Abstract
Reciprocal communication among cells of the tumor microenvironment contributes to cancer progression. Here, we show that a protumoral population of cultured bone marrow-derived cells (BMDC) containing Tie2+/CD45+/CD11b + cells responded to lung carcinoma cells and reciprocally stimulated them. These cells migrated via heterotrimeric G protein-dependent signaling pathways and strongly activated the PI3K/AKT, ERK and mTOR signaling cascades in response to conditioned media and chemotactic agonists. To get insight into the molecular machinery involved in BMDC migration, we revealed their repertoire of guanine nucleotide exchange factors for Rho GTPases (RhoGEFs) and G proteins in comparison with fresh bone marrow cells, proven that these cell populations had contrasting effects on tumor growth. BMDC exhibited a higher expression of G protein regulated RhoGEFs including P-Rex1, PDZ-RhoGEF, LARG, Trio and some less well characterized RhoGEFs such as ARHGEF5, ARHGEF17 and PLEKHG6. G proteins such as Gα12/13, Gαq, and the small GTPase RhoJ were also highly expressed in BMDC. Our results indicate that Tie2+/CD45+/CD11b + BMDC express a unique variety of chemotactic transducers and effectors potentially linked to their protumoral effect, warranting further studies to their characterization as molecular targets.
Keywords: Cell migration, GPCRs, Protumoral bone marrow-derived cells, RhoGEFs, Rho GTPases, Tie2-monocyte/macrophages
Introduction
The permissive pro-oncogenic role played by the tumor microenvironment is exacerbated by cells attracted from the bone marrow (Lyden et al. 2001). They contribute to enable the metastatic potential of cancer cells (Shaw et al. 2004). In addition to bone marrow-derived cells (BMDC), cancer associated fibroblasts, endothelial cells and multiple immune subpopulations are enrolled and their communication orchestrates a variety of coordinated local and systemic processes including tumor-induced angiogenesis, local immunosuppression, extracellular matrix remodeling for invasion and premetastatic niche establishment, which altogether support tumor growth and dissemination (De Palma et al. 2005; Jahangiri et al. 2018; Kazerounian and Lawler 2018; Peinado et al. 2012; Smith and Kang 2013; Vazquez-Prado et al. 2016).
Communication among cancer cells and those attracted to the tumor microenvironment include direct cellular interactions; however, a prominent role has been attributed to tumor secreted factors which generate complex oncogenic circuits and display systemic effects. Particularly, chemotactic factors and sophisticated macromolecular complexes released as exosomes promote cell mobilization and flag the site where cancer cells can find the right conditions to grow, out of the primary tumor (Hoshino et al. 2015; Kaur et al. 2018; Peinado et al. 2012; Ridge et al. 2018). Cells follow chemotactic gradients as an integrated response of their signaling effectors being activated by diverse receptor families, mainly belonging to G protein-coupled receptors (GPCRs) and tyrosine kinase receptors (Kitamura et al. 2015; Vazquez-Prado et al. 2016). Chemotactic membrane receptors promote cell migration by activating Rho GTPases via guanine nucleotide exchange factors (RhoGEFs) directly stimulated by signal transducers, second messengers and posttranslational modifications. In the case of BMDC, chemotactic agonists recognized by CXCR4 (Madlambayan et al. 2009) and CCR2 (Bonapace et al. 2014), among other GPCRs, promote cytoskeletal reorganization and assembly of contractile complexes during their journey towards evolving tumors and putative metastatic niches (Vazquez-Prado et al. 2016). Based on the fundamental role played by Rho family GTPases on the dynamics of the actin cytoskeleton, RhoGEFs are obligated participants on cell migration. They are multiple and exhibit complex and diverse multi-domain architectures, so their potential to integrate an array of signaling inputs widely varies among different cell populations (Lawson and Ridley 2018; Vazquez-Prado et al. 2016).
