Abstract
Transforming growth factor (TGF)-β1 mediates glycosaminoglycan (GAG) chain hyperelongation on secreted proteoglycans and these modifications are associated with increased lipid binding in the vessel wall and the development of atherosclerosis. In vascular smooth muscle cells (VSMCs), TGF-β1 regulated GAG elongation via extracellular signal-regulated kinase (ERK) and p38 as well as Smad2 linker region phosphorylation. In this study, our aim was to identify the TGF-β1 mediated signalling pathway involving reactive oxygen species (ROS) and Smad2 linker region phosphorylation that regulate the mRNA expression of GAG synthesizing enzymes, chondroitin 4-O-sulfotransferase 1 (CHST11) and chondroitin sulfate synthase 1 (CHSY1) which are the rate limiting enzymes involved in GAG chain elongation. Signalling molecules were assessed by western blotting, quantitative real-time PCR was used for analysis of gene expression and intracellular ROS level was measured by a fluorescence based assay. TGF-β1 induced ROS production in VSMCs. Nicotinamide adenine dinucleotide phosphate oxidase (Nox) inhibitors, diphenyleneiodonium (DPI) and apocynin blocked TGF-β1 mediated Smad2 linker region phosphorylation. TGF-β1 treatment increased the mRNA levels of CHST11 and CHSY1. Pharmacological inhibition of Nox blocked TGF-β1 mediated mitogen activated protein kinases (MAPKs) phosphorylation and TGF-β1 stimulated CHST11 and CHSY1 mRNA expression. These findings demonstrated that TGF-β1 mediated expression of CHST11 and CHSY1 can occur via Nox-dependent pathways and Smad2 linker region phosphorylation.
Keywords: Atherosclerosis, Nox, Reactive oxygen species, Mitogen activated protein kinases, Glycosaminoglycan
Introduction
Transforming growth factor β (TGF-β) is a pleiotropic growth factor that is strongly implicated in the pathophysiology of atherosclerosis (Bobik et al. 1999; Yang et al. 2010). In vascular smooth muscle cells (VSMCs), as a model of atherosclerosis, TGF-β1 stimulates an increase in the length of glycosaminoglycan (GAG) chains on the proteoglycan, biglycan (Little et al. 2002), leading to increased binding of atherogenic lipoproteins as the manifestation of the response to retention hypothesis of atherogenesis (Ballinger et al. 2010). GAG chain synthesis requires the combined action of GAG synthesizing enzymes. Characterizing the signalling pathways of the genes involved in GAG chain elongation are of interest as the identification of a pharmacological intervention will inhibit the hyperelongation of the GAG chains without interfering with the native length of chains (Anggraeni et al. 2011; Kamato et al. 2013a). GAG chain elongation occurs through the actions of glycosyltransferases and sulfotransferase (Silbert and Sugumaran 2002). In human VSMCs, TGF-β1 and thrombin treatment increase the expression of glycosyltransferase, chondroitin sulfate synthase 1 (CHSY1) and sulfotransferase chondroitin 4-O-sulfotransferase 1 (CHST11) (Rostam et al. 2016; Kamato et al. 2016). In a mouse model of atherosclerosis an increase in the mRNA expression of CHST11 correlated with an increase in plaque formation in vivo. Multiple signalling pathways are involved in regulating GAG chain elongation and regulating the gene expression of the enzymes involved (Afroz et al. 2018). These signalling pathway represent potential targets for therapeutic intervention of atherosclerosis.
TGF-β transduces its biological effects from the cell membrane to the nucleus through serine/threonine kinase cell surface receptors and their downstream effectors, Smad transcription factors in the process known as canonical signalling (Wrighton et al. 2009). To date, most studies on TGF-β-signalling pathways have focused on the rapid phosphorylation of Smad transcription factors (Smad2 or Smad3) at their extreme carboxyl terminus at a conserved motif (S-S-X-S) in the response of TGF-β type 1 receptors (TGFBR1) which are also known as Activin-like kinase 5 (Alk-5) (Souchelnytskyi et al. 1997; Massagué et al. 2005). However, recent studies have shown that the phosphorylation of R-Smads at serine and threonine residues within the central linker region (known as non-canonical or non-Smad signalling) can also regulate a wide range of cellular responses (Li et al. 2009; Jiang et al. 2010; Kamato et al. 2013b; Burch et al. 2011). In human VSMCs, TGF-β1 mediates the increase in the expression of CHST11 and CHSY1 mRNA expression, which are rate limiting enzymes for the elongation of GAG chains on biglycan, and this pathway involves Smad2 linker region phosphorylation (Afroz et al. 2018; Rostam et al. 2016). Multiple alternate pathways can be involved in TGF-β1 induced Smad linker region phosphorylation including mitogen-activated protein kinase (MAPK) (Kamato et al. 2013b).
Our earliest reports of TGF-β1 mediated Smad2 linker region phosphorylation to regulate proteoglycan synthesis were dependent on extracellular signal-regulated kinase (ERK) and p38 but not c-Jun N-terminal kinase (JNK) (Burch et al. 2010). This was later also correlated with the regulation of the rate limiting genes involved in the elongation of the GAG chain (Rostam et al. 2018; Rostam et al. 2016). MAPKs signalling pathways are activated by both mitogen and stress activated pathways (Son et al. 2011). Reactive oxygen species (ROS) is a regulator of signalling molecules (phosphatases, tyrosine kinases) and MAPKs (Thannickal and Fanburg 2000). Here we explore whether ROS facilitates GAG gene expression via enhancing MAPK activation which leads to the phosphorylation of Smad linker region.
The nicotinamide adenine dinucleotide phosphate oxidase (Nox) family consists of seven catalytic homologues (Nox1 to Nox5 and two dual oxidases, Duox-1 and Duox-2) that catalyze the reduction of molecular oxygen to superoxide anion by using NADPH as the reducing agent (Lassègue et al. 2012). The classical Nox (phagocytic oxidase) is a multi-subunit enzyme comprising two membrane-associated catalytic subunits containing gp91phox (newly termed Nox2) and p22phox and several cytosolic regulatory components composed of p40phox, p47phox, p67phox and Rac (Sirker et al. 2011). Four members of the Nox family, Nox1, Nox2, Nox4 and Nox5 are expressed in the vascular cells and they are known to be involved in atherosclerosis development and progression (Lassègue et al. 2012). Studies using mice deficient in the cytosolic subunit p47phox or catalytic subunits Nox1 or Nox2 revealed the role of specific subunits of Nox in atherogenesis (Barry-Lane et al. 2001; Miller et al. 2010; Sheehan et al. 2011). Studies also suggest Nox as a potential therapeutic target for the treatment of atherosclerosis in vitro and in vivo (Drummond et al. 2011). TGF-β induces Nox to produce ROS, which acts as a mediator for TGF-β induced downstream signalling (Jiang et al. 2014). The role of Nox and ROS in atherogenic signalling involving GAG elongation on biglycan is not known. It is essential to determine the role of Nox and ROS in TGF-β1 signalling as this may represent a novel therapeutic target for the treatment and prevention of atherosclerosis.
