Abstract
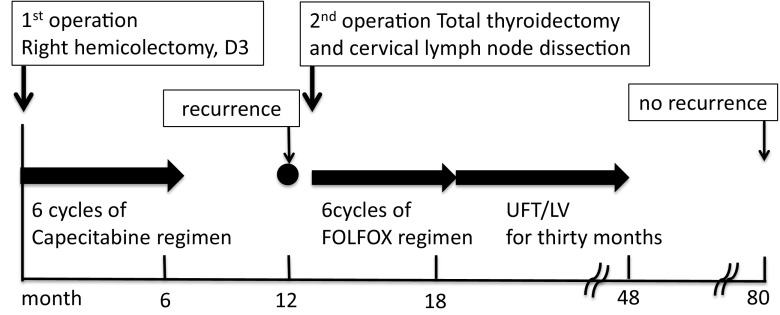
The patient was a 53-year-old male with a chief complaint of bloody stool. To treat the cecal colon cancer, right hemicolectomy was performed. Histological examination showed moderately differentiated adenocaricinoma, SE, N3, H0, P0, M0 Stage IIIb by Japanese Classification of the Colorectal Carcinoma. After the operation, the patient received a chemotherapy with 6 cycles of Capecitabine regimen. After 1 year later, computed tomography detected swelling of Virchow’s lymph node and tumor in the thyroid gland. By fine-needle aspiration cytology, thyroid gland tumor was diagnosed as papillary cancer and Virchow’s lymph node was detected adenocarcinoma which was metastasis of cecal cancer. Total thyroidectomy and cervical lymph node dissection were performed. After the operation, the patient received chemotherapy with 6 cycles of FOLFOX regimen. And the patient had taken UFT/LV for 30 months. Now he has no recurrence and keeps his quality of life high. He has been alive for 80 months since the first operation. Virchow lymph node dissection can be one of the options of treatment of metastasis.
Keywords: Colon cancer, Virchow lymph node dissection, Virchow lymph node metastasis
Introduction
As a result of the advances in the clinically available chemotherapeutic agents, the prognosis of patients with metastatic colorectal cancer has been improved. Nevertheless, colorectal cancer cannot be controlled completely, and the development of new therapies is necessary. In the cases of liver or lung metastasis, complete resection surgery is recommended. Distant lymph node metastasis, such as only Virchow’s lymph node metastasis due to colorectal cancer without any other solid organ metastasis, is extremely rare and poor prognosis and the evidence of the treatment is insufficient. We herein report a case of long-term survival in a patient with cecal cancer with Virchow’s lymph node.
Case report
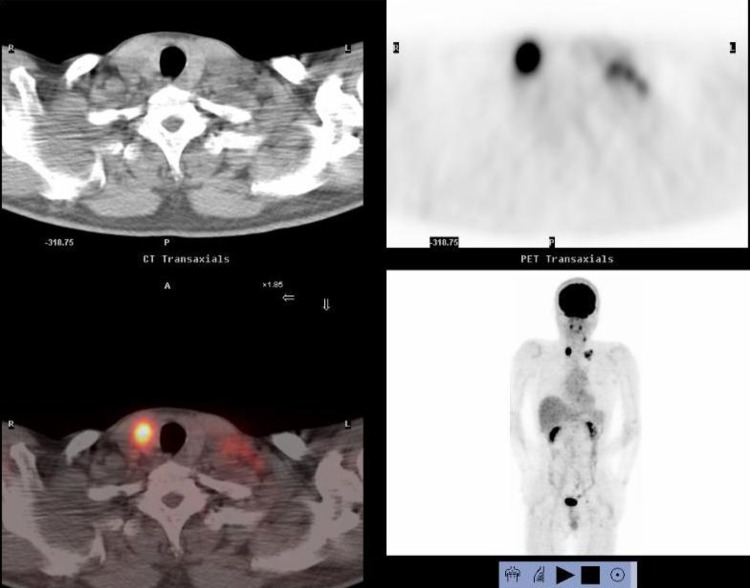
A 53-year-old male presented with a chief complaint of a bloody stool. Colonoscopy showed circumferential ulcer in cecum, and abdominal computed tomography (CT) showed wall thickening in cecum and enlarged lymph nodes. No metastasis was revealed in the liver and lungs. Radiographic contrast enema revealed apple-core-sign in cecum. Mild anemia was shown (Hb 11.2 g/dL), and carcino-embryonic antigen (CEA = 5.2) and carbohydrate antigen 19-9 (CA19-9 = 308.9) were elevated in blood examination. The preoperative diagnosis was colon cancer, C, SS, N2, H0, P0, M0 Stage IIIb by the Japanese Classification of the Colorectal Carcinoma (JCCC) [1]. Right