We recently demonstrated that P-Rex1, RGS-RhoGEFs and many other activators of Rho GTPases are expressed in VEGF-stimulated and tumor endothelial cells, suggesting an important participation of these multidomain proteins in tumor angiogenesis (Hernandez-Garcia et al. 2015). Furthermore, since inhibitors of the communication among cells of the tumor microenvironment have demonstrated a beneficial clinical effect, understanding how BMDC respond to tumor-derived factors constitutes a promising field to eventually develop therapeutic strategies aimed to interfere with BMDC contribution to tumor progression. Moreover, based on their tropism for tumors and metastatic niches, BMDC have also been visualized as potential therapeutic vehicles (Gao et al. 2008). With the long-term goal of identifying molecular targets mechanistically linked to the protumoral effects of BMDC, here we characterized the reciprocal communication between a protumoral population of BMDC and lung cancer cells and elucidated the repertoire of RhoGEFs and related signaling proteins expressed in BMDC. We found several highly expressed RhoGEFs that can be considered potential therapeutic targets.
Material and methods
Mouse tumor models
FVB/NJ (stock 001800), Tg(TIE2GFP)287Sato/J (stock 003658), and C57/BL6 (stock 000664) mice were obtained from Jackson Laboratories and maintained according to their instructions. All procedures were approved by UPEAL-Cinvestav ethical committee (protocols 33–13 and 0205–16). Lung carcinoma cells (alone or mixed either with cultured BMDC or fresh bone marrow cells) were suspended in 100 μL and inoculated subcutaneously, using 31G needles, in the dorsal region of FVB mice for LAP0297 lung carcinoma cells, or C57 mice for Lewis lung carcinoma cells (LLC). Tumor size was measured with caliper and volume was calculated based on the equation: width×length2 × π/6 as reported (Gao et al. 2008).
Cancer and endothelial cell cultures
Lung carcinoma cells (LAP0297) were kindly donated by Dr. Peigen Huang from the Department of Radiation Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, MA (Huang et al. 2008). Lewis lung carcinoma cells (LLC-GFP), kindly provided by Dr. Luisa Iruela-Arispe (Hernandez-Garcia et al. 2015), and PAE (porcine aortic endothelial) cells (Guzman-Hernandez et al. 2009) were cultured and maintained in Dulbecco’s Modified Eagle Medium (DMEM, Sigma) supplemented with 10% Fetal Bovine Serum (FBS) and antibiotics (Gibco).
Mouse bone marrow-derived cell (BMDC) cultures
Bone marrow-derived cell cultures enriched for Tie2+CD11b+CD45+ cells were prepared based on the protocol described by Asahara and colleagues (Sekiguchi et al. 2011), with minor modifications: the histopaque gradient was omitted and a short incubation with ammonium chloride was included to lyse erythrocytes before seeding the cells. Briefly, dissected femurs and tibias from C57 or FVB mice were flushed with 1X PBS. Extracted bone marrow cells were filtered through a 40 μm pore nylon cell strainer (BD Falcon, Tokyo, Japan; 352,340), incubated for 10 min with 1:4 ammonium chloride (NH4Cl, StemCell Technologies, 07800), washed with 1X PBS and collected by centrifugation at 1200 rpm. Bone marrow cells were suspended with EGM-2MV (EBM-2 supplemented with factors and 5% fetal bovine serum, FBS) and seeded on 10 μg/mL ProNectin-coated dishes (ProNectin, Fibronectin-like Engineered Protein Polymer-Plus Genetically Engineered, Sigma-Aldrich, F8141) during 24 h. BMDC cultures were established from non-adherent cells which were transferred to new ProNectin-coated dishes. Four days later, cells were washed and those that remained adherent were cultured for 14 days in EGM-2MV supplemented with 10% FBS (Sekiguchi et al. 2011).
