Abstract
We report the case of a 59-year-old man with thymic adenocarcinoma who was treated with colon cancer chemotherapy. He was referred to our hospital for an anterior mediastinal mass and multiple bone metastases that were found by computed tomography. Needle biopsy of the mediastinal tumor revealed a caudal-type homeobox 2 (CDX2)-positive adenocarcinoma. Neither upper nor lower gastrointestinal endoscopic examinations revealed any evidence of a primary tumor. The patient was administered CapeOX (capecitabine and oxaliplatin) and FOLFIRI (fluorouracil, leucovorin and irinotecan)/cetuximab. He died 6 months after diagnosis. Primary thymic adenocarcinoma was confirmed by autopsy. As far as we know, this is the first report in which colon cancer chemotherapy was used to treat CDX2-positive metastatic thymic adenocarcinoma.
Keywords: CDX2, Chemotherapy, Thymic adenocarcinoma
Introduction
Primary thymic adenocarcinoma is a rare tumor with no established treatment regimen. Recently, Moser et al. [1] reported a subtype of thymic adenocarcinoma with CDX2-positive enteric features. CDX2, a homeodomain transcription factor required for the development and maintenance of the intestinal epithelium, is overexpressed by human colon adenocarcinoma [2]. A previous study showed that in patients with carcinoma of unknown primary (CUP), CDX2-positive adenocarcinoma might benefit from chemotherapy normally administered for colon cancer [3]. Thus, colon cancer chemotherapy may also benefit patients with CDX2-positive primary thymic adenocarcinoma. In this study we report the use of a colon cancer regimen to treat a case of metastatic thymic adenocarcinoma expressing CDX2, and we review the relevant literature.
Case report
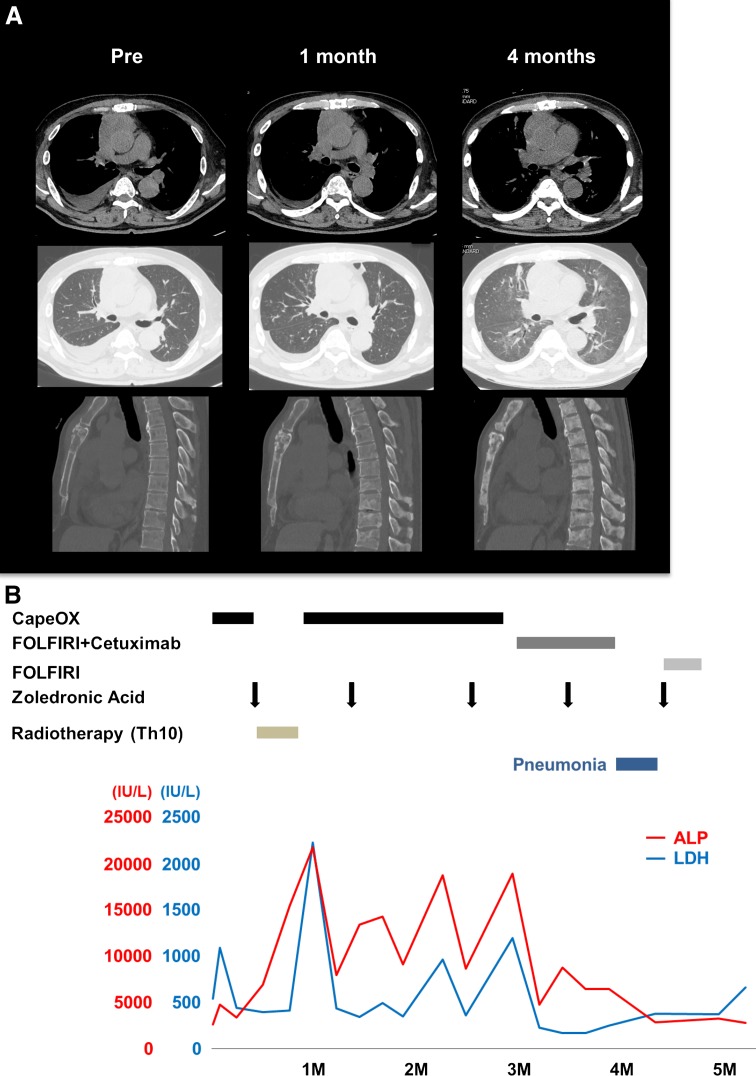
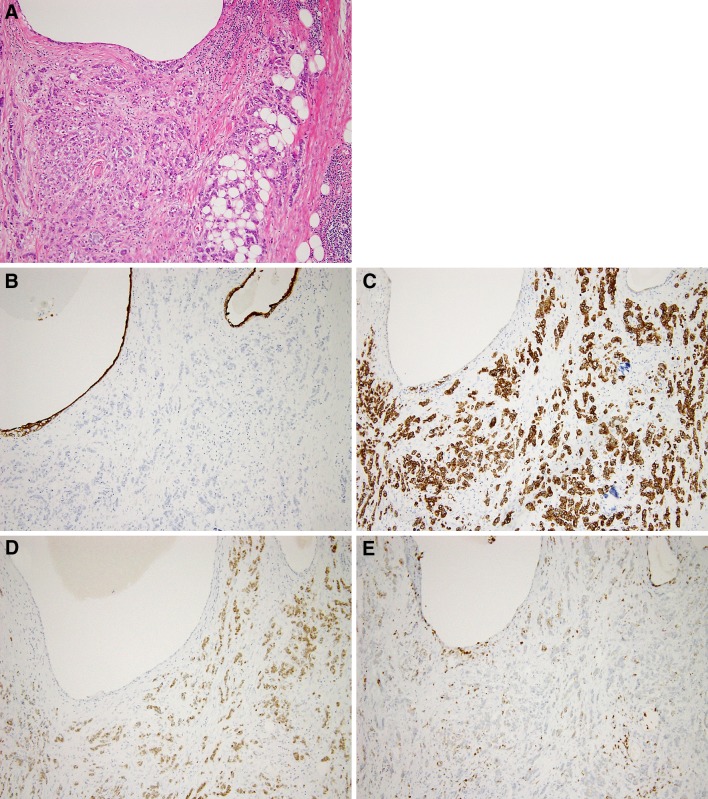
