Abstract
Anaplastic thyroid cancer is a fatal disease for which no effective therapeutic strategies exist. Lenvatinib, a tyrosine-kinase inhibitor that targets vascular endothelial growth factor receptor, has recently been approved in Japan for the treatment of patients with unresectable thyroid cancer including anaplastic thyroid cancer. Although lenvatinib, like the other tyrosine-kinase inhibitors, sunitinib and sorafenib, might also confer a risk of bleeding, fatal bleeding as a result of lenvatinib treatment for anaplastic thyroid cancer has not been described. A 61-year-old woman presented with a 7-cm mass in the right lobe of the thyroid, lymph node metastases to the neck and multiple lung metastases. Fine needle aspiration revealed that the tumor was anaplastic thyroid cancer. The TNM classification was T4aN1bM1, stage IVC. Shortly after local curative surgery, a tumor recurred in her neck that was treated with lenvatinib (24 mg/day). Nineteen days later, the common carotid artery ruptured and the lenvatinib was stopped. She received the best possible supportive care but died 40 days after stopping the lenvatinib. Autopsy findings showed that the tumor had invaded the adventitia of the common carotid artery at the region of the neck surgery, and an aneurysm had developed. However, the adventitia of the common carotid artery was preserved at the non-dissected area. Lenvatinib might confer risk for fatal bleeding in patients with recurrent anaplastic thyroid cancer after neck surgery, particularly with dissection around the common carotid artery.
Keywords: Anaplastic thyroid cancer, Lenvatinib, Common carotid artery, Rupture
Introduction
Anaplastic thyroid cancer (ATC) accounts for <2 % of all thyroid cancers, and it is usually lethal [1]. The six-month disease-specific survival rates of patients with stage IVA, B, or C common-type ATC are 60, 45 and 19 %, respectively [2, 3]. The American Thyroid Association Guidelines for ATC suggest that patients with stage IVA/IVB resectable disease have the best prognosis, particularly if the treatment approach is multimodal, whereas some with stage IVB unresectable cancer might respond to aggressive therapy, and those with stage IVC cancer should be considered for a clinical trial or palliative care, depending on their preference [4].
Treatment for aggressive forms of endocrine cancer has recently been transformed thanks to kinase inhibitors [5, 6]. The tyrosine-kinase inhibitors (TKIs), sorafenib and sunitinib, have induced partial responses among a few patients with radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC) [7–9]. On the other hand, a more recently developed drug, lenvatinib, induced much higher response rates for RAI-refractory DTC. Lenvatinib is an oral TKI targeting vascular endothelial growth factor (VEGF) receptor 1–3, fibroblast growth factor (FGF) receptor 1–4, ret proto-oncogene (RET), c-Kit, and platelet-derived growth factor (PDGF) receptor beta, and its anti-tumor effect is related to the control of tumor angiogenesis [10–12].
Lenvatinib treatment for RAI-refractory DTC is based on the results of the phase III Study of (E7080) Lenvatinib in Differentiated Cancer of the Thyroid (SELECT), in which lenvatinib extended the median progression-free survival of patients with RAI-refractory DTC from 3.6 to 18.3 months [13]. Takahashi et al. reported that lenvatinib was effective in 11 patients with ATC in a phase II study of lenvatinib; the objective response rate for patients with ATC treated with lenvatinib was 27.3 %, the median progression-free survival was 7.4 months, and the median overall survival was 10.6 months [14]. As a result, lenvatinib has been approved in Japan since May 2015 for the treatment of patients with unresectable thyroid cancer including ATC.
Over 97 % of patients taking lenvatinib in the SELECT trial had at least one treatment-related adverse event (AE), such as hypertension, diarrhea, or asthenia, but fatal bleeding was not reported [15]. Sunitinib and sorafenib have induced severe hemorrhagic complications in patients with various types of cancer [3, 16–20]. These findings indicate that treatment with TKI targeting VEGFR is associated with a significantly increased risk of bleeding. Similarly, lenvatinib is a VEGFR TKI, but a severe bleeding event during lenvatinib treatment of ATC has not been reported, as far as we investigated.
Here, we describe a patient who underwent neck surgery to treat ATC in whom the common carotid artery (CCA) ruptured during post-operative lenvatinib treatment for recurrent ATC.