Conditioned media, agonists and inhibitors
Conditioned media from BMDC and LAP0297/LLC were collected in serum-free media (EBM-2 or DMEM, respectively; 10 mL/p100 dish). Cells were initially washed (washed 5 times with 1X PBS) and media was collected 14 h later, centrifuged at 3000 rpm/20 min and stored at −70 °C. Agonists were from the following sources and used at the indicated concentrations: Stromal Derived Factor 1α (50 ng/mL, SDF-1α/CXCL12, PeproTech, 300-28A), Sphingosine 1-Phosphate (1 μM, S1P, Sigma-Aldrich, S9666), lysophosphatidic acid (1–5 μM, LPA, Biomol, LP-100), interleukin-8 (3 nM, IL-8, Sigma-Aldrich I1645), vascular endothelial growth factor (100 ng/mL, VEGF 165 human recombinant, Calbiochem, PF074), hepatocyte growth factor (10 ng/mL, HGF, R&D Systems, 294-HGN), epidermal growth factor (10 ng/mL, EGF, Gibco, 13,247–051), basic fibroblast growth factor (25 ng/mL, bFGF, R&D Systems, 234-FSE/CF); platelet derived growth factor (100 ng/mL, PDGF, Sigma-Aldrich, P3326). Antagonists and inhibitors were from the following sources: AMD3100, a CXCR4 antagonist (10 μM, Sigma-Aldrich A5602, (Fricker et al. 2006)); gallein, a Gβγ inhibitor (10 μM, Tocris 3090 (Lehmann et al. 2008)); and pertussis toxin, a heterotrimeric Gi inhibitor, (100 ng/mL, PTX, Calbiochem, 516,560 (Bonig et al. 2004)).
Cell migration assays
Cells, seeded on 0.02% gelatin-coated dishes (6 or 12 wells), were starved with serum-free media for 6 h (EBM-2 for BMDC) or 14 h (DMEM for LAP0297 and PAE cells). Two hours before stimulation, cells were pre-incubated with vehicle, pertussis toxin, gallein or AMD3100; in the case of LAP0297 and PAE cells, mitomycin C (12 μM, Sigma-Aldrich, M0440 (Cervantes-Villagrana et al. 2018)) was included. Migration assays were initiated by scraping cell monolayers with a pipette tip. Cells were washed three times with PBS and subjected to stimulation with conditioned media or the agonists indicated in figure legends. After 18 h, cells were fixed with 4% paraformaldehyde, stained with crystal violet, washed with PBS and photographed.
Western blot and Rac activation assay
Activation of Rac was assessed by pull down assays using recombinant GST-PAK-CRIB following the previously described procedure (Chavez-Vargas et al. 2016). BMDC were grown in P60 ProNectin-coated dishes, starved overnight in serum free-media, and stimulated with LAP0297 conditioned media. Protein lysates and pulldowns were separated on SDS-PAGE gels, transferred to Immobilon membranes (Millipore), blocked with 5% non-fat milk/TBS-Tween and incubated overnight at 4 °C, on a rocking platform, with the following primary antibodies: RhoJ (ab57584), Abcam; Rac1 (610651), PRKAR1a (610165BD), Transduction Laboratories; CREB (9197S), phospho-p42/44 MAP Kinase (ERK1/2) T202/Y204, (9191), pS6 ribosomal protein phosphoSer240/244 (5364); Vav2 (2848), Cell Signaling Technology; pan-Ras, (OP40), Millipore; Cdc42 (sc-8401), Gαq (sc-392), Gα12 (sc-409), Gβ (sc-261), ERK-2 (sc-154), phosphoAKT1/2/3 Ser473 (sc-7985-R), LARG/ARHGEF12 (sc-25,638), p115/Lsc RhoGEF/ARHGEF1 (sc-20,804), Rho A (sc-418), S6 (sc-74,459), Trio (sc-28,564), Santa Cruz Biotechnology; PKBα/AKT1 (P2482), P-Rex1 (HPA001927), PDZ-RhoGEF (HPA011026 and HPA014658), Sigma-Aldrich; and actin (Hernandez-Garcia et al. 2015). Membranes were washed with 1X TBS-Tween and incubated at room temperature for 1 h with anti-mouse or rabbit secondary antibodies (KPL, 074–1802 and 074–151) in milk/TBS-T. Again, membranes were washed and revealed using Immobilon Western Chemiluminescent HRP Substrate (Millipore).