A 59-year-old man with anterior chest swelling and pain was referred to our hospital. He was 161 cm tall and weighed 64 kg. He had an ECOG performance status of 0, body temperature of 36.6 °C, blood pressure of 145/106 mmHg, pulse rate of 116 beats per minute and SpO2 of 98 % (room air). Oral morphine was started for the management of chest and back pain. Physical examination revealed anterior chest swelling and a parietal mass. The patient had no significant previous medical history. An anterior mediastinal mass (76 × 63 × 31 mm), cardiac effusion and multiple bone metastases were found by computed tomography (Fig. 1a). Needle biopsy of the mediastinal tumor revealed adenocarcinoma. Immunohistochemically, the tumor was CK7 negative, CK20 positive, CDX2 positive, CD5 positive, thyroid transcription factor 1 (TTF-1) negative, and carcinoembryonic antigen (CEA) positive. No evidence of a primary tumor was found by upper or lower gastrointestinal endoscopic examination. The patient’s white blood cell count was 19,300 cells/μL, hemoglobin level was 12.4 g/dL, hematocrit level was 37.8 % and platelet count was 29.8 × 104/μL. His C-reactive protein level was 17.31 mg/dL. His blood cultures were negative. The serum CEA level was 18.6 ng/mL (normal: ≤6.0 ng/mL). AST, ALT, LDH, ALP and γ-GTP levels were also elevated. In particular, the ALP level was very high, at 2669 IU/L (normal: 106–322 IU/L). We suspected that he had thymic adenocarcinoma. However, primary thymic adenocarcinoma, especially with CDX2-positive enteric features, is a rare tumor. The treatment regimen for metastatic thymic adenocarcinoma has not yet been established. A previous report suggested that adenocarcinoma with a CK7-negative, CK20-positive and CDX2-positive colon cancer profile could benefit from a colon cancer chemotherapy regimen [4]. We therefore treated the patient as if he had CUP with a colon cancer profile. CapeOX (capecitabine 1000 mg/m2 bid from day 1 to day 14 and oxaliplatin 130 mg/m2 on day 1, every 3 weeks) was used as first-line chemotherapy. Radiotherapy of vertebral level