Abstract
Nab-paclitaxel (nab-PTX) is a nanoparticle albumin-bound paclitaxel and, as such, is free of solvents like ethanol and polyoxyethylene castor oil. The absence of solvents from this formulation has several practical advantages: it has a shorter infusion time, it negates the need for premedications for hypersensitivity reactions, and it can be administered to patients with alcoholic hypersensitivity. It is thought that nab-paclitaxel will be in widespread use in the near future because of its convenience and efficacy. Here, we report the case of a breast cancer patient who developed hemorrhagic cystitis potentially due to treatment with nab-paclitaxel. The patient was 69-year old lady with stage IIB left breast cancer. She was due to undergo neoadjuvant chemotherapy and started weekly treatment with nab-paclitaxel. On the second day of the first cycle of treatment, she experienced symptoms of cystitis, but was not hemorrhagic and the symptoms were managed with antibiotics. After the third cycle, the symptoms of cystitis became severe, and she was diagnosed with hemorrhagic cystitis and discontinued chemotherapy with nab-paclitaxel. This is the first case report of hemorrhagic cystitis associated with nab-paclitaxel.
Keywords: Locally advanced breast cancer, Nab-paclitaxel, Hemorrhagic cystitis
Introduction
Paclitaxel is one of the most effective cytotoxic agents used to treat breast cancer. Nab-paclitaxel (nab-PTX) is a nanoparticle albumin-bound paclitaxel and, as such, is free of solvents like ethanol and polyoxyethylene castor oil [1]. The absence of solvents from this formulation has several practical advantages: it has a shorter infusion time, it negates the need for premedications for hypersensitivity reactions, and it can be administered to patients with alcoholic hypersensitivity [1, 2]. This drug was first approved by the U.S. Food and Drug Administration in 2005, the European Medicines Agency in 2008, and the Japanese Pharmaceuticals and Medical Devices Agency in 2010 for breast cancer cases. More recently, positive results were published from a phase III trial in first-line non-small-cell lung cancer (NSCLC) [3] and advanced pancreatic cancer as a less toxic alternative to FOLFIRINOX [4]. Preliminary data also suggest possible applications of nab-paclitaxel as in combination therapies not only for breast cancer, but also for other types of cancer [5].
In a randomized phase III study in metastatic breast cancer, 3-week cycles of nab-paclitaxel was found to have improved efficacy and safety profile compared with conventional 3-week cycles of solvent-based paclitaxel [6]. However, in one phase III randomized study in advanced breast cancer, nab-paclitaxel given once per week was not superior compared with weekly solvent-based paclitaxel treatment [7]. In the other randomized phase III study of neoadjuvant chemotherapy for early breast cancer patients, substituting solvent-based paclitaxel with nab-paclitaxel on a weekly basis significantly increased the proportion of patients achieving a pathological complete response rate after anthracycline-based chemotherapy, most significantly in triple negative breast cancer patients [8]. Thus, the ideal target, dosing, treatment setting and schedule of nab-paclitaxel for breast cancer patients remain controversial, and further clinical trials are now ongoing and thought that nab-paclitaxel will be in widespread use, in the near future because of its convenience and efficacy. The most common adverse events reported in clinical trials were similar to those of conventional paclitaxel, such as alopecia, rash, sensory neuropathy, neutropenia, fatigue, and arthralgia [3, 9]. Here, we report one case of a very rare adverse event of hemorrhagic cystitis related to nab-paclitaxel use. We also discuss the incidence of hemorrhagic cystitis caused by taxane treatment.
Case report
A 69-year-old healthy woman with a left breast lump, that she had noticed 1 month earlier, was referred to our hospital from her local doctor. The left breast mass was 30 mm in diameter with positive dimpling sign in the upper outer quadrant by physical examination. The diagnosis of breast cancer was obtained from a core needle biopsy. Further imaging study with mammography, ultrasonography, and magnetic resonance imaging (MRI) revealed a mass with indistinct margin measuring 35 mm (Fig. 1a). A computed tomography scan revealed no distant metastasis, although several lymph nodes in the left axilla were clearly swelling indicating metastasis. Serum tumor markers of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA15-3) were within normal limits. The pretreatment detailed diagnoses indicated the tumor as invasive ductal carcinoma, and the cancer was shown by immunohistochemical study to be positive for estrogen receptor (ER) and progesterone receptor (PR) expression, and negative for human epidermal growth factor receptor type 2 (HER2), and was classified as clinically T2N1M0 stage IIB. The patient was alcohol intolerant, and had a past history of cystitis, but the cystitis was inactive at this time. She had a family history of breast cancer—her elder sister having developed breast cancer in her 40 s.