We investigated the role of ROS in TGF-β1 signalling in the context of an atherogenic response – the expression of the GAG synthesizing genes – in human VSMCs. We report that TGF-β1 increases ROS levels in VSMCs via its cognate receptor activation, TGF-β1 mediated increase in the expression of the genes for the enzymes which mediate the elongation of GAG chains on biglycan is dependent on ROS. ROS thus has a key role in TGF-β1 signalling.
Materials and methods
Materials
Ham’s F-12 K (Kaighn’s) medium and fetal bovine serum (FBS) was purchased from GIBCO (Invitrogen, Carlsbad, CA, USA). Dulbecco’s Modified Eagle Medium nutrient mixture-F12 (DMEM/F12), trypsin-EDTA and antibiotics (penicillin, streptomycin) were purchased from Bioidea (Tehran, Iran). The following chemicals were obtained from Sigma Aldrich (St Louis, MO, USA): SB431542, apocynin, diphenyleneiodonium chloride (DPI), 2`,7`-Dichlorofluorescence diacetate (H2DCFDA) dye, HEPES ≥99.5% (titration), sodium chloride (NaCl), sodium hydroxide, calcium chloride (CaCl2), magnesium chloride (MgCl2), D-(+)-glucose, sodium dodecyl sulfate (SDS), 2-mercaptoethanol and dimethyl sulphoxide. Potassium chloride (KCl) was from Chem-supply Pty Ltd. (SA, AUS). PierceTM bicinchoninic acid protein assay kit was purchased from ThermoFisher Scientific (IL, USA). Human recombinant transforming growth factor-β, anti-phospho-Smad2 (Ser245/250/255), anti-phospho-p38-MAPK (Thr180/Tyr182), anti-phospho-ERK (1/2) (Thr202/Tyr204), anti-rabbit immunoglobulin-G (IgG)-horseradish peroxidase (HRP) and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) rabbit monoclonal IgG antibody were purchased from Cell Signalling Technology (Beverly, MA, USA). The BioRad Trans-Blot® Turbo RTA transfer kit, polyvinylidene fluoride (PVDF) membrane, 30% acrylamide/bis-acrylamide solution, N,N,N`,N`-tetramethylehtylenediamine (TEMED), Ammonium persulfate (APS) and Quantity one imaging software were from BioRad laboratories (CA, USA). Primers (forward and reverse) for CHST11, CHSY1 and GAPDH were purchased from Takapouzist (Tehran, Iran).
Culture of human aortic smooth muscle cells
Human VSMCs were obtained from the Pasteur Institute (Tehran, Iran). These cells were grown in DMEM/F12 (1:1) culture medium supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C in 5% CO2. VSMCs were passaged to provide sufficient numbers for experimentation and cells used in experiments were between passages 10–20. VSMCs were seeded in 35 mm dishes at a density of 4 × 105/dish or onto 96 well plate (10,000 cells/plate) overnight in a total volume of 100 μl and maintained until confluency. Confluent cultures were serum starved in DMEM/F12 or Ham’s F-12 K medium containing 0.1% FBS and 1% penicillin-streptomycin for 24 h before treatment.
Western blotting
Total cell lysates with concentration of 20 μg were resolved by 10% SDS-PAGE and transferred onto PVDF membranes. Non-specific binding sites were blocked with 3% skim milk or 5% BSA and then incubated at 4 °C overnight with anti-phospho-Smad2 (Ser245/250/255), anti-phospho-p38-MAPK (Thr180/Tyr182) and anti-phospho-ERK (1/2) (Thr202/Tyr204) followed by peroxidase labeled anti-rabbit IgG and enhanced Chemiluminescence (ECL) detection. The membranes were then reprobed with anti-GAPDH. GAPDH was used as loading control.
Image densitometry
The analysis tools of BioRad ImageLab 5.2.1 software were used to quantify the volume density of the protein band of interest. The volume of background was subtracted from each of respective band of interest. The GAPDH was used as a loading control to quantify protein. The final density of the band of interest was obtained by normalizing the intensity of the band of interest with respective gel loading control GAPDH. The values were then normalised to basal and values were visualised in a histogram in GraphPad prism.
Gene expression analysis
The mRNA levels of GAG synthesizing enzymes (CHST11 and CHSY1) were determined by quantitative real-time polymerase chain reaction (q-RT-PCR). To measure gene expression, total RNA from cultured cells was extracted using RNeasy Mini kit (Qiagen, Iran) according to the manufacturer’s protocol, and RNA concentration and purity were checked using Nanodrop 2000 (Thermo Fisher Scientific). First strand cDNA was synthesized from 500 ng RNA using the Quanti Tect reverse transcription kit (Qiagen, Japan) according to the manufacturer’s protocol. qRT-PCR was performed using the QuantiFast™ SYBR® Green PCR kit (Qiagen, Iran) together with specific primers (see Table 1). Data was normalized to the GAPDH housekeeping gene. All experiments were performed at least three times and analysis performed in duplicate for each experiment. The delta-delta cycle-threshold (ΔΔCt) method was used to analyse the fold change in mRNA expression from qRT-PCR experiments. Ct values for all samples were recorded in a spreadsheet and normalised against the endogenous ribosomal GAPDH as a house-keeping gene. The Ct values of treated sample of interest was compared with a non-treated sample (basal). The following formula outline the analysis of mRNA expression of the target genes.
Table 1.