Th10 (30 Gy/10 fr) was added during cycles 1 and 2 of CapeOX to control back pain caused by bone metastases. Zoledronic acid was also used to prevent skeletal events. Although there was a transient decrease in the patient’s right pleural effusion (Fig. 1a) and serum biochemical parameters (Fig. 1b), these worsened again before the next cycle of CapeOX. Cancer pain progressed after cycle 4 of CapeOX. ALP, LDH and CEA levels were elevated to 18,937 IU/L, 1,197 IU/L and 47.4 ng/mL, respectively. We judged that the patient had developed progressive disease. Because the tumor was KRAS wild-type, cetuximab (250 mg/m2 after a loading dose of 400 mg/m2, weekly) combined with FOLFIRI (irinotecan 150 mg/m2 on day 1, l-leucovorin 200 mg/m2, fluorouracil 400 mg/m2 bolus and fluorouracil 2,400 mg/m2 46-h infusion, every 2 weeks) was chosen as the second-line therapy. After 1 cycle of FOLFIRI/cetuximab, LDH levels decreased to normal range and ALP levels decreased to 4,817 IU/L. After 2 cycles of FOLFIRI/cetuximab, the patient developed interstitial pneumonia with β-d glucan elevation. We suspected Pneumocystis jiroveci pneumonia and started sulfamethoxazole-trimethoprim and steroids. Although PCR did not detect Pneumocystis jiroveci, the patient’s pneumonia improved. We resumed FOLFIRI without cetuximab, however, after 1 cycle his performance status declined and the chemotherapy was discontinued. He died 6 months after diagnosis. Autopsy revealed that he had primary thymic adenocarcinoma (Figs. 2a, 3a), with lung, liver (Fig. 2b), bone (Fig. 2c), right adrenal gland and bladder metastases. Immunohistochemistry images from the anterior tumor are shown in Fig. 3. The tumor was CK7 negative, CK20 positive, CDX2 positive and CD5 positive (Fig. 3b–e).
Fig. 1.
Time-dependent changes. a Computed tomography of the chest at pretreatment, 1 month after initial treatment (after 1 cycle of CapeOX) and 4 months after initial treatment (after 2 cycles of FOLFIRI/cetuximab) using a plain mediastinal window (top), plain pulmonary window (middle) and bone window (bottom). b Serum biochemical parameters