Case report
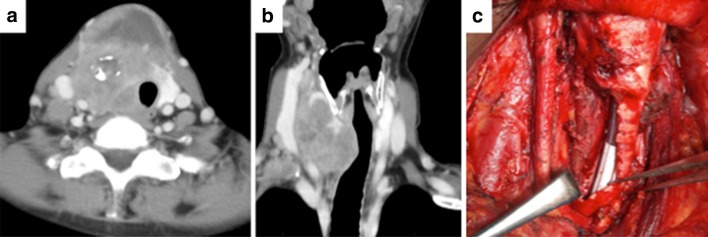
A 61-year-old woman with no significant medical history visited her primary physician with a neck mass, and was referred to our hospital 2 months later. Computed tomography (CT) revealed a tumor measuring 7 cm in the right lobe of the thyroid gland that involved the tracheal wall. Lymph node metastases at levels II, III, and IV were identified in the right neck (Fig. 1a, b) and multiple lung metastases were found. Fine needle aspiration revealed that the thyroid tumor was ATC. The TNM classification was T4a (trachea) N1bM1 (lung), stage IVC. The patient had airway stenosis and symptoms of dyspnea, and she and her family desired aggressive curative treatment including local disease control. Thus, locally curative surgery included right thyroid lobectomy and right neck dissection with resection of the tracheal wall and the internal jugular vein, including the sheath of the CCA (Fig. 1b). A tracheo-cutaneous fistula was constructed using a delto-pectoral flap. The tumor showed no adhesions to the CCA. The diagnosis was pathologically confirmed as ATC.
Fig. 1.
a, b On computed tomography, the neck tumor measures 7 cm in the right lobe of the thyroid and involves the tracheal wall. Lymph node metastases are identified at level II, III, and IV in the right neck. c Locally curative surgery, including right thyroid lobectomy and right neck dissection with resection of the tracheal wall, the internal jugular vein, and the sheath of the common carotid artery (CCA)
Four weeks after surgery, local recurrence was noted, and the multiple lung metastases showed rapid progression according to CT. The patient subsequently underwent chemotherapy consisting of weekly paclitaxel (wPTX; 80 mg/m2/week, one course consisting of three administrations). The lymph node in the right neck and the multiple lung metastases showed progression after two courses of wPTX, and we suspended wPTX administration. Three weeks later, we began treatment with lenvatinib (24 mg/day/body). Significant reduction of the recurrent tumor and the lung metastases was seen at 1 week after initiation of lenvatinib (Fig. 2a, b). She had a grade 2 AE of high blood pressure [Common Terminology Criteria for Adverse Events (CTCAE) version 4.0], but did not have thrombocytopenia. Nineteen days after the initiation of lenvatinib, the recurrent tumor of the right neck had necrotized. Further, the CCA was exposed, and an aneurysm of the CCA was noted (Fig. 3). Treatment with lenvatinib was then stopped. On CT, the lung metastases showed partial response. The CCA ruptured on the following day and was ligated. After stopping the lenvatinib treatment, the tumor rapidly increased in size. She received the best possible supportive care and died 40 days later after the lenvatinib treatment was stopped.
Fig. 2.
Significant reduction of the lung metastases was seen at 1 week after initiation of lenvatinib. a Before treatment of lenvatinib. b One week after treatment of lenvatinib
Fig. 3.

At 19 days after the initiation of lenvatinib, recurrent tumor of the right neck has necrotized, the common carotid artery (CCA) is now exposed, and an aneurysm (red arrow) of the CCA has appeared
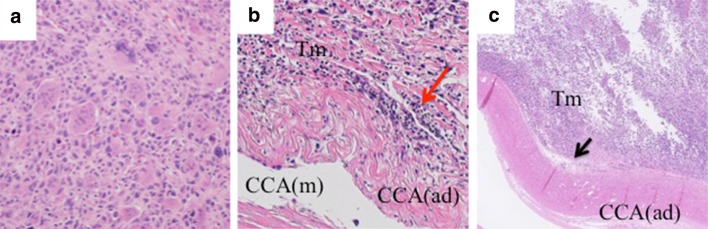
In the surgical specimen, pathological findings invaded that spindle atypical cell and multinucleated giant cell infiltrated extensively. Additionally, necrosis, ghost-like papillary carcinoma and irregular nuclear fission were found (Fig. 4a). Diagnosis was ATC. An autopsy revealed extension of the neck tumor to the mediastinum under the skin and metastases to the lungs, kidneys and mediastinal lymph node. The pathological findings showed that the tumor had invaded the adventitia of the CCA in the region of the neck surgery, but not the media. The ATC did not invade the adventitia of the CCA at the non-dissected area (Fig. 4b, c).
