016). Statistical analyses and database search were performed by DeCyder™ MS (GE Healthcare) and Mascot Search (Matrix Science).
Quantitative real-time PCR (qRT-PCR) and Western blot analysis
Total RNA was collected using the GF-1 Total RNA Extraction kit (Vivantis), and cDNA was synthesized with the RevertAid Reverse Transcriptase (Thermo scientific). PCR reaction was performed using the ThunderBird SYBR qPCR Mix (Toyobo). Forward and reverse primers for qRT-PCR (5′–3′) were: NOTCH1, CTCACCTGGTGCAGACCCAG and GCACCTGTA- GCTGGTGGCTG; HEY1, ACGAGAATGGAAACTTGAGTTC and AACTCCGATAGT-CCATAGCAAG; HEY2, ATGAGCATAGGATTCCGAGAGTG and GGCAGGAGGCAC- TTCTGAAG; NRARP, GGGCTGCATAGAAAATTGGA and CCCTTTTTAGCCTCCCAG- AG; 14-3-3 theta, CCTACAAGAACGTGGTCGGG and CGATCATCACCACACGCAAC; β-actin, CGAGGCCCAGAGCAAGAGAG and CTCGTAGATGGGCACAGTGTG. For Western blots, cells were lysed and probed following SDS/PAGE using antibodies from the Pro-Survival (9941), Pro-Apoptosis (9942), 14-3-3 Family (9769) Antibody Sampler Kits (Cell Signaling).
Immunocytochemistry
Cells were plated into 24-well plates at 5 × 105 cells/well and cultured for 24 h. Next, cells were fixed in 4% formaldehyde in PBS for 15 min, permeabilized in PBS with 0.1% Triton X-100 (PBS-T) for 10 min and blocked with 20% fetal bovine serum in PBS-T for 30 min. After washing, cells were incubated with the anti-Notch1 antibody (1:200) overnight at 4 °C and then with the fluorescently labelled secondary antibody. Cells were washed and observed via fluorescence microscopy.
siRNA knockdown of 14-3-3 theta
Cells were plated at 1000 cells/well in 24-well or 25,000 cells/well in 6-well plates and were transiently transfected with 10 nM of scrambled or 14-3-3 siRNA (sc-29,586, Santa Cruz Biotech) using Lipofectamine™ 3000 (Invitrogen). After 48 and 72 h, cells were collected for qRT-PCR and Western blot analysis.
Statistical analysis
All values are represented as mean ± S.E.M. Statistical differences between the two groups were determined with the Student’s t test.
Results
Overexpression of N1ICD variants in cholangiocarcinoma cells
In order to investigate the role of Notch signaling in CCA progression, intrahepatic CCA cell lines, RMCCA-1 and HuCCA-1, were introduced with N1ICD constructs encoding either full N1ICD or N1ICD that lacks the RAM domain (ΔRAM) using the retroviral system that was constructed by Dr. Warren Pear’s laboratory (Fig. 1a). The MSCV-IRES-GFP retroviral vector was used to drive the expression of N1ICD and GFP in CCA cells. CCA cells were successfully transduced with the constructs and expressed N1ICD variants in both mRNA and protein levels (Fig. 1b–d). We first determined the Notch1 mRNA in CCA cells. As expected, N1ICD is localized in the nuclei of transduced CCA cells (70%) while ΔRAM, which also lacks one of the nuclear localization signals (NLS), was found more in the cytoplasm (50%) (Fig. 1e and f).
Fig. 1.
a Schematic of full-length human NOTCH1 protein, NOTCH1 intracellular domain (N1ICD), and N1ICD without the RAM domain (ΔRAM). EGF, epidermal growth factor; LNR, Lin12/Notch repeat; HDN and HDC, N-terminal and C-terminal heterodimerization domains; TM, transmembrane domain; RAM, RBP-J-associated module; NLS, nuclear localization signal; ANK, Ankyrin repeats; TAD, transactivation domain; PEST, PEST sequence. b The cholangiocarcinoma cell line RMCCA-1 was successfully transduced with retroviruses encoding N1ICD variants as shown in green from GFP fusion. Scale bars: 200 μm. c and d Expression of N1ICD variants were confirmed by quantitative RT-PCR and Western blotting. e and f Analysis of NOTCH1 localization quantification in RMCCA-1 cells. Immunocytochemistry of NOTCH1 in RMCCA-1 cells expressing N1ICD variants showed NOTCH1 localization in both nuclei and cytoplasm. Blue: nucleus; red: NOTCH1. g, h, i mRNA levels of Notch target genes, HEY1, HEY2, and NRARP in RMCCA-1 cells expressing N1ICD variants by quantitative RT-PCR. All experiments were performed in triplicate and repeated at least twice. Data represent means + S.E.M., and Student’s t test was performed, * p value <0.05, ** p value <0.001, and *** p value <0.0001
Gene expression analyses revealed that Notch target genes, including HEY1, HEY2, and NRARP, were upregulated in a similar fashion for both N1ICD and ΔRAM, suggesting that some direct targets of Notch signaling can be RBP-J-independent (Fig. 1g and i).