Sequences of primers
| CHST11 | Forward | 5′-GGCCCTGCGCAAAG-3′ |
| Reverse | 5′-GGGTGTGTGGGTCGATGAG-3′ | |
| CHSY1 | Forward | 5’-CCCGCCCCAGAAGAAGTC-3’ |
| Reverse | 5’-TCTCATAAACCATTCATACTTGTCCAA-3’ | |
| GAPDH | Forward | 5’-CAAGTTCAACGGCACAGTCAAG-3’ |
| Reverse | 5’-CATACTCAGCAC CAGCATC ACC-3’ |
Intracellular ROS detection assay
VSMCs were washed with 100 μl of Krebs-HEPES buffer (10 mM HEPES, 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 11 mM D-(+)-glucose and pH 7.4) and incubated for 45 min with 10 μM H2DCF-DA at 37 °C and 5% CO2. The dye was gently removed and VSMCs were then treated with or without agonist/antagonist of interest. The fluorescence was measured intensity using Ensight Multimode Plate Reader at excitation/emission = 485/535 nm. The fluorescence intensity was used to calculate the fold change.
Statistical analysis
Data was normalised and shown as the mean ± standard error of the mean (SEM) of three independent experiments performed, unless stated otherwise. A One-Way Analysis of Variance was used to calculate statistical significance of normalised data as stated, followed by least significant different post-hoc analysis. Results considered significant when the probability was less than 0.05 (*p < 0.05) and 0.01 (**p < 0.01).
Results
TGF-β1 stimulates the phosphorylation of Smad2 linker region
Canonical TGF-β1 signalling involves carboxy terminal phosphorylation of Smad (Derynck and Zhang 2003) but we have recently described a role for non-canonical Smad linker region phosphorylation in the expression of genes that are involved in the elongation of GAG chains on the proteoglycan, biglycan (Rostam et al. 2016). There are 3 serine residues and one threonine residue in the Smad2 linker region which are the target of many different kinases and we used an antibody which recognizes the phosphorylation of the cluster of serine residues (Ser245, Ser250 and Ser255). VSMCs were treated with TGF-β1 (2 ng/ml) for 30, 60 and 120 min (Fig. 1). TGF-β1 treatment of VSMCs caused an increase in the phosphorylation of Smad2 linker region to 2.7-fold (p < 0.05) at 30 min. The cellular level of Smad2 linker region phosphorylation was lower at 60 and 120 min post-TGF-β1 treatment, 2.1-fold (p < 0.05) and 1.6-fold respectively (Fig. 1). Stimulation with TGF-β1 at 30 min was used in further experiments.
Fig. 1. TGF-β1 promotes phosphorylation of Smad2 linker region in human VSMCs.
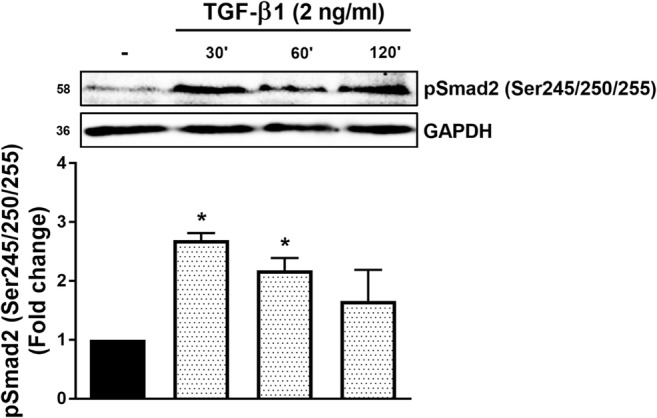
VSMCs were stimulated for 0.5, 1 and 2 h with TGF-β1 (2 ng/ml), harvested and total protein was extracted. Proteins were resolved by 10% SDS-PAGE and then transferred to PVDF membrane. Membranes were incubated with anti-phospho-Smad2 (Ser245/250/255) (1:1000) and then followed by incubation with peroxidase labeled anti-rabbit IgG (1:10000) and ECL detection. Anti-GAPDH was used as loading control. Normalized data in each case are shown as mean ± SEM from three independent experiments and statistical significance was determined by One-way ANOVA followed by least significant difference post-hoc analysis. *p < 0.05 compared with untreated control
TGF-β1 treatment increases ROS levels in VSMCs
To study the role of ROS in this signalling pathway the first question was to assess if TGF-β1 treatment increases ROS levels in VSMCs. VSMCs were treated with TGF-β1 (2 ng/ml) for 30 min in the presence and absence of the TGFBR1 antagonist, SB431542 (10 μM) and the Nox inhibitor, DPI (20 μM) (Fig. 2). TGF-β1 treatment increased the steady state level of ROS by 1.2-fold (p < 0.01) in 30 min and this increase was completely inhibited in cells treated with either SB431542 or DPI (p < 0.01) (Fig. 2). This data clearly establishes that TGF-β1 treatment increases intracellular ROS level in human VSMCs and this effect is mediated via its receptor and most likely activation of Nox enzymes.
Fig. 2. TGF-β1 stimulates a Nox-dependent increase in ROS in human vascular smooth muscle cells.