Fig. 4.
a In the surgical specimen, spindle atypical cell and multinucleated giant cell infiltrate extensively. Necrosis, ghost-like papillary carcinoma and irregular nuclear fission are found. b Autopsy reveals that the tumor invades the adventitia of the common carotid artery (CCA) but not to the media in the region of neck surgery (red arrow). c The adventitia of the CCA is preserved at the non-dissected area in the left neck (black arrow). CCA common carotid artery, Tm tumor, ad adventitia, m media
Discussion
This case report suggests that lenvatinib treatment for a locally recurrent ATC tumor after neck surgery can cause fatal bleeding. Our patient was diagnosed with possibly inoperable anaplastic thyroid cancer stage IVC. However, we proceeded with surgery and subsequent chemotherapy. Complete resection is considered the most significant factor for favorable outcomes of ATC [21], and our patient wished to undergo an aggressive curative approach that included surgery. Lenvatinib was administered postoperatively to treat the recurrent ATC tumor. Thereafter, the CCA ruptured and the treatment for the ATC was stopped.
Antiangiogenic TKI, including lenvatinib, has become an important part of therapy for progressive and advanced RAI-resistant DTC [13]. Takahashi et al. reported that lenvatinib could be effective in patients with ATC [14]. On the other hand, most of patients taking lenvatinib have had a treatment-related AE [15]. Lenvatinib, like other TKIs that target VEGFR such as sunitinib and sorafenib, might also have a risk of bleeding. However, as far as we can ascertain, the present report is the first to describe a severe bleeding event associated with lenvatinib treatment for ATC.
Sunitinib and sorafenib are associated with a 2.4 % risk of high-grade bleeding when administered to treat various types of cancer [18]. VEGF is important to the survival of endothelial cells as it helps to maintain the architecture and integrity of the microvasculature. Therefore, blocking VEGFR might alter the reparability and renewability of endothelial cells in response to trauma, which could increase the risk of bleeding [16, 18].
The difference between lenvatinib and the other TKIs is its potent ability to inhibit FGFR-1, which offers a potential opportunity to block at least one established mechanism of resistance to VEGF/VEGFR inhibitors [22]. The inhibition of FGFR-1 might comprise the mechanism through which lenvatinib can shrink tumors. Additionally, anti-angiogenic TKI administered to patients with extant risk factors, including radiotherapy, surgery, and tumor invasion of vital structures, plays a role in the development of aero-digestive fistulae by impairing the growth of new blood vessels and delaying wound healing [20]. The recurrent tumor in the neck of our patient became necrotic in response to powerful VEGFR inhibition induced by lenvatinib and resulted in cavity formation, followed by exposure and rupture of the CCA.
On the other hand, an aneurysm developed in the CCA at the site of the neck surgery in our patient, and autopsy revealed tumor invasion of the adventitia of the CCA. The adventitia is the outermost layer of the arterial wall, and it behaves like a stiff tube that prevents rupture [23]. Surgical removal of the CCA sheath resulted in reduced protection against tumor invasion into the adventitia of the CCA.
We considered that the rupture of the CCA in our patient was caused by the following factors. Surgical removal of the CCA sheath allowed the tumor to infiltrate the adventitia of the CCA, which allowed lenvatinib to necrotize the recurrent ATC. Damage to the adventitia resulted in the formation of an aneurysm that ruptured due to wide exposure of the CCA.
Clinicians should carefully administer lenvatinib to patients with recurrent ATC tumors after surgery, particularly after dissection around the CCA, or when a recurrent ATC tumor is in close proximity to blood vessels. In such situations, patients even with the lethal disease should receive adequate information and counseling about the possibility of fatal bleeding.
In conclusion, lenvatinib can efficiently shrink tumors, but the risk of bleeding might be high for patients with recurrent ATC after neck surgery. Further studies are needed to clarify the safety and indications for administering lenvatinib to treat ATC.