colectomy with D3 lymph node dissection was performed (Fig. 1). Histological examination showed colon cancer, C, type 2, 45 × 30 mm, 1/2 circ, tub2 > tub1 > por1 > por2, SE, int, ly2, v1, N3 [No201(10/13), 211(0/4), 202(1/2), 203(1/3)], H0, P0, M0, PM0, DM0, RM0, Stage IIIb by JCCC. After the operation, the patient received chemotherapy with Capecitabine regimen (starting dose: Xeloda® 4200 mg/body orally on day1–14, every 3 weeks). During 3 cycles of Capecitabine regimen, due to the onset of icterus (Grade2), we managed to continue Capecitabine regimen with the extension of interval period and dose reduction. But after 6 cycles of Capecitabine regimen, icterus was exacerbated. The chemotherapy was aborted. After 1 year later, CT detected swelling of Virchow’s lymph node and tumor in the thyroid gland (Fig. 2). Positron emission tomography showed that increased standardized uptake values (SUV) of 28.0 and 7.0 were observed in the thyroid gland tumor and left cervical region (Fig. 3). By fine-needle aspiration cytology, thyroid gland tumor was diagnosed as papillary carcinoma and Virchow’s lymph node was detected adenocarcinoma which was metastasis of cecal cancer. We diagnosed, thyroid papillary carcinoma, T3, N1b, M0, Stage IVa and recurrence of cecal cancer in Virchow’s lymph node. In selecting surgical therapy or chemotherapy, chemotherapy has side effects of icterus due to Capecitabine, there was a possibility that chemotherapy was difficult to continue. In surgical treatment, cervical dissection was possible in the treatment of thyroid papillary carcinoma, and no organ metastasis in the whole clinical course and no other sites of nodal metastasis except Virchow’s node at the time of the recurrence. From these points, we thought that surgical treatment was better than chemotherapy. After informed consent from surgeon and otolaryngologist, the patient wished surgical treatment. Total thyroidectomy and cervical lymph node dissection was performed. Finally histological examination revealed that Virchow’s lymph node was moderately differentiated adenocarcinoma as a metastatic cecal cancer (Fig. 4). After the operation, the patient received chemotherapy with 6 cycles of FOLFOX regimen (5-FU bolus 720 and 4250 mg/body, leucovorin 360 mg/body and oxaliplatin 150 mg/body). And the patient had taken UFT/LV for 30 months. After UFT/LV regimen, the patient selected follow-up without chemotherapy. Now he has no recurrence and keeps his quality of life high. He has been surviving for 80 months since the first operation (Fig. 5).
Fig. 1.
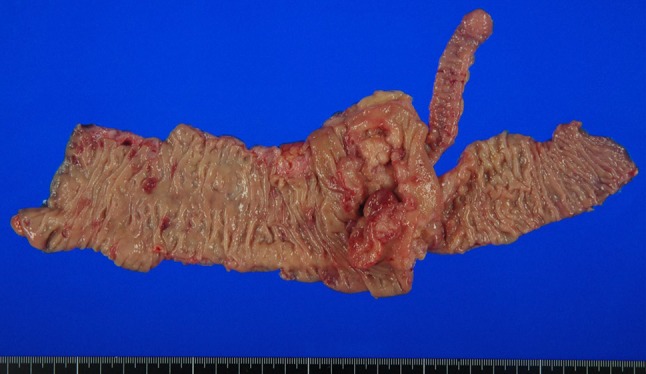
The resected specimen of cecal cancer
Fig. 2.