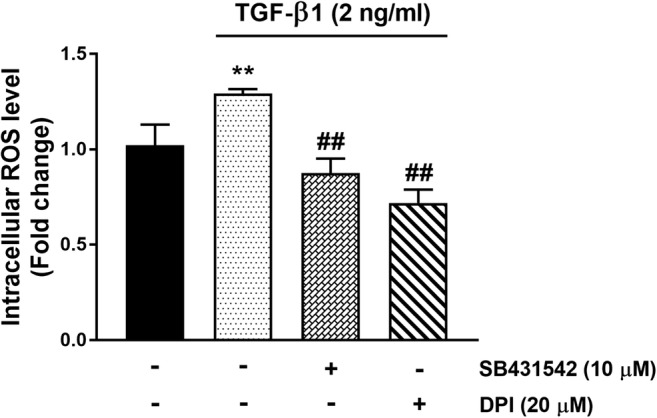
VSMCs were treated with TGF-β1 (2 ng/ml) for 30 min in the presence and absence of the TGFBR1 antagonist, SB431542 (10 μM) and the Nox inhibitor, DPI (20 μM). Histogram represents fluorescence intensity minus the baseline, expressed as fold per basal. Results are expressed as mean ± SEM from three independent experiments and statistical significance was determined by One-way ANOVA followed by least significant difference post-hoc analysis. **p < 0.01 compared with untreated control and ##p˂0.01 compared with TGF-β1
TGFBR1/Alk-5-mediated ROS signalling pathway in human VSMCs involves phosphorylated Smad2 linker region
In order to elucidate the role of Nox in the phosphorylation of Smad2 linker region, two inhibitors of Nox (DPI and apocynin) were used to assess the effect of TGF-β1 on Smad2 linker region phosphorylation. DPI is a broad-spectrum inhibitor of Nox; apocynin is a widely used inhibitor of Nox but its status as a Nox inhibitor in non-phagocytic cells is an area of some contention (Vejrazka et al. 2005; Heumuller et al. 2008). When VSMCs were treated with TGF-β1 (2 ng/ml) for 30 min Smad2 linker region phosphorylation was elevated 2.7-fold (p < 0.01) compared to non-treated controls (Fig. 3a). In the presence of DPI (1–20 μM), the TGF-β1 mediated Smad2 linker region phosphorylation was inhibited in a partially dose-dependent manner with a maximal inhibitory effect (approximating 100% inhibition) at DPI concentration of 20 μM (p < 0.01) (Fig. 3a). The established TGFBR1 inhibitor, SB431542 (10 μM), almost completely blocked the response to TGF-β1 (p < 0.05) (Fig. 3a). Then, we tested apocynin, a compound which prevents translocation of p47phox to plasma membrane and interferes with Nox activation in VSMCs (Kinkade et al. 2013). TGF-β1 treatment caused an increase of Smad2 linker region phosphorylation after 30 min. In the presence of 1 and 10 μM of apocynin TGF-β1 mediated Smad2 linker region phosphorylation was slightly inhibited at the lower concentration of apocynin and the higher concentration caused partial but statistically significant inhibition (approximating 50%) (p < 0.05) (Fig. 3b). These data suggest that TGF-β1 mediated Smad2 linker region phosphorylation involves ROS.
Fig. 3. Nox-dependent signalling regulates TGFBR1/Alk-5 mediated Smad2 linker region phosphorylation in human VSMCs.
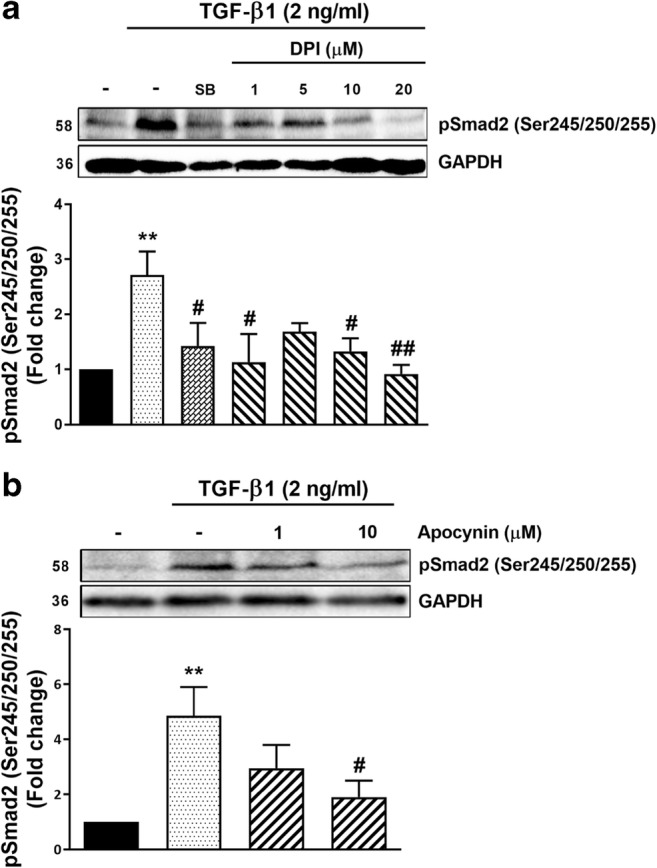
a VSMCs were treated with TGF-β1 (2 ng/ml) for 30 min in the presence and absence of the TGFBR1 antagonist, SB431542 (SB) (10 μM) and the Nox inhibitor, DPI (1–20 μM) b VSMCs were treated with TGF-β1 (2 ng/ml) for 30 min in the presence and absence of the Nox inhibitor, apocynin (1 and 10 μM). Membranes were incubated with anti-phospho-Smad2 (Ser245/250/255) (1:1000) followed with peroxidase labeled anti-rabbit IgG (1:10000) and ECL detection. Anti-GAPDH was as loading control. Normalised data in each case are shown as mean ± SEM from three independent experiments and statistical significance was determined by One-way ANOVA followed by least significant difference post-hoc analysis. *p < 0.05 and **p < 0.01 compared with untreated control, #p < 0.05 and ##p < 0.01 compared with TGF-β1
TGF-β mediated MAPKs (ERK and p38) phosphorylation is Nox-dependent in human VSMCs
We have previously shown that TGF-β1-mediated GAG hyperelongation on the proteoglycan, biglycan as well as the stimulation of the expression of the genes for the enzymes which are rate limiting for the process of GAG chain elongation are dependent on MAPK, specifically p38 and ERK but not JNK in human VSMCs (Burch et al. 2010; Dadlani et al. 2008). To further unravel the role of ROS in TGF-β1 mediated Smad2 linker region phosphorylation we investigate whether MAPK mediated Smad2 linker region phosphorylation is downstream of ROS release. The phosphorylation of ERK and p38 were investigated in the presence and absence of Nox inhibitor, DPI (20 μM) and putative Nox inhibitor, apocynin (20 μM) (Fig. 4). TGF-β1 treatment of human VSMCs caused a 2.9-fold (p < 0.01) increase in the cellular level of pERK1/2 and this response was almost completely attenuated by both DPI and apocynin (p < 0.01) (Fig. 4a). TGF-β1 treatment caused a 2.7-fold (p < 0.01) increase in the level of pp38 which was partially but not statistically significantly attenuated by DPI but was almost completely blocked by apocynin (p < 0.01) (Fig. 4b). These data are consistent with our earlier findings of the stimulatory role of TGF-β1 on ERK and p38 but we now show that these responses are blocked by ROS inhibitors and are therefore downstream of Nox in the signalling pathway.
Fig. 4. TGF-β mediated MAPK (ERK and p38) phosphorylation is Nox-dependent signalling in human VSMCs.