Effects of N1ICD variants on CCA cell proliferation, survival, and migration
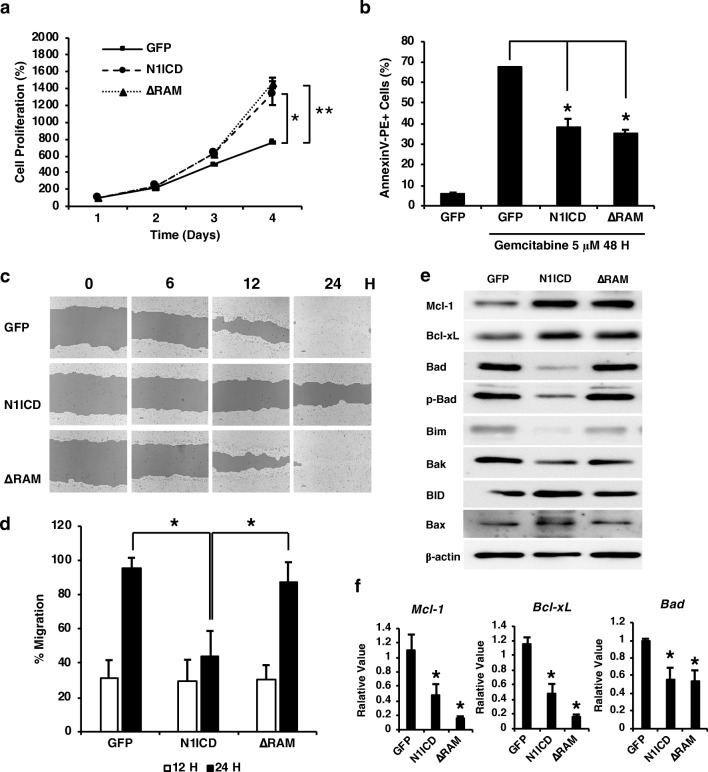
Next, we investigated the effects of overexpression of N1ICD variants on CCA cell proliferation, survival, and migration. The proliferation rate of CCA cells over a 4-day period was evaluated using MTT assays. Our results demonstrated that CCA cells overexpressing N1ICD or ΔRAM had a higher proliferation rate than the control group, suggesting that the RAM domain or RBP-J was dispensable for the activity of NOTCH1 in cell proliferation (Fig. 2a). Cell viability under the treatment of an anti-cancer drug, gemcitabine, was also assessed as a model for CCA cell survival under stress condition. Similar to the effects on cell proliferation, both N1ICD and ΔRAM overexpression significantly decreased the number of apoptotic CCA cells treated with gemcitabine (30 and 35%, respectively) (Fig. 2b). Our results demonstrated that Notch activation by N1ICD decreased CCA cell migration by 53% in the wound-healing assay (Fig. 2c and d); however, the effect was absent in ΔRAM, implicating that the inhibitory effect of Notch activation on cell migration was through the RBP-J-dependent targets. These data indicated that Notch play an oncogenic role in promoting cell proliferation and survival, but not cell migration, in cholangiocarcinoma possibly through a RBP-J-independent manner.
Fig. 2.