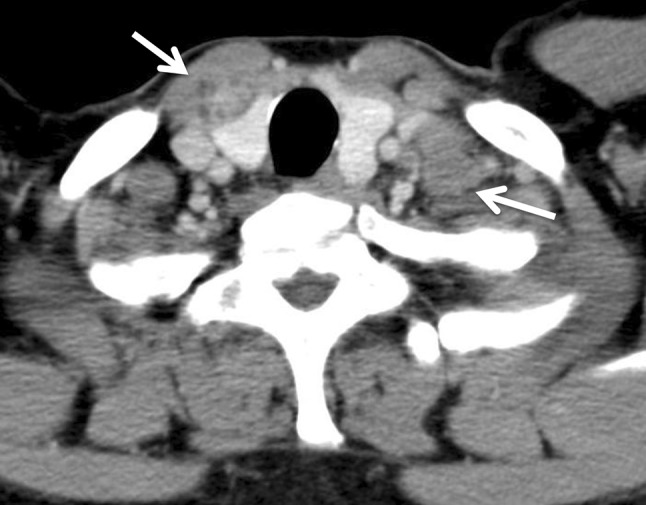
Cervical to abdominal CT showed swelling of Virchow’s lymph node (right arrow) and tumor in the thyroid gland (left arrow)
Fig. 3.
Positron emission tomography showed that increased standardized uptake values (SUV) of 28.0 and 7.0 were observed in the thyroid gland tumor and left cervical region
Fig. 4.

Pathological findings of immunohistochemical steining: adenocarcinoma of Virchow lymph node is CDX2(+), CK20(+), thyroglobulin(−), TTF-1(−). Papillary carcinoma of thyroid gland is CDX2(−), CK20(−), thyroglobulin(+), TTF-1(+). Cecal cancer (primary lesion) is CDX2(+), CK20(+), thyroglobulin(−), TTF-1(−)
Fig. 5.
The clinical course
Discussion
In western countries as well as in Japan, the incidence of colorectal cancer is substantially high compared to various other malignancies. As a result of the advances in the clinical availability of chemotherapeutic agents, such as 5-FU, leucovorin, and oxaliplatin as a combination chemotherapy regimen (FOLFOX), and 5-FU, leucovorin, and irinotecan as an alternative combination chemotherapy (FOLFIRI), the median overall survival time of patients with metastatic colorectal cancer is approximately 20 months [2]. Furthermore, bevacizumab and anti-epidermal growth factor receptor (EGFR) monoclonal antibodies have also been reported to improve the survival times of patients [3, 4]. Nevertheless, colorectal cancer cannot be completely controlled, and the development of new therapies is necessary.
The sites of metastasis of colorectal cancer are the liver (85%), lungs (4%), and peritoneum (10%) [5]. Regional lymph node metastasis is observed in 35–40% of colorectal cancer [6]. However, distant lymph node metastasis such as cervical lymph node metastasis of colorectal cancer without any other solid organ metastasis is very uncommon. Cervical lymph node metastasis is likely caused by malignant tumors of the thyroid gland, lungs, breasts, and head and neck region [7].