Fig. 1.
MRI (MIP) a and ultrasonography b revealed a mass, measuring 35 mm in the upper and outside of the left breast before treatment. Ultrasonography demonstrated the shrinkage of the left breast mass after one cycle of nab-paclitaxel treatment (c)
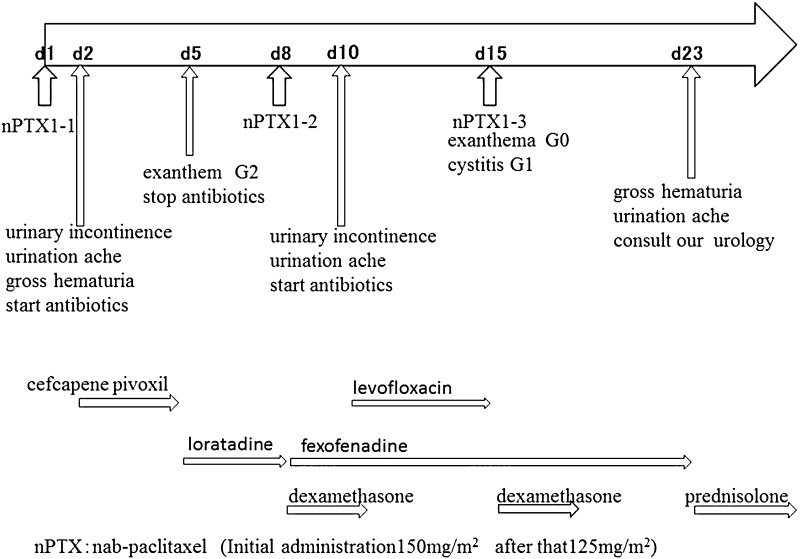
The treatment of this patient was planned to undergo neoadjuvant chemotherapy with taxane for 4 cycles followed by fluorouracil, epirubicin, and cyclophosphamide (FEC) for 4 cycles. Nab-paclitaxel was chosen as a taxane treatment instead of conventional paclitaxel or docetaxel, because she was alcohol intolerant, and nab-paclitaxel was to be administered at a dose of 150 mg/m2 intravenously on days 1, 8, and 15, and every 28 days for 4 cycles. The first cycle of nab-paclitaxel was administered at a dose of 150 mg/m2 with 1 mg of granisetron hydrochloride, but without any antiallergic premedication. The patient experienced urinary incontinence, urination ache and gross hematuria on the second day of the first cycle of nab-paclitaxel administration, and she visited local urologist (Fig. 2). She was diagnosed with cystitis and started to take cefcapene pivoxil orally at a dose of 300 mg per day. On day 5, the patient visited us exhibiting exanthem on the trunk, upper body, and neck. At presentation, the cystitis was not evident, and the grade 2 exanthema, according to NCI–CTC criteria, was thought to have been a side effect of the cefcapene pivoxil or nab-paclitaxel, so the antibiotics were stopped and oral loratadine at 10 mg/day was administered for 4 days. On day 8, exanthema had improved to grade 1, and there was no sign of cystitis. Nab-paclitaxel was administered at reduced dose of 125 mg/m2 with antiallergic medication of oral dexamethasone (2 mg/day) for 3 days, and oral fexofenadine 60 mg/day for 7 days. On day 10, the urinary incontinence and urination ache returned, and the patient visited her local urologist again. She was again diagnosed with cystitis and prescribed oral levofloxacin at 500 mg/day. On day 15, with exanthema at grade 0, and cystitis at grade 1, nab-paclitaxel was administered at a dose of 125 mg/m2 together with dexamethasone at 2 mg/day for 3 days, and oral fexofenadine at 60 mg/day for 7 days. On day 23, the patient visited her local urologist, having noticed macroscopic hematuria and experiencing continuous urination ache. The local urologist referred the patient to the urologist at our hospital. Urine analysis detected occult bleeding 3+, protein 3+, and leukocyte−. The patient underwent a cystoscopy, which revealed edema of the mucous membrane, causing inflammation and bleeding (Fig. 3). Bladder capacity was reduced to approximately 100 ml, but neither blood clots nor bladder tumors were detected, and urine cytodiagnosis revealed no malignant cells. The patient was diagnosed with drug-induced hemorrhagic cystitis caused by nab-paclitaxel. Bladder washing and oral prednisolone treatment (7.5 mg/day) were provided (Fig. 2). The hemorrhagic cystitis was relieved after 2 weeks, and tumor shrinkage was observed by ultrasonography (Fig. 1b). The continuation of the neoadjuvant chemotherapy was considered to be inappropriate, and the choice of immediate surgery or neoadjuvant hormonal treatment was proposed. The patient chose further preoperative treatment with aromatase inhibitor. After 6 months of hormonal treatment, she underwent right mastectomy and axillary lymph node dissection. The pathological findings revealed minimal invasive residual disease of 4 × 2 mm, and surrounding intraductal component of 28 × 18 × 5 mm. There was no lymph node metastasis (0/26). The cancer cells were positive for ER and PR and negative for HER2 by IHC, and classified as ypT1aN0M0, stage I. The patient underwent adjuvant radiotherapy of 50 Gy in 25 fractions to the right conserved breast, then boost of 10 Gy in 5 fractions to the tumor bed, and was given an aromatase inhibitor.