a The proliferation of RMCCA-1 cells. Cells were seeded on a 96-well plate and cultured for 1, 2, 3 and 4 Days. RMCCA-1 proliferation was quantified by an MTT assay. b RMCCA-1 cells were treated with 5 μM gemcitabine for 48 h. Cell apoptosis was determined by flow cytometry. c and d Cell migration and TScratch quantification was determined by the scratch assay at 0, 6, 12, and 24 h. e Western blot analysis showed differential levels of pro-apoptotic (Bad, phospho-Bad, Bim, Bak, BID, Bax) and anti-apoptotic (Mcl-1, Bcl-xL) proteins in RMCCA-cells expressing N1ICD variants. f mRNA levels of Mcl-1, Bcl-xL, and Bad were determined by quantitative RT-PCR. All experiments were performed in triplicate and repeated at least twice. Data represent means + S.E.M., and Student’s t test was performed, * p value <0.05, ** p value <0.001, and *** p value <0.0001
NOTCH1 activation suppresses apoptosis and induces expression of anti-apoptotic proteins
It is well established that Notch signaling plays a major role in cell survival and apoptosis in several cell types. We further investigated the downstream effectors of Notch activation in CCA by determining the levels of key pro- and anti-apoptotic proteins and found that CCA expressing N1ICD or ΔRAM showed a comparable increase in anti-apoptotic proteins, Mcl-1 and Bcl-xL (Fig. 2e), which is consistent with their effects on cell proliferation and survival. We also determined the transcript levels of Mcl-1, Bcl-xL, and Bad and found that both Mcl-1 and Bcl-xL were significantly decreased to about 40% with N1ICD and 15% with ΔRAM (Fig. 2f). Bad expression was also shown to reduce to about 50% in both forms of Notch activation (Fig. 2f). Interestingly, the significant reduction of mRNA levels of these pro- and anti-apoptotic genes contradict with our Western blot data, suggesting that N1ICD may not only regulate these genes at the transcriptional level. Alternatively, Notch signaling may be involved in the regulation of other factors that enhance the stability, or diminish the degradation, of Mcl-1 and Bcl-xL. N1ICD appeared to decrease the levels of Bad and p-Bad and had no impact on other pro-apoptotic proteins; however, this phenomenon was not observed in the CCA cells expressing ΔRAM (Fig. 2e). Thus, it is likely that the roles of Notch in regulating pro-apoptotic proteins and promoting CCA cell proliferation and survival are separate and independent from each other.
Identification of candidate proteins for Notch targets by LC-MS/MS proteomic analysis
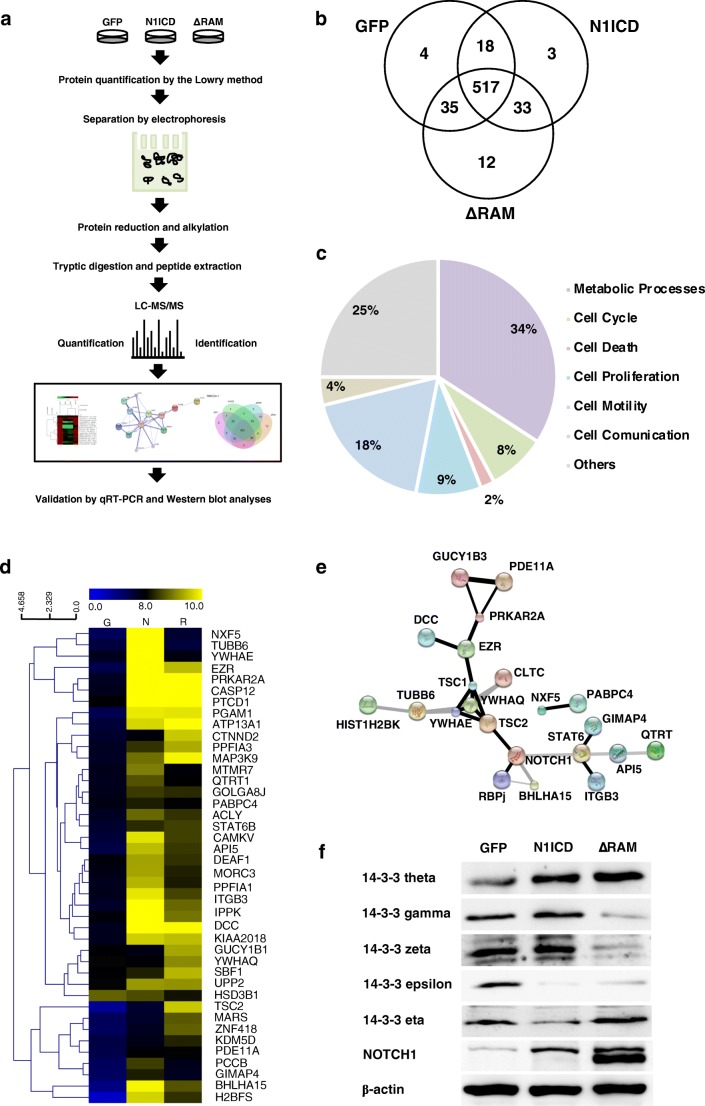
We attempted to determine candidate proteins that were regulated by Notch signaling and played roles in cell proliferation and survival using proteomic approaches. A comparative SDS-PAGE of protein lysates of CCA cells expressing N1ICD variants was performed and further used in GeLC-MS/MS analysis (Fig. 3a). Upon in-gel tryptic digestions coupled with LC-MS/MS analysis of the protein lysates, 622 differentially expressed proteins were identified. Only 517 proteins were found in all groups and classified into 7 groups according to their reported biological processes synergized by UniProtKB, using the PANTHER classification system (Fig. 3b and c). These proteins were categorized as metabolic processes (34%), cell cycle (25%), cell death (4%), cell proliferation (18%), cell motility (9%), cell communication (2%), and others (8%). Among these proteins, we further analyzed proteins in the functional groups of cell cycle, cell death, and cell proliferation to determine the relative expression by reciprocal common fraction between peptide intensities of the different groups. Next, we selected only those proteins that were consistent with our cell proliferation and survival data and showed relative expression profiles in Fig. 3d. The Search Tool for Interactions of Chemicals (STITCH) analysis showed potential interactions between candidate proteins and NOTCH1 and molecules that are involved in the Notch pathway (Fig. 3e). Our interaction analysis demonstrated that the 14-3-3 protein family has been known to be associated with the Notch signaling pathway, and found that one of its members, 14-3-3 theta (YWHAQ), was significantly upregulated in CCA expressing N1ICD variants. 