Fig. 2.
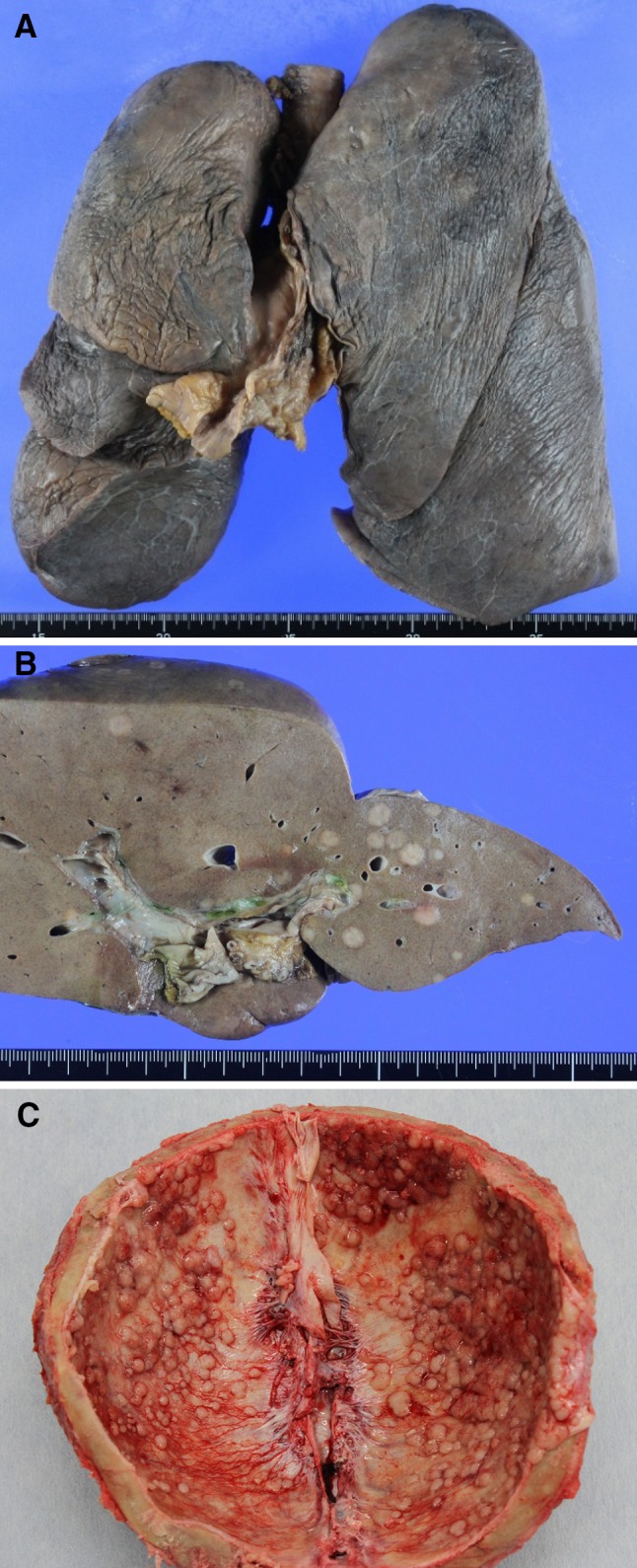
Macroscopic autopsy findings: thymus (a), liver (b) and skeletal bone (c)
Fig. 3.
H&E staining (a, ×100) and immunohistochemical examination (b–e, ×100) of the anterior mediastinal tumor demonstrated positive results for CK7 (b clone OV-TL 12/30, DAKO), CK20 (c clone Ks20.8, DAKO), CDX2 (d clone DAK-CDX2, DAKO) and CD5 (e clone 4C7, Novocastra)
Discussion
CDX2 is overexpressed by gastrointestinal carcinoma [5]. Varadhachary et al. [4] reported that CUP with a CK7-negative, CK20-positive and CDX2-positive colon cancer profile could benefit from a colon cancer chemotherapy regimen. In another report they suggested that chemotherapy for colon cancer might be effective for CDX2-positive CUP [3]. Combination therapies, such as platinum/taxane and platinum/gemcitabine, are often used for CUP, but these have not been useful for colon cancer. If the CDX2 expression of a carcinoma correlates with its sensitivity to colon cancer regimens such as FOLFOX, CapeOX and FOLFIRI, these therapies may also benefit patients with CDX2-positive primary thymic adenocarcinoma.
There have been 46 reported cases of thymic adenocarcinoma [1, 6] in addition to our own. Of the 47 cases, 12 (25.5 %) were primary thymic adenocarcinoma with a CDX2-positive phenotype. We reviewed 11 cases whose characteristics and clinical courses were reported (Table 1) [1, 6–11]. The median age was 41 years and the male-to-female ratio was 6:5. The most common morphologic subtype was mucinous adenocarcinoma. All were TTF-1 negative and 10 were CD5 positive. The CK7 positive:negative ratio was 7:4. There was no correlation between CK7 and CDX2 expression in primary thymic adenocarcinoma. CEA was assessed by immunohistochemistry in 7 cases, and all were positive.
Table 1.