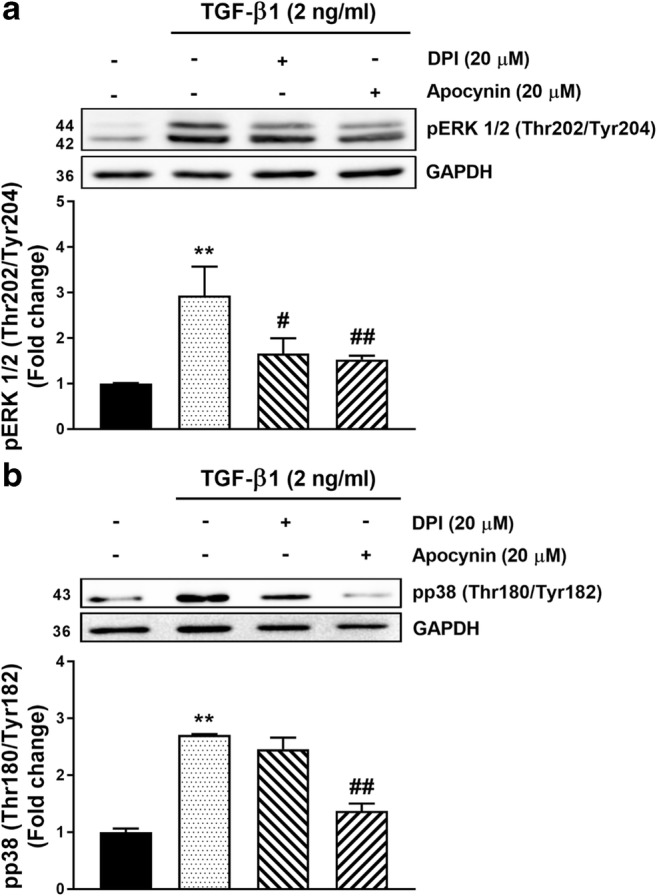
VSMCs were pre-incubated with DPI (20 μM) and apocynin (20 μM) for 30 min before being treated with TGF-β (2 ng/ml) for 5 min. Membranes were incubated with a Anti-phospho-ERK (1/2) (Thr202/Tyr204) (1:4000) followed by followed by peroxidase labeled anti-rabbit IgG (1:2000). b Anti-phospho-p38-MAPK (Thr180/Tyr182) (1:1000) followed by followed by peroxidase labeled anti-rabbit IgG (1:2000) and ECL detection. Anti-GAPDH was as loading control. Normalized data in each case are shown as mean ± SEM from three independent experiments and statistical significance was determined by One-way ANOVA followed by least significant difference post-hoc analysis. **p˂0.01 compared with untreated control, #p˂0.05 and ##p˂0.01 compared with TGF-β
TGF-β1 stimulated GAG synthesizing enzymes mRNA expression is ROS dependent
We then investigated the involvement of Nox enzymes in the TGF-β1 mediated expression of GAG synthesizing enzymes. VSMCs were treated with TGF-β1 (2 ng/ml) for 6 h resulting in an up-regulation of the mRNA expression of CHST11 to 2.2-fold (p < 0.01) compared to untreated cells. The TGF-β1 mediated CHST11 mRNA expression was completely blocked by DPI (20 μM) (p < 0.01) and apocynin (20 μM) (p < 0.05) (Fig. 5a). The TGFBR1 inhibitor, SB431542 abolished TGF-β1-stimulated CHST11 mRNA expression (p < 0.01). Similarly, VSMCs were treated with TGF-β1 (2 ng/ml) for 6 h result in an increase in the mRNA expression of CHSY1 to 2.3-fold (p < 0.01) compared to untreated cells and treatment of VSMCs with DPI (20 μM), apocynin (20 μM) and SB431542 (10 μM) completely blocked the TGF-β1 mediated CHSY1 mRNA expression (p < 0.01) (Fig. 5b). These findings indicate that Nox and most likely ROS are involved in of TGF-β1 stimulation of the mRNA expression of GAG synthesizing enzymes. Taken together the data presented in this manuscript is consistent with our earlier reports of the role of pERK1/2 and pp38 on Smad linker region phosphorylation and the expression of mRNA for GAG elongation enzymes (CHST11 and CHSY1) but we now show a role for Nox in these responses.
Fig. 5. TGF-β1 mediated mRNA expression of GAG genes (A) CHST11 and (B) CHSY1 is mediated via Nox dependent pathways.
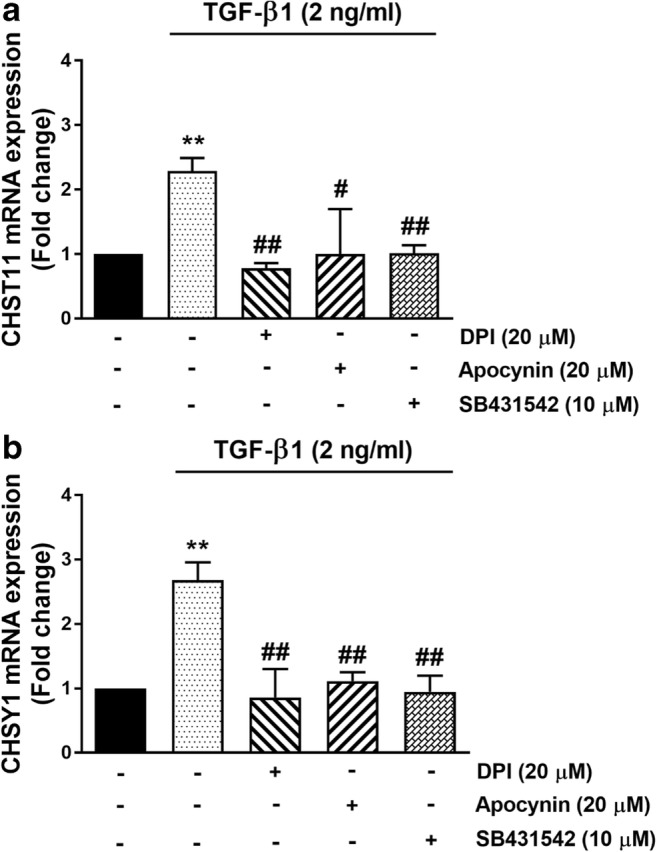
VSMCs were pre-incubated with the following inhibitors: Nox inhibitors DPI (20 μM) and apocynin (20 μM) for 2 h and TGFBR1 antagonist, SB431542 (10 μM) for 30 min before being treated with TGF-β1 (2 ng/ml) for 6 h. Total RNA was harvested and the mRNA of a CHST11 and b CHSY1 were analyzed using qRT-PCR. GAPDH was used as a house keeping gene. Results are expressed as mean ± SEM from three independent experiments and statistical significance was determined by One-way ANOVA followed by least significant difference post-hoc analysis. **p < 0.01 compared with untreated control, #p < 0.05 and ##p < 0.01 compared with TGF-β1
Discussion
We provide a clear but preliminary demonstration of the role of Nox and ROS in TGF-β1 signalling; specifically, non-canonical Smad linker region phosphorylation and downstream expression of the genes for the enzymes which are rate limiting in the process of GAG elongation on the proteoglycan, biglycan. We provide an insight into a specific signalling pathway from TGF-β1/TGFBR1 to Nox to MAPKs and then Smad linker region phosphorylation leading to the expression of mRNA linked to chondroitin sulfate GAG chain synthesizing enzymes, CHST11 and CHSY1 in human VSMCs.