Conflict of interest
The author Kazufumi Obata received supervision fees from Company Eisai. Iwao Sugitani received research funding from Company Eisai and lecture fees from Company Eisai, Bayer and Genzyme.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. E Gland Surg. 2015;4:44–51. doi: 10.3978/j.issn.2227-684X.2014.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugitani I, Miyauchi A, Sugino K, et al. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg. 2012;36:1247–1254. doi: 10.1007/s00268-012-1437-z. [DOI] [PubMed] [Google Scholar]
- 3.Onoda N, Sugitani I, Higashiyama T, et al. Concept and design of a nationwide prospective feasibility/efficacy/safety study of weekly paclitaxel for patients with pathologically confirmed anaplastic thyroid cancer (ATCCJ–PTX–P2) BMC Cancer. 2015;15:475–479. doi: 10.1186/s12885-015-1490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 5.Gruber JJ, Colevas AD. Differentiated thyroid cancer: focus on emerging treatments for radioactive iodine-refractory patients. Oncologist. 2015;20:113–126. doi: 10.1634/theoncologist.2014-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marotta V, Sciammarella C, Vitale M, et al. The evolving field of kinase inhibitors in thyroid cancer. Crit Rev Oncol Hematol. 2015;93:60–73. doi: 10.1016/j.critrevonc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Takami H, Ito K, Sugino K. Development of molecular targeted drugs for advanced thyroid cancer in Japan. Endocr J. 2014;61:833–839. doi: 10.1507/endocrj.EJ14-0107. [DOI] [PubMed] [Google Scholar]
- 8.Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG–PET–positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16:5260–5268. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machiels JP, Henry S, Zanetta S, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006–01. J Clin Oncol. 2010;28:21–28. doi: 10.1200/JCO.2009.23.8584. [DOI] [PubMed] [Google Scholar]
- 10.Tohyama O, Matsui J, Kodama K et al (2014) Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014:638747. doi:10.1155/2014/638747 [DOI] [PMC free article] [PubMed]
- 11.Yeung KT, Cohen EE. Lenvatinib in advanced, radioactive iodine-refractory, differentiated thyroid carcinoma. Clin Cancer Res. 2015;21:5420–5426. doi: 10.1158/1078-0432.CCR-15-0923. [DOI] [PubMed] [Google Scholar]
- 12.Krajewska J, Kukulska A, Jarzab B. Drug safety evaluation of lenvatinib for thyroid cancer. Expert Opin Drug Saf. 2015;14:1935–1943. doi: 10.1517/14740338.2015.1102883. [DOI] [PubMed] [Google Scholar]
- 13.Schlumberger M, Tahara M, Wirth LJ. Lenvatinib in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:1868. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi S, Tahara M, Kiyota N et al (2014) Phase II study of lenvatinib, a multi-targeted tyrosine kinase inhibitor, in patients with all histologic subtypes of advanced thyroid cancer (differentiated, medullary, and anaplastic). ESMO Meeting Abstr 4933
- 15.Kiyota N, Schlumberger M, Muro K, et al. Subgroup analysis of Jpanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differiated thyroid cancer. Cancer Sci. 2015;106:1714–1721. doi: 10.1111/cas.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui EP, Ma BB, King AD, et al. Hemorrhagic complications in a phase II study of sunitinib in patients of nasopharyngeal carcinoma who has previously received high-dose radiation. Ann Oncol. 2011;22:1280–1287. doi: 10.1093/annonc/mdq629. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Zhang HL, Zhang Y, et al. Digestive tract hemorrhage due to complications with gastrointestinal stromal tumor treated with sunitinib: a case report. Oncol Lett. 2013;5:699–701. doi: 10.3892/ol.2012.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967–974. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- 19.Brose MS, Frenette CT, Keefe SM, et al. Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol. 2014;41:S1–S16. doi: 10.1053/j.seminoncol.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Blevins DP, Dadu R, Hu M, et al. Aerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancer. Thyroid. 2014;24:918–922. doi: 10.1089/thy.2012.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passler C, Scheuba C, Prager G, et al. Anaplastic (undifferentiated) thyroid carcinoma (ATC): a retrospective analysis. Langenbecks Arch Surg. 1999;384:284–293. doi: 10.1007/s004230050205. [DOI] [PubMed] [Google Scholar]
- 22.Stjepanovic N, Capdevila J. Multikinase inhibitors in the treatment of thyroid cancer: specific role of lenvatinib. Biologics. 2014;8:129–139. doi: 10.2147/BTT.S39381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khamdaengyodtai P, Vafai K, Sakulchangsatjatai P, et al. Effects of pressure on arterial failure. J Biomech. 2012;45:2577–2588. doi: 10.1016/j.jbiomech.2012.07.032. [DOI] [PubMed] [Google Scholar]