Ten reported cases of CDX2-positive thymic adenocarcinoma
| Ref. no. | Age/sex | Histological type | Immunohistochemistry | Serum CEA | |||||
|---|---|---|---|---|---|---|---|---|---|
| CK7 | CK20 | CDX2 | TTF1 | CD5 | CEA | ||||
| 1 | 41/M | NOS | − | + | + | − | − | + | N/A |
| 39/F | Mucinous | + | + | + | − | + | + | N/A | |
| 6 | 59/F | Tubular | Focal + | + | + | − | + | N/A | + |
| 7 | 41/M | Mucinous | Focal + | + | + | − | Focal + | + | N/A |
| 8 | 52/F | Mucinous | − | + | + | − | + | + | − |
| 38/M | Mucinous | + | + | + | − | Focal + | + | + | |
| 55/M | Mucinous | − | + | + | − | Focal + | + | + | |
| 9 | 36/F | Mucinous | + | + | Focal + | − | + | N/A | N/A |
| 10 | 55/M | Papillotubular | + | + | Focal + | − | + | N/A | − |
| 11 | 28/F | Mucinous | Focal + | + | + | − | + | N/A | − |
| Current case | 59/M | NOS | − | + | + | − | + | + | + |
| Ref. no. | Age/sex | Histological type | Primary therapy | Therapy for metastasis or recurrence | |||||
|---|---|---|---|---|---|---|---|---|---|
| Primary surgery | Adjuvant CTx | Adjuvant RT | Outcome | Lesion | Treatment | outcome | |||
| 1 | 41/M | NOS | Yes | None | None | Alive without disease after 18 months | |||
| 39/F | Mucinous | Yes | None | None | 2 recurrences | Mediastinal | Surgery | Alive 159 months after diagnosis | |
| 6 | 59/F | Tubular | Yes | Yes (Palliative chemoradiotherapy and radiotherapy, no detail) | Lung and bone (at diagnosis) | No detail | Alive with aggravated bone and lung metastases after 11 months of follow-up | ||
| 7 | 41/M | Mucinous | Yes | Yes (No detail) | Yes | 2 recurrences | No detail | Surgery | Alive without disease 1 year after 2nd surgery |
| 8 | 52/F | Mucinous | Yes | Yes (CDDP + ETP) | Yes | Recurrence at 7 months | Lung and cervical lymph nodes | CBDCA + PTX | Alive 4 months after recurrence |
| 38/M | Mucinous | Yes | Yes (CBDCA + DTX) | Yes | Recurrence | Bone, malignant pleural and pericardial effusions | No detail | Died 12 months after initial diagnosis | |
| 55/M | Mucinous | Yes | Yes (CBDCA + DTX) | Yes | Recurrence | Lung, sacrum, liver, right adrenal gland and mediastinal lymph nodes | Weekly PTX, CPT-11 | Died 24 months after initial diagnosis | |
| 9 | 36/F | Mucinous | Yes | Yes (CDDP + PTX) | Yes | Recurrence at 1 year | Lung with malignant pleural effusion | No detail | Died 3 months after recurrence |
| 10 | 55/M | Papillotubular | Yes | None | Yes | Alive in 14 months after surgery | |||
| 11 | 28/F | Mucinous | Yes | None | None | 2 recurrences over 2 years’ follow-up | No detail | GEMOX and RT | Alive without disease 6 months after treatment |
| Current case | 59/M | NOS | None | Bone (at diagnosis) | CapeOX, FOLFIRI + cetuximab | Died 6 months after diagnosis | |||
CBDCA carboplatin, CDDP cisplatin, CTx chemotherapy, DTX docetaxel, ETP etoposide, GEMOX gemcitabine and oxaliplatin, N/A not assessed, NOS not otherwise specified, PTX paclitaxel, RT radiotherapy
Nine cases with a CDX2-positive thymic adenocarcinoma had limited disease and received surgical resection as primary therapy (Table 1). Of the 9 resected cases, 5 received adjuvant chemotherapy and radiotherapy, 1 received adjuvant radiotherapy only, and 3 received no adjuvant therapy. Adjuvant chemotherapy regimens were cisplatin/etoposide, carboplatin/docetaxel and cisplatin/paclitaxel. There was recurrence in 7 cases: 4/5 (80 %) who were administered adjuvant chemotherapy and radiotherapy, 1/1 (100 %) who received adjuvant radiotherapy, and 2/3 (66.7 %) who underwent surgery only. These data suggest that adjuvant chemotherapy and/or radiotherapy were not sufficiently beneficial. Three patients received local therapy for recurrence: 2 underwent surgery alone, and 1 received chemotherapy (GEMOX) and radiotherapy. Four recurrence cases had multiple lesions. Of these, 2 underwent chemotherapy, 1 received paclitaxel/carboplatin, which was ineffective, and 1 received weekly paclitaxel and irinotecan sequentially, which had no effect and little effect, respectively. In our case, CapeOX resulted in transient disease control, and FOLFIRI/cetuximab improved LDH and ALP levels. If our patient had not developed interstitial pneumonia, his disease control period might have been longer. These findings suggested that regimens containing oxaliplatin or irinotecan might be an optimal choice for CDX2-positive thymic adenocarcinoma.