Fig. 2.
Clinical course of the chemotherapy, urological symptom and concomitant treatment
Fig. 3.
Cystoscopy revealed edema, inflammation and bleeding of the mucous membrane of the bladder, and it led up to the diagnosis of hemorrhagic cystitis
Discussion
Nab-paclitaxel is an anticancer cytotoxic agent approved for the treatment of breast cancer. Paclitaxel, a member of the taxane family of microtubule-stabilizing agents and the first licensed formation of paclitaxel (CrEL-paclitaxel), has been used for over 20 years, but its hydrophobic nature results in poor solubility. The CrEL formulation can cause hypersensitivity reactions, and to avoid this adverse effect, additional use of steroids and antihistamines is recommended [10]. Nab-paclitaxel improves the pharmacokinetic properties of paclitaxel, which is thought to increase its antitumor effects [11]. In fact, clinical trials have shown that the efficacy of nab-paclitaxel is significantly better than that of CrEL-paclitaxel with almost every safety profile [6].
Hemorrhagic cystitis is a serious complication that can occur in patients undergoing treatment with cytotoxic drugs for cancer. It is known that the risk of developing acute hemorrhagic cystitis is related to the duration of the contact time with cytotoxic drugs, and their concentration or dose [12]. It is also known that long-term oral administration of a lower dose of cytotoxic drugs may induce late-onset hemorrhagic cystitis [13]. Besides hematuria, the common symptoms of hemorrhagic cystitis are the same as nonhemorrhagic cystitis, such as frequent urination, difficulty in urination, compelling need to urinate, and pain on urination. However, it is not uncommon to suddenly develop asymptomatic gross hematuria without such prior symptoms. The removal of blood clots in the bladder, continuous bladder irrigation with normal saline, and adequate hydration, are the first choice for treatment. Hyperbaric oxygen therapy is reported to be effective with radiation-induced hemorrhagic cystitis, and is also expected to be of benefit in the treatment of drug-induced hemorrhagic cystitis, but this is yet to be reported and needs further research [14]. Although it may not be considered as a standard treatment, there is a report that steroid therapy is effective in the treatment of radiation-induced hemorrhagic cystitis [15].
Cyclophosphamide, ifosfamide, and busulfan are well known to be causative drugs of hemorrhagic cystitis [16], but reports on taxane-related hemorrhagic cystitis are very rare. In the Japanese postmarketing surveillance report of nab-paclitaxel for breast cancer patients (n = 934), only one case of grade 1 urinary incontinence and 2 cases of grade 1 hematuria were reported, but there have been no cases reported of hemorrhagic cystitis [17]. Nippon Kayaku reports the adverse events by `Paclitaxel ⌈NK⌋’, generic drug of solvent-based paclitaxel. In the report, among 2186 adverse events in 1688 patients, 6 cases of hemorrhagic cystitis including 1 case as severe adverse event were reported [18]. In regard to other taxane, a 73-year-old man with bone metastasis from prostate cancer was reported to have developed hemorrhagic cystitis after the third cycle of docetaxel treatment. The patient ceased docetaxel treatment, undertook bladder irrigation, and recovered after 2 weeks [19]. Four cases of hemorrhagic cystitis during cabazitaxel for metastatic castration-resistant prostate cancer treatment have been reported. Those 4 cases were previously treated with pelvic radiation therapy, and it was speculated that those were due to a radiation recall syndrome induced by cabazitaxel [20]. We believe that the present case is the first report of hemorrhagic cystitis caused by nab-paclitaxel.
The metabolism of nab-paclitaxel does not provide an explanation for the occurrence of hemorrhagic cystitis. In a pharmacokinetic research, a much higher fraction of unbound paclitaxel in blood was demonstrated for nab-paclitaxel versus solvent-based paclitaxel (6.3 vs. 2.4 %, p < 0.001) [21]. Hepatic metabolism (CYP2C8 forming major metabolite and minor metabolites via CYP3A4) accounts for majority of elimination of nab-paclitaxel, while renal excretion of unchanged nab-paclitaxel accounts for about 4 % [22]. There was no obvious hepatic dysfunction for this patient, but a past history of usual cystitis could have been a possible cause for this adverse event.