14-3-3 theta has been reported to bind to tuberous sclerosis complex 2 (TSC2), which is regulated by Notch signaling (Li et al. 2002; Kapuria et al. 2012). Further verification of the protein level of 14-3-3 theta by Western blot analysis confirmed that it was upregulated in CCA cells expressing N1ICD and ΔRAM in compliance with the expression profile from LC-MS/MS (Fig. 3f). The mRNA level of 14-3-3 theta was also increased in Notch-activated cells, suggesting that Notch regulates the expression of 14-3-3 theta at transcriptional level. In humans, there are seven isoforms of 14-3-3 proteins (theta, eta, zeta, beta, gamma, epsilon, and sigma). We identified five isoforms (theta, gamma, epsilon, zeta and sigma) in CCA cells via LC-MS/MS analysis, and our results revealed that the expression of 14-3-3 proteins theta, gamma, and zeta were significantly increased in CCA cells expressing N1ICD variants while 14-3-3 proteins eta and epsilon was downregulated (Fig. 3f). Altogether, our results implied that the 14-3-3 theta, especially 14-3-3 theta, may be involved in the proliferation and survival of CCA.
Fig. 3.
a Experimental workflow for GeLC-MS/MS proteomic analysis in RMCCA-1 expressing N1ICD variants. Proteins were collected from cells and separated in SDS-PAGE before in-gel tryptic digestion and peptide extraction. Then, LC-MS/MS analysis of protein lysates was performed, followed by protein identification, quantification, and validation by quantitative RT-PCR and Western blotting. b The Venn diagram represents unique and overlapping identified proteins in CCA cells expressing N1ICD variants. c Molecular functions and biological processes of identified proteins were categorized according to the UniProt database using the PANTHER Classification System. d A cluster heat map of expressed proteins in CCA cells expressing N1ICD variants were compared and performed by MultiExperimental Viewer (MeV) software. Columns represent proteins from GFP control (G), N1ICD (N), and ΔRAM (R), respectively. e Protein interactions between identified proteins and known Notch-interacting and Notch target proteins were analyzed by STITCH. f Expression levels of the 14-3-3 family proteins. To validate LC-MS/MS proteomic results, Western blotting was performed to determine the protein levels of 14-3-3 theta, gamma, zeta, epsilon, and eta in CCA cells expressing N1ICD variants
Functional validation of 14-3-3 theta as a Notch target candidate in promoting CCA cell proliferation and survival
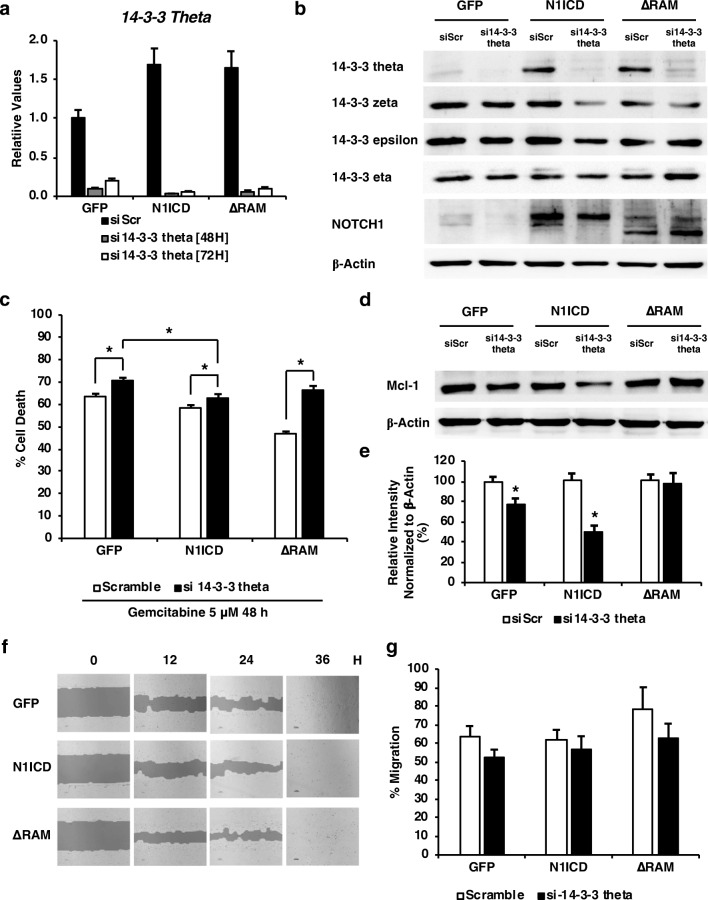
From our proteomic analysis and expression verification, we hypothesized that 14-3-3 theta promotes CCA cell proliferation and survival under a direct regulation of RBP-J-independent Notch signaling. 