The best treatment for metastasis of colorectal cancer currently depends on the site and pattern of disease progression. In the cases of liver or lung metastases, complete resection via surgery is the recommended course of treatment with 5-year survival rates reported to be above 60% [8, 9]. In the cases of peritoneal carcinomatosis, the combination of multimodal therapy and cytoreductive surgery has been widely accepted and the use of this approach yields median survival times exceeding 60 months [10]. Although in limited cases, surgical resection is an acceptable option for patients with peritoneal carcinomatosis from colorectal cancer. Distant lymph node metastasis to the nodes such as the Virchow lymph node is thought to be an extremely poor prognostic risk factor and evidence for successful treatment options for this is lacking. There are some reports about synchronous or metachronous metastasis of the Virchow lymph node that were treated with chemotherapy or chemoradiotherapy and achieved good prognosis [11–15]. Some reports described that aggressive salvage surgery for Virchow’s lymph node recurrence [16, 17] led to long-term recurrence free survival (Table 1). In our case, there were metastatic recurrences in the Virchow lymph node from cecal cancer and thyroid gland cancer. It was difficult to administer adequate chemotherapy owing to side effects such as icterus, and the lesion could be dissected at the time of cervical lymph node dissection. We performed surgical treatment and achieved a good response. Although whether to perform surgery is controversial, based on this case, we believe that the option of surgical intervention could be acceptable in cases where (1) there is only one organ of metastasis and the patient has shown stable disease after chemotherapy, or (2) there is difficulty with the management of chemotherapeutic side effects. After R0 surgery for Virchow lymph node recurrence, the necessity of chemotherapy may be debatable. For long-term survival of patients with advanced colorectal cancer, it is important to consider the use of all key drugs, such as 5-FU, LV, oxaliplatin, irinotecan, and molecular targeted treatments. In our particular case, after complete resection for metastasis of the Virchow lymph node, we administered FOLFOX and UFT as post-operative chemotherapy. As a result, currently, there is no recurrent lesion upon radiological examination. The data in this report should help guide therapeutic decisions in cases with a recurrent lesion, without sufficient data about long-term prognosis, such as the status of the Virchow lymph node.
Table 1.
Long-term survival of cases with Virchow lymph node metastasis of colorectal cancer
| No | Author | Year | Age/gender | Primary locationa | Vircow LN metastasis on synchronous or metachronous | Metastasis of another organ | Resection of Virchow LN | Postoperative chemotherapy | Disease free survival time | Overall survival time |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Baba | 1998 | 48/F | S | Synchronous | Paraaorta LN | + | UFT/5y | 6 years | 6 years |
| 2 | Mio | 2004 | 48/F | C | Synchronous | Paraaorta LN | – | 5FU + LV | – | 3 years |
| 3 | Takahashi | 2009 | 73/M | C | Synchronous | Paraaorta LN, lung | – | 5FU + LV, UFT/LV, FOLFOX, FOLFIRI | – | 3 years |
| 4 | Honma | 2002 | 63/F | S | Synchronous | – | – | 5-DFUR + CPT-11 | – | 5 years 4 months |
| 5 | Hirose | 2010 | 57/M | R | Synchronous | – | – | FOLFOX, FOLFIRI, FOLFOX + b-mab | – | 3 years 2 months |
| 6 | Ando | 2013 | 63/M | S | Synchronous | – | – | FOLFOX, FOLFIRI, Capecitabine | – | 5 years 9 months |
| 7 | Imamura | 2013 | 55/M | R | Metachronous | – | + | CPT-11 + c-mab | 1 year 7 months | 5 years |
| 8 | Koyanagi | 2014 | 69/M | R | Metachronous | Liver, pelvic LN | – | FOLFOX, FOLFIRI, Capecitabine + B-mab | – | 3 years 1 month |
| 9 | Takeshita | 2015 | 66/F | R | Metachronous | Liver, paraaorta LN | + | FOLFIRI, TS-1, FOLFOX, Capecitabine + B-mab | 1 year 6 month | 8 years |
| 10 | Our case | 2016 | 53/M | C | Metachronous | – | + | Capecitabine, FOLFOX, UFT/LV | 5 years 6 months | 6 years 8 months |
aPrimary location S sigmoid, C cecum, R rectum
Author contributions
HK and DF, KK, MM, YH, KK, TG designed the reports, HK, MM, TG performed the treatment for this case; and HK wrote the paper.
Conflict of interest
We have no financial relationships to disclose.