Conclusion
We report herein a case of hemorrhagic cystitis that occurred during nab-paclitaxel treatment of a breast cancer patient. Physicians should be aware that hemorrhagic cystitis is a potential adverse event related to taxane treatment.
Acknowledgments
We thank Brian K Purdue for his native speaker revision. We are particularly grateful for the insightful and constructive comments given by Dr. Nishiyama Hiroyuki from University of Tsukuba.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–1044. [PubMed] [Google Scholar]
- 2.Ibrahim NK, Samuels B, Page R, Doval D, Patel KM, Rao SC, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23(25):6019–6026. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montana M, Ducros C, Verhaeghe P, Terme T, Vanelle P, Rathelot P. Albumin-bound paclitaxel: the benefit of this new formulation in the treatment of various cancers. J Chemother. 2011;23(2):59–66. doi: 10.1179/joc.2011.23.2.59. [DOI] [PubMed] [Google Scholar]
- 6.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 7.Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Leung E, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance) J Clin Oncol. 2015;33(21):2361–2369. doi: 10.1200/JCO.2014.59.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17(3):345–356. doi: 10.1016/S1470-2045(15)00542-2. [DOI] [PubMed] [Google Scholar]
- 9.Yamada K, Yamamoto N, Yamada Y, Mukohara T, Minami H, Tamura T. Phase I and pharmacokinetic study of ABI-007, albumin-bound paclitaxel, administered every 3 weeks in Japanese patients with solid tumors. Jpn J Clin Oncol. 2010;40(5):404–411. doi: 10.1093/jjco/hyp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finley RS, Rowinsky EK. Patient care issues: the management of paclitaxel-related toxicities. Ann Pharmacother. 1994;28(5 Suppl):S27–S30. doi: 10.1177/10600280940280S507. [DOI] [PubMed] [Google Scholar]
- 11.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 12.de Vries CR, Freiha FS. Hemorrhagic cystitis: a review. J Urol. 1990;143(1):1–9. doi: 10.1111/j.1365-2826.1990.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 13.Stillwell TJ, Benson RC, Jr, Burgert EO., Jr Cyclophosphamide-induced hemorrhagic cystitis in Ewing’s sarcoma. J Clin Oncol. 1988;6(1):76–82. doi: 10.1200/JCO.1988.6.1.76. [DOI] [PubMed] [Google Scholar]
- 14.Payne H, Adamson A, Bahl A, Borwell J, Dodds D, Heath C, et al. Chemical- and radiation-induced haemorrhagic cystitis: current treatments and challenges. BJU Int. 2013;112(7):885–897. doi: 10.1111/bju.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanagi M, Nishimura T, Kurita S, Lee C, Kondo Y, Yamazaki K. A case of prednisolone therapy for radiation-induced hemorrhagic cystitis. Nihon Hinyokika Gakkai Zasshi. 2011;102(3):600–602. doi: 10.5980/jpnjurol.102.600. [DOI] [PubMed] [Google Scholar]
- 16.Traxer O, Desgrandchamps F, Sebe P, Haab F, Le Duc A, Gattegno B, et al. Hemorrhagic cystitis: etiology and treatment. Prog Urol. 2001;11(4):591–601. [PubMed] [Google Scholar]
- 17.Report of use-results survey for nab-pacitaxel conducted as all-case surveillance in Japan (2013). http://www.taiho.co.jp/index.html
- 18.MINK Web. Medical information by Nippon Kayaku. https://mink.nipponkayaku.co.jp/index2.html. 28 June 2016
- 19.Ntekim AI, Ajekigbe A. Hemorrhagic cystitis in a patient receiving docetaxel for prostate cancer. Clin Med Insights Oncol. 2010;4:11–13. doi: 10.4137/CMO.S4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grellety T, Houédé N, Hoepffner JL, Rivière J, Mérino C, Lieutenant V, et al. Hemorrhagic cystitis in patients treated with cabazitaxel: a radiation recall syndrome? Ann Oncol. 2014;25(6):1248–1249. doi: 10.1093/annonc/mdu132. [DOI] [PubMed] [Google Scholar]
- 21.Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14(13):4200–4205. doi: 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release. 2013;170(3):365–372. doi: 10.1016/j.jconrel.2013.05.041. [DOI] [PubMed] [Google Scholar]