14-3-3 theta knockdown in CCA cells using siRNA was performed, and they exhibited a significant decrease in 14-3-3 theta mRNA levels to less than 10% when compared with scrambled control at 48 and 72 h (Fig. 4a). The protein level of 14-3-3 theta, but not other 14-3-3 isoforms, was reduced, confirming the successful knockdown of our candidate protein (Fig. 4b). The 14-3-3 theta knockdown CCA cells exhibited a significant increase in apoptotic cell number under gemcitabine treatment, abolishing the pro-survival effects of N1ICD and ΔRAM to 10 and 15%, respectively (Fig. 4c). Mcl-1 was slightly decreased due to the knockdown, which corresponds to the increased apoptosis (Fig. 4d). Quantification of the Western blot data showed that 14-3-3 theta knockdown via siRNA resulted in a significant decrease in Mcl-1 protein levels in GFP- and N1ICD-expressing cells by 20 and 50%, respectively (Fig. 4e). However, the knockdown did not have any significant effect in ΔRAM-expressing cells, implicating that 14-3-3 theta may not be the only protein that plays a role in CCA cell survival, especially in RAM-dependent Notch activation. In addition, knockdown cells appeared to migrate normally in the wound-healing experiment (Fig. 4f and g). These data suggested that Notch signaling regulates cell proliferation and survival in part through 14-3-3 theta. However, the moderate degree to which 14-3-3 theta knockdown could reduce cell survival implicates that Notch must exert its effects through other mediators or signaling pathways besides the 14-3-3 family.
Fig. 4.
Knockdown of 14-3-3 theta in RMCCA-1 expressing N1ICD variants. Cells were transfected with si-scrambled (siScr) or si-14-3-3 theta (si14-3-3 theta) using lipofectamine for 48 and 72 h. a and b The mRNA and protein levels of 14-3-3 theta, zeta, epsilon, and eta, were determined by quantitative RT-PCR and Western blotting. c Knockdown of 14-3-3 theta increased the number of CCA cell death under gemcitabine treatment. Cells were transfected with siScr or si14-3-3 theta and treated with 5 μM gemcitabine for 48 h. Cell viability/death was quantified by the MTT assay. d The level of Mcl-1 was decreased in 14-3-3 theta knockdown cells. Levels of Mcl-1 protein in 14-3-3 knockdown RMCCA-1 cells were analyzed by Western blotting. e The relative quantification for Mcl-1 expression in RMCCA-1 cell lysates normalized with respect to b-actin was determined by ImageJ. f and g Cell migration and TScratch quantification was determined by the scratch assay at 0, 6, 12, and 24 h. All experiments were performed in triplicate and repeated at least twice. Data represent means + S.E.M., and Student’s t test was performed, * p value <0.05, ** p value <0.001, and *** p value <0.0001
Discussion
Notch signaling is evolutionarily conserved and controls numerous developmental processes. It is a key signaling element in cell proliferation, differentiation, and apoptosis. Notch signaling is known to be essential for the differentiation of hepatocytes into biliary lineage cells during the early stages of intrahepatic CCA development as well as promotes hepatocellular carcinoma development (Fan et al. 2012). CCA often acquires more aggressive phenotypes at later stages, with increased cell growth, survival, and unresponsiveness or resistance to drugs. Therefore, inhibition or suppression of these properties of cancer cells should have a significant impact on cancer treatment and disease outcome improvement. To understand the role of Notch in CCA cell progression, we activated Notch signaling using N1ICD and assess its effects on cancer cell progression. Additionally, RBP-J or non-canonical Notch signaling has emerged as an important route for the action of the Notch pathway, thus we also compared such effects with RBP-J-independent Notch activation using ΔRAM. Our results suggested that some of the classical Notch targets, including HEY1, HEY2, NRARP, were induced by both N1ICD variants, while others, such as HEYL and HES5, were not induced in the absence of the RAM domain. These data demonstrate a shift in the concept that some direct Notch targets may be regulated in an RBP-J-independent fashion, which raises an important question of how Notch regulates the expression of those targets and what the functions it may serve in the context of cancer cell progression.