FACS analysis
Expression of surface markers was assessed in cells incubated for 30 min on ice with the following antibodies: anti-mouse Tie2-PE (12–5987), CD45-PE-Cyanine7 (25–0451), CD34-eFluor450 (48–0341), all from Affymetrix (eBioscience); anti-CD11b-FITC (553310) or CD11b-BV605 (563015), from BD Bioscience. Controls included fluorescent isotype antibodies and unstained samples. For analysis, dissected lung and tumors were minced and digested with Collagenase II at 37 °C for 45–60 min and filtered through a 40 μm strainer to obtain single cell suspensions. Fresh bone marrow cells (BM) were isolated from tibias and depleted from red cells. Cultured BMDC were detached with trypsin. Cells were washed with 5% FBS/1X PBS before immunostaining. Labeled cell populations were analyzed with a BD-Fortessa cytometer or a Coulter-Beckman system (Beckton Dickinson). FACS data were analyzed with Summit software (Beckman Coulter).
RT-PCR analysis
Expression of 62 DH-RhoGEFs (total found in the mouse genome; http://smart.embl-heidelberg.de/) and cell markers was assessed by RT-PCR analysis of total RNA isolated from fresh bone marrow and BMDC cultures using the primers and procedures previously reported (Hernandez-Garcia et al. 2015). Ideal primers were designed in the Primer3 web platform (http://bioinfo.ut.ee/primer3-0.4.0/primer3/), using cDNA and open reading frame sequences of DH-RhoGEFs obtained from the NCBI/nucleotide database. Primers, selected to detect all possible splice variants, were confirmed to be specific by BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Briefly, total RNA from BM and BMDC was prepared with the Trizol method (Invitrogen). Erythrocyte-free fresh bone marrow cells, collected in conic polypropylene tubes, were lysed with 1 mL Trizol; BMDC were lysed in their culture dishes. Each RT-PCR reaction was done with 100 ng of total RNA; cDNA was prepared using the Superscript III kit (Invitrogen) and amplified with JumpStart RED Taq ReadyMix PCR Reaction Mix kit (Sigma-Aldrich, P0982). PCR reactions started with an initial step of 10 min at 94 °C, followed by 30 cycles (94 °C, 30″; 61 °C 30″; 72 °C, 1′), and a final step of 10 min at 72 °C. Samples were maintained at 4 °C before being analyzed by 2% agarose gel electrophoresis. Ethidium bromide-stained gels were visualized with a BioDoc-It imaging system (UVP Inc., Upland, CA). Intensity of amplified DNA fragments was quantitated with the NIH/ImageJ software (https://imagej.nih.gov/ij/index.html). Expression of β-actin, amplified from serial dilutions of cDNA, served as quality control and to normalize the expression of different markers. Expression of each RhoGEF was normalized to the summed value of all RhoGEFs. Primers for cell markers shown in Fig. 1i were: CD31 TGCAGGAGTCCTTCTCCACT and ACGGTTTGATTCCACTTTGC; Ang1 AGGCTTGGTTTCTCGTCAGA and TCTGCACAGTCTCGAAATGG; VEGFR2 GGCGGTGGTGACAGTATCTT and GTCACTGACAGAGGCGATGA; VE-cadherin ATTGAGACAGACCCCAAACG and TTCTGGTTTTCTGGCAGCTT; CD133 GAAAAGTTGCTCTGCGAACC and TCTCAAGCTGAAAAGCAGCA; CD34 ACCACAGACTTCCCCAACTG and CGGATTCCAGAGCATTTGAT; Id1 CCAGTGGGTAGAGGGTTTGA and AGAAATCCGAGAAGCACGAA; c-Kit TTATCCTTTAGGCCGTGTGG and TGTGGCCCCTTAAGTACCTG; CXCR4 TCAGTGGCTGACCTCCTCTT and TTTCAGCCAGCAGTTTCCTT; PDGFRβ AATTCCGTGCCGAGTGACAG and ACGTAGCCATTCTCGATCACA; CD45 CCTGCTCCTCAAACTTCGAC and GACACCTCTGTCGCCTTAGC; and β-actin TCAAGATCATTGCTCCTCCTGAGC and TACTCCTGCTTGCTGATCCACATC.