In summary, no previous reports have described the use of a colon cancer regimen for CDX2-positive primary thymic adenocarcinoma. Complete resection is the best therapy for primary and limited metastatic lesions, even in cases of recurrence. Adjuvant chemotherapy and/or radiotherapy is not recommended. A colon cancer regimen might be effective for CDX2-positive thymic adenocarcinoma. Further reports are awaited.
Conflict of interest
Mizuno received a research funding from Chugai Pharmaceutical Co., Ltd. Others have no conflict of interest to declare.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Because the patient died of thymic adenocarcinoma, informed consent was obtained from his family.
References
- 1.Moser B, Schiefer AI, Janik S, Marx A, Prosch H, Pohl W, Neudert B, Scharrer A, Klepetko W, Mullauer L. Adenocarcinoma of the thymus, enteric type: report of 2 cases, and proposal for a novel subtype of thymic carcinoma. Am J Surg Pathol. 2014 doi: 10.1097/PAS.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 2.Witek ME, Nielsen K, Walters R, Hyslop T, Palazzo J, Schulz S, Waldman SA. The putative tumor suppressor Cdx2 is overexpressed by human colorectal adenocarcinomas. Clin Cancer Res. 2005;11(24 Pt 1):8549–8556. doi: 10.1158/1078-0432.CCR-05-1624. [DOI] [PubMed] [Google Scholar]
- 3.Varadhachary GR, Karanth S, Qiao W, Carlson HR, Raber MN, Hainsworth JD, Greco FA. Carcinoma of unknown primary with gastrointestinal profile: immunohistochemistry and survival data for this favorable subset. Int J Clin Oncol. 2014;19(3):479–484. doi: 10.1007/s10147-013-0583-0. [DOI] [PubMed] [Google Scholar]
- 4.Varadhachary GR, Raber MN, Matamoros A, Abbruzzese JL. Carcinoma of unknown primary with a colon-cancer profile-changing paradigm and emerging definitions. Lancet Oncol. 2008;9(6):596–599. doi: 10.1016/S1470-2045(08)70151-7. [DOI] [PubMed] [Google Scholar]
- 5.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27(3):303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Jung HY, Cho H, Chung JH, Bae SB, Lee JH, Lee HJ, Jang SH, Oh MH. A rare case of primary tubular adenocarcinoma of the thymus, enteric immunophenotype: a case study and review of the literature. J Pathol Transl Med. 2015;49(4):331–334. doi: 10.4132/jptm.2015.04.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapur P, Rakheja D, Bastasch M, Molberg KH, Sarode VR. Primary mucinous adenocarcinoma of the thymus: a case report and review of the literature. Arch Pathol Lab Med. 2006;130(2):201–204. doi: 10.5858/2006-130-201-PMAOTT. [DOI] [PubMed] [Google Scholar]
- 8.Maeda D, Ota S, Ikeda S, Kawano R, Hata E, Nakajima J, Mori M, Fukayama M. Mucinous adenocarcinoma of the thymus: a distinct variant of thymic carcinoma. Lung Cancer (Amsterdam, Netherlands) 2009;64(1):22–27. doi: 10.1016/j.lungcan.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Ghafar J, Yong SJ, Kwon W, Park IH, Jung SH. Primary thymic mucinous adenocarcinoma: a case report. Korean J Pathol. 2012;46(4):377–381. doi: 10.4132/KoreanJPathol.2012.46.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teramoto K, Kawaguchi Y, Hori T, Ishida M, Hashimoto M, Kitamura S, Motoishi M, Hanaoka J, Tezuka N, Okabe H. Thymic papillo-tubular adenocarcinoma containing a cyst: report of a case. Surg Today. 2012;42(10):988–991. doi: 10.1007/s00595-012-0161-5. [DOI] [PubMed] [Google Scholar]
- 11.Maghbool M, Ramzi M, Nagel I, Bejarano P, Siebert R, Saeedzadeh A, Daneshbod Y. Primary adenocarcinoma of the thymus: an immunohistochemical and molecular study with review of the literature. BMC Clin Pathol. 2013;13(1):17. doi: 10.1186/1472-6890-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]