In this study we used two human CCA cell lines, RMCCA-1 and HuCCA-1, to demonstrate a differential response on N1ICD variants. The retroviral system was selected as it can transduce cells to express our transgenes in a minimal, consistent level, which we believe is appropriate and highly essential for the study of Notch signals to lessen the possibilities of indirect, irrelevant effects of gene overexpression. The expression of either N1ICD variant exerted a clear impact on cell proliferation and survival under gemcitabine treatment (5 μM, 48 h). Interestingly, our results showed that Notch activation via N1ICD significantly decreased CCA cell migration. It is not uncommon for the multifaceted signaling pathway like Notch to exhibit conflicting roles in cancer progression as it has been well established that Notch often serves an oncogenic function in tumor cells but negatively regulates cell proliferation and migration of endothelial cells in tumors. Nevertheless, ΔRAM fails to inhibit CCA cell migration, implicating the importance of RBP-J-dependent, canonical signals in the migration process. Therefore, in this study we focused on the role of Notch in cell proliferation and survival and further investigated the molecular mechanisms to identify its downstream effectors. Notch signaling has been reported to regulate different sets of genes in different cell types during embryogenesis by genome-wide transcriptomic analyses (Meier-Stiegen et al. 2010). In addition to the Notch protein itself, Notch targets can also act in both positive and negative fashions, suggesting one mechanism through which Notch activation can lead to opposite effects in different contexts. Aster and colleagues reported that induction of T-ALL required the ANK and TAD domains, while the RAM and PEST domains are dispensable (Aster et al. 2000). In this study, we found that N1ICD-expressing CCA cells exhibited a reduction of gemcitabine-induced apoptosis, but this effect was not observed in other cell lines, including HEK 293 T (data not shown). We also investigated the apoptosis process to confirm the results in cell proliferation and survival. Our results showed that NOTCH1 activation resulted in increased levels of anti-apoptotic Mcl-1 and Bcl-xL and decreased pro-apoptotic Bad. Previous evidence has shown that N1ICD overexpression correlated well with the upregulation of anti-apoptotic protein Mcl-1 in macrophages (Palaga et al. 2013).
Recently, two-dimensional LC-MS/MS analysis in the hamster liver with chronic or advanced opisthorchiasis revealed a high expression of 14-3-3 eta (Haonon et al. 2016). Also, secretomic analysis identified secreted proteins from CCA cells in a three-dimensional culture system, including S100A4, 14-3-3 sigma, 14-3-3 zeta, and 14-3-3 epsilon (TIT-OON et al. 2014). Furthermore, patients with intrahepatic CCA recurrence showed high levels of 14-3-3 zeta (Zhang et al. 2015). 14-3-3 proteins are able to interact with many different proteins based on their specific phosphor-serine/phosphor-threonine binding activity. 14-3-3 proteins usually bind to their partners at defined phosphorylation motifs and can function in regulating different pathways via intra- and intercompartmental sequestration of proteins, activation or inhibition of enzyme activities, and promotion or disruption of protein interactions (Hermeking 2003; Reinhardt and Yaffe 2013; Dar et al. 2014). Protein kinase C, Bad, and CDC25C have been demonstrated to associate with 14-3-3 proteins in a phosphorylation-dependent fashion (Hermeking 2003). Our proteomics analysis showed differentially expressions of the 14-3-3 protein family, including 14-3-3 theta (YWHAQ), 14-3-3 epsilon (YWHAE), 14-3-3 gamma (YWHAG), and 14-3-3 sigma (SFN) after activation of Notch signaling. Validation of proteomic data revealed differential levels of candidate proteins, 14-3-3 sigma, theta, epsilon, gamma, and zeta in different N1ICD variant CCA cells, confirming that these 14-3-3 family proteins were under the regulation of RBP-J-independent Notch signals.