Carboxy terminal phosphorylation of Smad2 and Smad3 occurs as a direct result of the kinase activity of TGFBR1. Phosphorylated Smad2/3 can interact with Smad4 and then these activated complexes translocate into the nucleus to regulate transcription of target genes (Massague 2003). In contrast to carboxy terminal phosphorylation, Smad2 linker region phosphorylation occurs via activation of multiple serine/threonine kinase intermediates (Rezaei et al. 2012; Kamato et al. 2014; Burch et al. 2010; Rostam et al. 2016; Kamato et al. 2013b). The role of Smad2 linker region phosphorylation in cell biology is expanding. Whereas all previous studies of Smad linker region phosphorylation have involved direct activation of TGFBR1 (Rostam et al. 2016) or indirect activation of TGFBR1 resulting from transactivation by a G protein-coupled receptors (GPCR) (Kamato et al. 2016) such it is accompanied by TGFBR1 mediated Smad carboxy terminal phosphorylation. We have recently reported that, in keratinocytes, GPCR activation results in Smad2 linker region phosphorylation without carboxy terminal phosphorylation (Talati et al. 2018). This new data indicates that linker region phosphorylation is a signalling pathway in its own right and thus a re-evaluation of the role of Smad carboxy terminal phosphorylation in cellular signalling is warranted (Derynck and Zhang 2003; Massagué et al. 2005).
Several reports suggest the Nox signalling pathways may be connected with the phosphorylation of Smad2 and Smad3 (Yang et al. 2013; Cucoranu et al. 2005; Choi et al. 2014). In human cardiac fibroblasts, Nox4 is an essential mediator of Smad2/3 transcription factor phosphorylation and activation in response to TGF-β (Cucoranu et al. 2005). In our work we used an antibody for phosphorylated Smad2 linker to observe the role of ROS signalling in human VSMCs. We observed that blocking Nox with inhibitors, DPI and apocynin, did not alter the levels of Smad2 phosphorylation at the carboxy terminal (data not shown), demonstrating as expected that Nox is not involved in a direct inhibition of TGFBR1. Our results showed that DPI and apocynin dose dependently block the phosphorylation of Smad2 in its linker region. These results demonstrate clearly that TGF-β1 stimulated phosphorylation of Smad2 linker region involves ROS. Smad linker region phosphorylation occurs via the upstream activation of serine/threonine kinases. In TGF-β1 treated VSMCs Smad2 linker region phosphorylation was dependent on ERK and p38 but not JNK. Smad2 linker region phosphorylation lead to the synthesis of proteoglycan biglycan (Burch et al. 2010) and an increase in the gene expression of the enzymes involved in the elongation of the GAG chain (Rostam et al. 2016). A previous study on pancreatic carcinoma cells revealed an involvement of Nox in the regulation of TGF-β1 stimulated biglycan expression involving p38 activation. (Groth et al. 2005) reported that Rac-1, a cytosolic subunit of the Nox complex, is an important signalling intermediate in TGF-β1-induced biglycan expression, and shows that DPI, as a pharmacologic inhibitor of Nox, suppressed biglycan expression.
Pharmacological inhibition of Nox enzymes blocked TGF-β1 mediated phosphorylation of ERK and p38. In addition, H2O2 increased the phosphorylation of p38 in VSMCs (Burch et al. 2010) and ERK in renal tubular epithelial cells (Rhyu et al. 2005). In human pancreatic carcinoma cells, DPI suppressed TGF-β induced biglycan expression and p38 phosphorylation (Groth et al. 2005). Similarly, in rat VSMCs angiotensin II mediated phosphorylation of p38 and not ERK is inhibited by Nox inhibitor DPI (Touyz et al. 2003). ROS scavenging in human bronchial epithelial cells inhibited TGF-β mediated phosphorylation of p38, JNK and ERK (Ge et al. 2016). In our in vitro model of atherosclerosis ERK and p38 but not JNK are involved in proteoglycan synthesis and GAG chain elongation (Burch et al. 2010). Taken together our results shown that ROS scavenging effectively suppressed TGF-β1 mediated Smad2 linker region phosphorylation and GAG chain elongation, which demonstrates a role of ROS upstream of MAPK pathway.
Proteoglycan synthesis in VSMCs is of interest, because the binding of atherogenic lipoproteins to modified proteoglycans in the blood vessel wall is an initiating event in the development of atherosclerosis (Little et al. 2002; Ballinger et al. 2004; Grande-Allen et al. 2007; Burch et al. 2011). Recent studies indicate that stimulation of VSMCs with TGF-β1 leads to increase in biglycan expression and GAG synthesizing enzymes expression involving the phosphorylation of Smad2 linker region. GAG synthesizing enzymes (CHST11 and CHSY1) are responsible for sulfation and GAG chain hyperelongation (Afroz et al. 2018; Rostam et al. 2018; Rostam et al. 2016). The mRNA expression of these genes increases in atherosclerotic lesions in vivo (Anggraeni et al. 2011). From the results obtained in this study, Nox inhibitors (DPI and apocynin) inhibited the mRNA levels of these enzymes indicating an involvement of Nox-dependent signalling in regulating the mRNA expression of GAG synthesizing enzymes. Our findings provide a deeper understanding of the complex signalling pathways controlling GAG synthesizing enzymes and which adds further insight into the atherosclerotic process. However; other studies are necessary to elucidate the molecular mechanisms of Nox functions in TGF-β1 induced activation of Smad2 linker region and the mRNA expression of GAG synthesizing enzymes in human VSMCs.