Fig. 1.
Cultured BMDC enriched with Tie2+/CD45+/CD11b+cells promote tumor growth. a Experimental design to evaluate the effect of cultured BMDC and fresh bone marrow cells co-inoculated with lung carcinoma cells on tumor growth in immunocompetent mice. b Cultured BMDC promote LAP0297 tumor growth in FVB mice. Mice were inoculated with cancer cells (0.5X106 lung carcinoma LAP0297) alone or either with BMDC (1X106 BMDC +0.5X106 LAP0297) or fresh bone marrow cells (1X106 BM + 0.5X106 LAP0297). Data represent the mean ± SEM; LAP (n = 12), LAP+BMDC (n = 6) and LAP+BM (n = 10); *p < 0.05; ***p < 0.001 (vs LAP); two-way repeated measures ANOVA followed by Tukey test. c BMDCs promote Lewis lung carcinoma (LLC) tumor growth in C57 mice. LLC cells (106) alone or with BMDC (106) were inoculated into immunocompetent C57 mice and tumors allowed to developed for two weeks. Data represent the mean ± SEM of 6 to 7 animals per group. **p < 0.01, ***p < 0.001; LLC vs (LLC + BMDC) analyzed by two-way repeated measures ANOVA followed by Tukey test. d Freshly isolated bone marrow cells attenuate LAP0297 tumor growth. FVB mice were inoculated with LAP0297 cells alone or with freshly isolated bone marrow cells and tumors were monitored for two weeks. Data represent the mean ± SEM of 4 to 5 animals per group. *p < 0.05, ***p < 0.001, two-way repeated measures ANOVA followed by Tukey test. e Rate of green fluorescent cells isolated from Tie2-GFP+ transgenic mice. Bone marrow cells from wild type and FVB-Tie2-GFP mice were analyzed by flow cytometry (plots a and b, respectively); plot c shows the rate of fluorescent cells from lungs of FVB-Tie2-GFP transgenic mice. f Cultured BMDC are enriched with Tie2+ cells. BMDC and freshly isolated bone marrow cells (BM) from wild type FVB mice were stained with anti Tie2 antibodies and subjected to FACS analysis. g Cultured Tie2+/CD45+ BMDC express the monocyte marker CD11b. BMDC were stained with antibodies for Tie2 (PE), CD45 (PE-Cy7) and CD11b (FITC) and analyzed by FACS. h Rate of Tie2+/CD11b+/CD45+ cells in LAP0297 tumors. FVB mice were inoculated with 106 LAP0297 cells and tumors allowed to grow for two weeks. Expression of Tie2 (PE), CD45 (PE-Cy7) and CD11b (BV605) in dissected tumors, analyzed as single cell suspensions, was done by FACS. Background fluorescence was assessed with the FITC channel. i Expression of cell markers in BMDCs and freshly isolated bone marrow cells. RT-PCR analysis for the indicated cell markers was performed in fresh bone marrow cells (BM) isolated from FVB mice and in cultured BMDC. Data represent the mean ± SEM of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001; two-way ANOVA followed by the Tukey test
Statistical analysis
Densitometric quantitation and cell migration assays were analyzed with the ImageJ software. Data are presented as the mean ± S.E.M. of at least three independent experiments as indicated in figure legends. Data were analyzed either with the Student’s t test or one-way ANOVA followed by Dunnett or Tukey for multiple comparison tests. Comparative analysis of tumor volumes was done by two-way repeated measures ANOVA followed by the Tukey test. Sigma Plot 11.0 software was used for statistical analysis and GraphPad Prism V6.05 software to prepare the graphs. Statistical significance was considered for values of p < 0.05.