Informed consent
The patient provided informed written consent prior to treatment and publication.
References
- 1.Japanese Society for Cancer of the Colon and Rectum (2009) Japanese classification of colorectal carcinoma, 2nd English edn. Kanehara and Co, Tokyo
- 2.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 5.Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999;6:651–657. doi: 10.1007/s10434-999-0651-x. [DOI] [PubMed] [Google Scholar]
- 6.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97(1):72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 7.Bulent A, Lutfi D, Niyazi K, Salim D. Cervical lymphadenopathy as the first presentation of sigmoid colon cancer. Middle East J Cancer. 2013;4(4):185–188. [Google Scholar]
- 8.Ko S, Jo H, Yun S, Park E, Kim S, Seo HI. Comparative analysis of radiofrequency ablation and resection for resectable colorectal liver metastases. World J Gastroenterol. 2014;20:525–531. doi: 10.3748/wjg.v20.i2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiono S, Ishii G, Nagai K, Yoshida J, Nishimura M, Murata Y, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg. 2005;79:278–282. doi: 10.1016/j.athoracsur.2004.06.096. [DOI] [PubMed] [Google Scholar]
- 10.Sugarbaker PH, Chang D, Koslowe P. Prognostic features for peritoneal carcinomatosis in colorectal and appendiceal cancer patients when treated by cytoreductive surgery and intraperitoneal chemotherapy. Cancer Treat Res. 1996;81:89–104. doi: 10.1007/978-1-4613-1245-1_9. [DOI] [PubMed] [Google Scholar]
- 11.Fujie Y, Ikeda M, Seshimo I, Ezumi K, Hata T, Shingai T, et al. Complete response of highly advanced colon cancer with multiple lymph node metastases to irinotecan combined with UFT: report of a case. Surg Today. 2006;36:1133–1138. doi: 10.1007/s00595-006-3315-5. [DOI] [PubMed] [Google Scholar]
- 12.Ohchi T, Akagi Y, Kinugasa T, Ishibashi Y, Tanaka N, Fujino S, et al. Virchow lymph node metastatic recurrence of sigmoid colon cancer with severe lymph node metastases successfully treated using systemic chemotherapy combined with radiotherapy. Anticancer Res. 2013;33:2935–2940. [PubMed] [Google Scholar]
- 13.Ando M, Fujiya K, Kamikozuru H, Ganno H, Amagasa H, Takeuchi S, et al. Long-term survival of a case with advanced sigmoid colon cancer and Virchow’s lymph node metastasis. Jpn J cancer Chemother. 2013;40(12):2083–2085. [PubMed] [Google Scholar]
- 14.Oyanagi H, Kameyama H, Nogami H, Shimada Y, Nakano M, Nakano M, et al. A case of long-term survival in a patient with rectal cancer with Virchow lymph node metastasis, liver metastases and lung metastases. Jpn J Cancer Chemother. 2014;41(12):1674–1676. [PubMed] [Google Scholar]
- 15.Imamura H, Iino S, Sakamoto A, Kuwahata T, Yonemori K, Onishi S, et al. A long-term survival case of rectal cancer with Virchow’s lymph node metastasis by multimodality therapy. Jpn J Cancer Chemother. 2014;41(12):1674–1676. [Google Scholar]
- 16.Takeshita N, Fukunaga T, Kimura M, Sugamoto Y, Tasaki K, Hoshino I, et al. Successful resection of metachronous para-aortic, Virchow lymph node and liver metastatic recurrence of rectal cancer. World J Gastroenterol. 2015;21(44):12722–12728. doi: 10.3748/wjg.v21.i44.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imamura H, Iino S, Sakamoto A, Kuwahata T, Yonemori K, Onishi S, et al. A case of rectal cancer which showed complete response to chemotherapy for para-aortic lymph node metastases remaining after surgery for which resection of Virchow lymph node recurrence led to long-term survival. Jpn J Cancer Chemother. 2013;40(9):1233–1236. [PubMed] [Google Scholar]