Conclusions
TGF-β1 acting through its receptor leads to the phosphorylation of the transcription factor Smad2 linker region, a response associated with TGF-β1-mediated mRNA expression of GAG synthesizing enzymes CHSY1 and CHST11 that are closely associated with GAG chain elongation in human VSMCs. Our study shows that ROS is a key mediator in this pathway. Pharmacological inhibition of Nox blocked TGF-β1-mediated the levels of Smad2 linker region phosphorylation. These findings provide a better understanding of the signalling pathways controlling the length of GAG on proteoglycans, which facilitate increased lipoprotein binding in the vessel wall and the development of atherosclerosis. The demonstration of the role of ROS in TGF-β1 signalling further extends the pathophysiological roles of TGF-β1 but also provides another potential therapeutic target to prevent ROS-mediated ageing of human biological systems.
Acknowledgements
This work was supported by the Ahvaz Jundishapur University of Medical Sciences (Grant no. HLRC-9708) and by the University of Queensland Early Career Grant (DK) (Grant no. 1832825).
Abbreviations
- CHST11
Chondroitin 4-Ο-sulfotransferase 1
- CHSY1
Chondroitin synthase 1
- DPI
Diphenyleneiodonium
- ERK
Extracellular signal-regulated kinase
- GAG
Glycosaminoglycan
- JNK
C-Jun N-terminal kinase
- MAPKs
Mitogen activated protein kinases.
- Nox
Nicotinamide adenine dinucleotide phosphate oxidase
- ROS
Reactive oxygen species
- TGFBR1
Transforming growth factor-β receptor type 1
- TGF-β1
Transforming growth factor β1
- VSMCs
Vascular smooth muscle cells
Contributor Information
Hossein Babaahmadi-Rezaei, Email: babaahmadi-h@ajums.ac.ir.
Peter J. Little, Phone: +61 7 3346 1701, Email: p.little@uq.edu.au
References
- Afroz R, Cao Y, Rostam MA, Ta H, Xu S, Zheng W, Osman N, Kamato D, Little PJ. Signalling pathways regulating galactosaminoglycan synthesis and structure in vascular smooth muscle: implications for lipoprotein binding and atherosclerosis. Pharmacol Ther. 2018;187:88–97. doi: 10.1016/j.pharmthera.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Anggraeni VY, Emoto N, Yagi K, Mayasari DS, Nakayama K, Izumikawa T, Kitagawa H, Hirata K-I. Correlation of C4ST-1 and ChGn-2 expression with chondroitin sulfate chain elongation in atherosclerosis. Biochem Biophys Res Commun. 2011;406:36–41. doi: 10.1016/j.bbrc.2011.01.096. [DOI] [PubMed] [Google Scholar]
- Ballinger ML, Nigro J, Frontanilla KV, Dart AM, Little PJ. Regulation of glycosaminoglycan structure and atherogenesis. Cell Mol Life Sci. 2004;61:1296–1306. doi: 10.1007/s00018-004-3389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger ML, Osman N, Hashimura K, De Haan JB, Jandeleit-Dahm K, Allen T, Tannock LR, Rutledge JC, Little PJ. Imatinib inhibits vascular smooth muscle proteoglycan synthesis and reduces LDL binding in vitro and aortic lipid deposition in vivo. J Cell Mol Med. 2010;14:1408–1418. doi: 10.1111/j.1582-4934.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry-Lane PA, Patterson C, Van Der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE−/−mice. J Clin Investig. 2001;108:1513–1522. doi: 10.1172/JCI200111927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobik A, Agrotis A, Kanellakis P, Dilley R, Krushinsky A, Smirnov V, Tararak E, Condron M, Kostolias G. Distinct patterns of transforming growth factor-beta isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-beta in fibrofatty lesion development. Circulation. 1999;99:2883–2891. doi: 10.1161/01.CIR.99.22.2883. [DOI] [PubMed] [Google Scholar]
- Burch ML, Yang SN, Ballinger ML, Getachew R, Osman N, Little PJ. TGF-beta stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad2. Cell Mol Life Sci. 2010;67:2077–2090. doi: 10.1007/s00018-010-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch ML, Zheng W, Little PJ. Smad linker region phosphorylation in the regulation of extracellular matrix synthesis. Cell Mol Life Sci. 2011;68:97–107. doi: 10.1007/s00018-010-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Kim DH, Kim SJ, Kim J, Jeong SI, Chung CH, Yu KY, Kim SY. Decursin attenuates hepatic fibrogenesis through interrupting TGF-beta-mediated NAD(P) H oxidase activation and Smad signaling in vivo and in vitro. Life Sci. 2014;108:94–103. doi: 10.1016/j.lfs.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- Dadlani H, Ballinger ML, Osman N, Getachew R, Little PJ. Smad and p38 MAP kinase-mediated signaling of proteoglycan synthesis in vascular smooth muscle. J Biol Chem. 2008;283:7844–7852. doi: 10.1074/jbc.M703125200. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge A, Ma Y, Liu Y-N, Li Y-S, Gu H, Zhang J-X, Wang Q-X, Zeng X-N, Huang M. Diosmetin prevents TGF-β1-induced epithelial-mesenchymal transition via ROS/MAPK signaling pathways. Life Sci. 2016;153(1–8):1–8. doi: 10.1016/j.lfs.2016.04.023. [DOI] [PubMed] [Google Scholar]
- Grande-Allen KJ, Osman N, Ballinger ML, Dadlani H, Marasco S, Little PJ. Glycosaminoglycan synthesis and structure as targets for the prevention of calcific aortic valve disease. Cardiovasc Res. 2007;76:19–28. doi: 10.1016/j.cardiores.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Groth S, Schulze M, Kalthoff H, Fandrich F, Ungefroren H. Adhesion and Rac1-dependent regulation of biglycan gene expression by transforming growth factor-beta. Evidence for oxidative signaling through NADPH oxidase. J Biol Chem. 2005;280:33190–33199. doi: 10.1074/jbc.M504249200. [DOI] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Wu H, Zhang X, Gan H, Sun J, Chen Q, Guo M, Zhang Z. Role of cross-talk between the Smad2 and MAPK pathways in TGF-β1-induced collagen IV expression in mesangial cells. Int J Mol Med. 2010;26:571–576. doi: 10.3892/ijmm_00000501. [DOI] [PubMed] [Google Scholar]