Results
An in vitro expanded population of Tie2+/CD45+/CD11b + BMDC contribute to tumor growth
To initiate the characterization of signaling pathways underlying the reciprocal communication between lung cancer cells and protumoral bone marrow-derived cells (BMDC) and define the molecular profile of RhoGEFs and related signaling proteins in BMDC, we expanded a BMDC population according to a reported protocol based on differential adhesion properties and selective media (Sekiguchi et al. 2011). Since bone marrow-resident endothelial progenitors and Tie2+ monocytes are known as effective promoters of tumor growth (De Palma et al. 2005; Gao et al. 2008), we aimed to obtain a bone marrow-derived culture enriched with Tie2+ protumoral cells. The protumoral effect of BMDC was tested in immunocompetent FVB and C57/BL6 mice co-inoculated either with LAP0297 or LLC lung cancer cells, respectively (Fig. 1a). BMDC significantly promoted tumor growth (Fig. 1b, c). In contrast, fresh bone marrow cells (BM) co-inoculated with equal amounts of tumor cells (5 X 105 or 106 cells/mouse) attenuated tumor growth (Fig. 1b, d). Using bone marrow from Tie2-GFP-FVB mice and the protocol described by Asahara and colleagues (Sekiguchi et al. 2011), we expanded a Tie2+ population (based on GFP fluorescence) from <0.2%, in the fresh bone marrow, to more than 30% of Tie2+ in cultured BMDC (Fig. 1e, panel b and Fig. 1f, Tie2+ shown in red; cells from lungs were almost 10% GFP+ endothelial cells, Fig. 1e, panel c (Ohle et al. 2012). According to FACS analysis for additional markers, protumoral BMDC cultures had abundant Tie2+/CD45+/CD11b+ cells. This cell population corresponded to more than 30% of total cultivated cells (Fig. 1g). Cytometry analysis revealed that CD45 and CD11b were well expressed, while CD34 was absent (not shown), indicating the monocytic lineage of these cultured protumoral BMDC (De Palma et al. 2005). To analyze the incorporation of these cells in primary tumors developed in FVB mice, tumors grown for two weeks were excised, disaggregated and analyzed by cytometry. Consistent with the integration of Tie2+/CD45+/CD11b+ cells in the tumor stroma, 7% of cells isolated from LAP0297 tumors exhibited the profile of markers characterized in BMDC cultures (Fig. 1h). Comparative analysis of markers detected at the RNA level between protumoral BMDC and fresh bone marrow cells revealed a small difference. PDGFRβ was increased in BMDC (likely corresponding to Tie2+ mesenchymal progenitors (De Palma et al. 2005)), while CD31, CD133, CD34 and c-kit were decreased (Fig. 1i). The high expression of CD45 and low expression of CD31, VEGFR2 and progenitor markers (CD133, CD34) indicated that cultured protumoral BMDC mainly contained a hematopoietic population, whereas endothelial progenitor cells were likely absent (De Palma et al. 2005; Kitamura et al. 2015; Rafii and Lyden 2003).