Several studies have demonstrated the role for Notch signaling in the regulation of genes that are important in cancer cell progression. Our studies provided novel evidence that Notch activation promotes CCA cell proliferation and survival through the 14-3-3 protein family. The role of 14-3-3 in CCA progression is likely not limited to the 14-3-3 theta isoform but may be associated with other 14-3-3 family members. Nevertheless, among the candidate proteins, 14-3-3 theta was highly expressed in Notch-activated CCA cells, and 14-3-3 theta knockdown significantly abolished the pro-survival effects of Notch activation and sensitized the cells to gemcitabine cytotoxicity and cell death. Our results clearly indicated that 14-3-3 protein theta is one of the RBP-J-independent Notch-mediated downstream targets, which could serve as a potential candidate for diagnostic markers and therapeutic targets in CCA treatment.
Acknowledgments
The work was supported by the Thailand Research Fund (TRG5780200) and Mahidol University (T.K.). The authors thank the Central Instrument Facility, Center of Nanoimaging, Faculty of Science, Mahidol University and Sucheewin Krobthong for technical assistance.
References
- Aster JC, Xu L, Karnell FG, Patriub V, Pui JC, Pear WS. Essential roles for ankyrin repeat and transactivation domains in induction of T-cell leukemia by notch1. Mol Cell Biol. 2000;20:7505–7515. doi: 10.1128/MCB.20.20.7505-7515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aster JC, Blacklow SC, Pear WS. Notch signalling in T-cell lymphoblastic leukaemia/lymphoma and other haematological malignancies. J Pathol. 2011;223:262–273. doi: 10.1002/path.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A, Wu D, Lee N, Shibata E, Dutta A. 14-3-3 proteins play a role in the cell cycle by shielding cdt2 from ubiquitin-mediated degradation. Mol Cell Biol. 2014;34:4049–4061. doi: 10.1128/MCB.00838-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R, Turner D. Vertebrate hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene. 2001;20(58):8342–8357. doi: 10.1038/sj.onc.1205094. [DOI] [PubMed] [Google Scholar]
- Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebäck T, Schulz MMP, Koumoutsakos P, Detmar M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. BioTechniques. 2009;46:265–274. doi: 10.2144/000113083. [DOI] [PubMed] [Google Scholar]
- Grabher C, Boehmer von H, Look AT. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2006;6:347–359. doi: 10.1038/nrc1880. [DOI] [PubMed] [Google Scholar]
- Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–1762. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- Greenwald I (2005) LIN-12/Notch signaling in C. elegans. WormBook : the online review of C elegans biology 1–16. 10.1895/wormbook.1.10.1 [DOI] [PMC free article] [PubMed]
- Haonon O, Rucksaken R, Pinlaor P, Pairojkul C, Chamgramol Y, Intuyod K, Onsurathum S, Khuntikeo N, Pinlaor S. Upregulation of 14-3-3 eta in chronic liver fluke infection is a potential diagnostic marker of cholangiocarcinoma. Proteom Clin Appl. 2016;10:248–256. doi: 10.1002/prca.201500019. [DOI] [PubMed] [Google Scholar]
- Hermeking H. The 14-3-3 cancer connection. Nat Rev Cancer. 2003;3:931–943. doi: 10.1038/nrc1230. [DOI] [PubMed] [Google Scholar]
- Kapuria S, Karpac J, Biteau B, Hwangbo DS, Jasper H. Notch-mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage. PLoS Genet. 2012;8:e1003045–e1003014. doi: 10.1371/journal.pgen.1003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovall RA, Blacklow SC. Chapter two - mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol. 2010;92(92):31–71. doi: 10.1016/S0070-2153(10)92002-4. [DOI] [PubMed] [Google Scholar]
- Kurooka H, Honjo T. Functional interaction between the mouse notch1 intracellular region and histone acetyltransferases PCAF and GCN5. J Biol Chem. 2000;275:17211–17220. doi: 10.1074/jbc.M000909200. [DOI] [PubMed] [Google Scholar]