- Jiang F, Liu G-S, Dusting GJ, Chan EC. NADPH oxidase-dependent redox signaling in TGF-β-mediated fibrotic responses. Redox Biol. 2014;2:267–272. doi: 10.1016/j.redox.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamato D, Babaahmadi Rezaei H, Getachew R, Thach L, Guidone D, Osman N, Roufogalis B, Duke CC, Tran VH, Zheng W, Little PJ. (S)-[6]-Gingerol inhibits TGF-beta-stimulated biglycan synthesis but not glycosaminoglycan hyperelongation in human vascular smooth muscle cells. J Pharm Pharmacol. 2013;65:1026–1036. doi: 10.1111/jphp.12060. [DOI] [PubMed] [Google Scholar]
- Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25:2017–2024. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Kamato D, Rostam MA, Piva TJ, Babaahmadi Rezaei H, Getachew R, Thach L, Bernard R, Zheng W, Little PJ, Osman N. Transforming growth factor beta-mediated site-specific Smad linker region phosphorylation in vascular endothelial cells. J Pharm Pharmacol. 2014;66:1722–1733. doi: 10.1111/jphp.12298. [DOI] [PubMed] [Google Scholar]
- Kamato D, Thach L, Getachew R, Burch M, Hollenberg MD, Zheng W, Little PJ, Osman N. Protease activated receptor-1 mediated dual kinase receptor transactivation stimulates the expression of glycosaminoglycan synthesizing genes. Cell Signal. 2016;28:110–119. doi: 10.1016/j.cellsig.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Kinkade K, Streeter J, Miller FJ. Inhibition of NADPH oxidase by apocynin attenuates progression of atherosclerosis. Int J Mol Sci. 2013;14:17017–17028. doi: 10.3390/ijms140817017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassègue B, San Martín A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zeng B, Chai Y, Cai P, Fan C, Cheng T. The linker region of Smad2 mediates TGF-β-dependent ERK2-induced collagen synthesis. Biochem Biophys Res Commun. 2009;386:289–293. doi: 10.1016/j.bbrc.2009.05.084. [DOI] [PubMed] [Google Scholar]
- Little PJ, Tannock L, Olin KL, Chait A, Wight TN. Proteoglycans synthesized by arterial smooth muscle cells in the presence of transforming growth factor-beta1 exhibit increased binding to LDLs. Arterioscler Thromb Vasc Biol. 2002;22:55–60. doi: 10.1161/hq0102.101100. [DOI] [PubMed] [Google Scholar]
- Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:2993–2997. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Miller AA, De Silva TM, Judkins CP, Diep H, Drummond GR, Sobey CG. Augmented superoxide production by Nox2-containing NADPH oxidase causes cerebral artery dysfunction during hypercholesterolemia. Stroke. 2010;41:784–789. doi: 10.1161/STROKEAHA.109.575365. [DOI] [PubMed] [Google Scholar]
- Rezaei HB, Kamato D, Ansari G, Osman N, Little PJ. Cell biology of Smad2/3 linker region phosphorylation in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2012;39:661–667. doi: 10.1111/j.1440-1681.2011.05592.x. [DOI] [PubMed] [Google Scholar]
- Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh S-T, Lee HB. Role of reactive oxygen species in TGF-β1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- Rostam MA, Kamato D, Piva TJ, Zheng W, Little PJ, Osman N. The role of specific Smad linker region phosphorylation in TGF-beta mediated expression of glycosaminoglycan synthesizing enzymes in vascular smooth muscle. Cell Signal. 2016;28:956–966. doi: 10.1016/j.cellsig.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Rostam MA, Shajimoon A, Kamato D, Mitra P, Piva T, Getachew R, Cao Y, Zheng W, Osman N, Little PJ. Flavopiridol inhibits TGF-beta-stimulated biglycan synthesis by blocking linker region phosphorylation and nuclear translocation of Smad2. J Pharmacol Exp Ther. 2018;365:156–164. doi: 10.1124/jpet.117.244483. [DOI] [PubMed] [Google Scholar]
- Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis. 2011;216:321–326. doi: 10.1016/j.atherosclerosis.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert JE, Sugumaran G. Biosynthesis of chondroitin/dermatan sulfate. IUBMB Life. 2002;54:177–186. doi: 10.1080/15216540214923. [DOI] [PubMed] [Google Scholar]
- Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchelnytskyi S, Tamaki K, Engström U, Wernstedt C, Ten Dijke P, Heldin C-H. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-β signaling. J Biol Chem. 1997;272:28107–28115. doi: 10.1074/jbc.272.44.28107. [DOI] [PubMed] [Google Scholar]
- Talati N, Kamato D, Piva TJ, Little PJ, Osman N. Thrombin promotes PAI-1 expression and migration in keratinocytes via ERK dependent Smad linker region phosphorylation. Cell Signal. 2018;47:37–43. doi: 10.1016/j.cellsig.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Cruzado M, Tabet F, Yao GY, Salomon S, Schiffrin EL. Redox-dependent MAP kinase signaling by Ang II in vascular smooth muscle cells: role of receptor tyrosine kinase transactivation. Can J Physiol Pharmacol. 2003;81:159–167. doi: 10.1139/y02-164. [DOI] [PubMed] [Google Scholar]
- Vejrazka M, Micek R, Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta. 2005;1722:143–147. doi: 10.1016/j.bbagen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Wrighton KH, Lin X, Feng X-H. Phospho-control of TGF-β superfamily signaling. Cell Res. 2009;19:8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Burch ML, Tannock LR, Evanko S, Osman N, Little PJ. Transforming growth factor-beta regulation of proteoglycan synthesis in vascular smooth muscle: contribution to lipid binding and accelerated atherosclerosis in diabetes. J Diabetes. 2010;2:233–242. doi: 10.1111/j.1753-0407.2010.00089.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang Z, Yang H, Wang L, Gillespie SR, Wolosin JM, Bernstein AM, Reinach PS. TRPV1 potentiates TGFβ-induction of corneal myofibroblast development through an oxidative stress-mediated p38-SMAD2 signaling loop. PLoS One. 2013;8:e77300. doi: 10.1371/journal.pone.0077300. [DOI] [PMC free article] [PubMed] [Google Scholar]