- Li Y, Inoki K, Yeung R, Guan K-L. Regulation of TSC2 by 14-3-3 binding. J Biol Chem. 2002;277:44593–44596. doi: 10.1074/jbc.C200510200. [DOI] [PubMed] [Google Scholar]
- Meier-Stiegen F, Schwanbeck R, Bernoth K, Martini S, Hieronymus T, Ruau D, Zenke M, Just U. Activated Notch1 target genes during embryonic cell differentiation depend on the cellular context and include lineage determinants and inhibitors. PLoS One. 2010;5:e11481. doi: 10.1371/journal.pone.0011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurette O, Stylianou S, Rock R, Collu GM, Gilmore AP, Brennan K. Notch activation induces Akt signaling via an autocrine loop to prevent apoptosis in breast epithelial cells. Cancer Res. 2009;69:5015–5022. doi: 10.1158/0008-5472.CAN-08-3478. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, McFadden DG, Nakagawa M, et al. Members of the HRT family of basic helix-loop-helix proteins act as transcriptional repressors downstream of Notch signaling. Proc Natl Acad Sci U S A. 2000;97:13655–13660. doi: 10.1073/pnas.250485597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paemanee A, Wikan N, Roytrakul S, Smith DR (2016) Application of GelC-MS/MS to proteomic profiling of Chikungunya virus infection: preparation of peptides for analysis. In: Application of GelC-MS/MS to proteomic profiling of Chikungunya virus infection: preparation of peptides for analysis. Springer New York, New York, pp 179–193 [DOI] [PubMed]
- Palaga T, Ratanabunyong S, Pattarakankul T, Sangphech N, Wongchana W, Hadae Y, Kueanjinda P. Notch signaling regulates expression of Mcl-1 and apoptosis in PPD-treated macrophages. Cell Mol Immunol. 2013;10:444–452. doi: 10.1038/cmi.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumalsamy LR, Nagala M, Sarin A. Notch-activated signaling cascade interacts with mitochondrial remodeling proteins to regulate cell survival. Proc Natl Acad Sci U S A. 2010;107:6882–6887. doi: 10.1073/pnas.0910060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- Rattanasinganchan P, Leelawat K, Treepongkaruna S-A, Tocharoentanaphol C, Subwongcharoen S, Suthiphongchai T, Tohtong R. Establishment and characterization of a cholangiocarcinoma cell line (RMCCA-1) from a Thai patient. World J Gastroenterol. 2006;12:6500–6506. doi: 10.3748/wjg.v12.i40.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt HC, Yaffe MB. Phospho-Ser/Thr-binding domains: navigating the cell cycle and DNA damage response. Nat Rev Mol Cell Biol. 2013;14:563–580. doi: 10.1038/nrm3640. [DOI] [PubMed] [Google Scholar]
- Sade H, Krishna S, Sarin A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem. 2004;279:2937–2944. doi: 10.1074/jbc.M309924200. [DOI] [PubMed] [Google Scholar]
- Sirisinha S, Tengchaisri T, Boonpucknavig S, Prempracha N, Ratanarapee S, Pausawasdi A. Establishment and characterization of a cholangiocarcinoma cell line from a Thai patient with intrahepatic bile duct cancer. Asian Pac J Allergy Immunol. 1991;9:153–157. [PubMed] [Google Scholar]
- Stylianou S, Clarke R, Brennan K. Aberrant activation of Notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- Tit-oon P, Chokchaichamnankit D, Khongmanee A, et al. Comparative secretome analysis of cholangiocarcinoma cell line in three-dimensional culture. Int J Oncol. 2014;45:2108–2116. doi: 10.3892/ijo.2014.2636. [DOI] [PubMed] [Google Scholar]
- Zender S, Nickeleit I, Wuestefeld T, Sörensen I, Dauch D, Bozko P, el-Khatib M, Geffers R, Bektas H, Manns MP, Gossler A, Wilkens L, Plentz R, Zender L, Malek NP. A critical role for Notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013;23:784–795. doi: 10.1016/j.ccr.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Zhang C, Liu L-X, Dong Z-R, Shi GM, Cai JB, Zhang PF, Ke AW, Yu JX, Zhou J, Fan J. Up-regulation of 14-3-3ζ expression in intrahepatic cholangiocarcinoma and its clinical implications. Tumour Biol. 2015;36:1781–1789. doi: 10.1007/s13277-014-2780-5. [DOI] [PubMed] [Google Scholar]