Tumor cells and BMDC reciprocally control their migration activating GPCR-dependent signaling pathways
Based on our initial results and the reported paracrine effect of BMDC (De Palma et al. 2005), we wanted to characterize how BMDC and LAP0297 cells stimulate each other by their conditioned media (Figs. 2 and 3, respectively). As shown in Fig. 2a, BMDC conditioned media (BMDC-CM) stimulated LAP0297 lung cancer cells activating AKT, ERK and mTOR signaling pathways in a time-dependent manner, and induced their migration (Fig. 2b). In contrast, conditioned media from irrelevant cells, such as HEK293T, did not stimulate LAP0297 cell migration (not shown). Then we tested the effect of specific agonists as potential promoters of LAP0297 cell migration, our results showed a significant positive effect of LPA, HGF and, as positive control, fetal bovine serum (FBS) (Fig. 2c). BMDC-derived conditioned media and LPA induced a Gi-dependent (PTX-sensitive) migratory response (Fig. 2d), mediated by Gβγ (Fig. 2e), as indicated by the inhibitory effect of gallein, a Gβγ inhibitor (Lehmann et al. 2008).
Fig. 2.
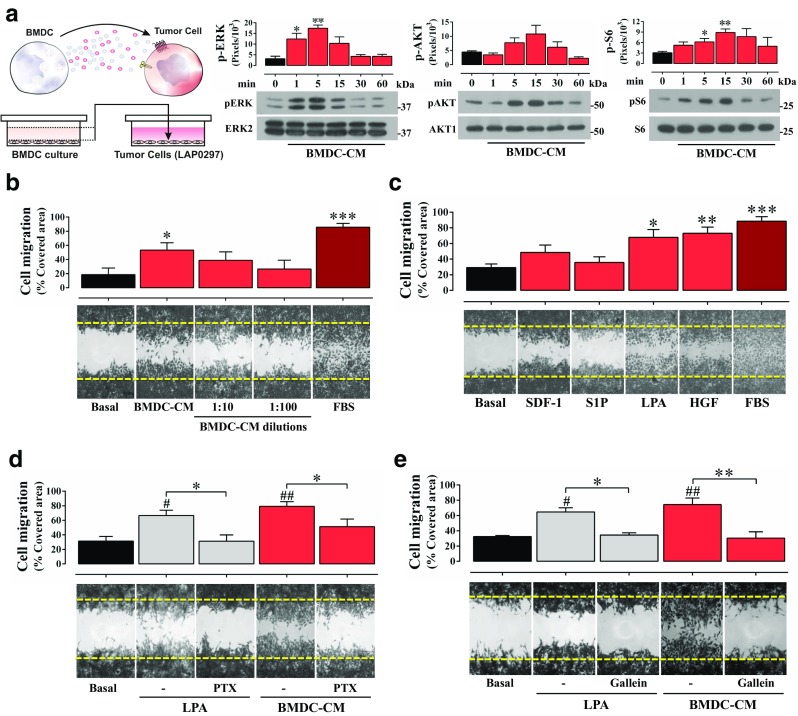
Protumoral BMDC-derived conditioned media stimulate lung cancer cell migration and signaling. a BMDC conditioned media (BMDC-CM) stimulate AKT, ERK and mTOR signaling cascades in lung cancer cells. LAP0297 cells, maintained overnight in serum-free EBM-2 media, were stimulated with BMDC-CM for the indicated times and subjected to western blot analysis to detect the activation of ERK, AKT and mTOR (using pS6, as readout). Data represent the mean ± SEM of three independent experiments. pERK: *p < 0.05, **p < 0.01 (all vs basal), one-way ANOVA followed by Dunnett test. pS6: *p < 0.05, **p < 0.01 (all vs basal), t-test. b BMDC-CM promotes lung cancer cell migration. Serum starved LAP0297 cells, in six-well plate dishes, were subjected to cell migration assays for 18 h. Cells were stimulated with BMDC-CM. 10% FBS served as positive control. Data represent the mean ± SEM of four independent experiments. *p < 0.05 (all vs basal), ***p < 0.001, t-test. c LPA and HGF promote LAP0297 cell migration. Cell migration assays were done with cells stimulated with agonists for GPCRs: SDF-1 (50 ng/mL), S1P (1 μM) and LPA (5 μM); or tyrosine-kinase receptors: HGF (10 ng/mL). Graph represents the mean ± SEM of four independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (vs. basal); one-way ANOVA followed by Tukey. d Pertussis toxin inhibits LAP0297 cell migration induced by BMDC-CM. Cells were stimulated with LPA (5 μM) or BMDC-CM in the absence or presence of PTX (100 ng/mL) to inhibit heterotrimeric Gi protein. Data represent three independent experiments, *p < 0.05 (-PTX vs + PTX); #p < 0.05, ##p < 0.01 (vs basal); one-way ANOVA followed by Tukey, *p < 0.05 (CM vs. CM-PTX), t-test. e Inhibition of Gβγ by gallein prevents LAP0297 cell migration induced by BMDC-CM. Cells were stimulated with LPA (5 μM) or BMDC-CM, in the absence of presence of gallein (10 μM). Data represent three independent experiments. *p < 0.05, **p < 0.01 (-Gal vs + Gal); #p < 0.05, ##p < 0.01 (basal vs stimuli); one-way ANOVA followed by Tukey
Fig. 3.
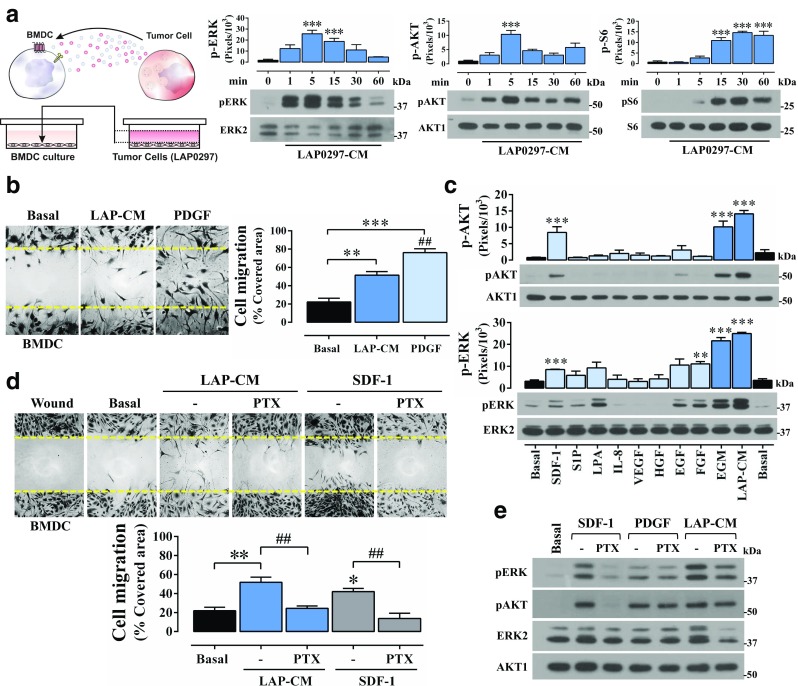
Lung cancer cell conditioned media promote BMDCs migration and signaling. a Conditioned media collected from LAP0297 lung cancer cells (LAP0297-CM) stimulate AKT, ERK and mTOR signaling cascades in BMDC. Cultured BMDC were starved for 14 h, stimulated with LAP0297-CM and analyzed by western blot to assess the activation of ERK, AKT and mTOR (with pS6 as readout) signaling pathways. Data represent the mean ± SEM of three independent experiments. ***p < 0.001 (all vs basal), one-way ANOVA followed by the Tukey test. b BMDC migration is induced by lung cancer cell conditioned media and PDGF. Serum-starved BMDC cultures were scratched with a pipette tip, stimulated with LAP-CM and PDGF (100 ng/mL) and cells allowed to migrate for 18 h. Data represent the mean ± SEM of 3 to 4 independent experiments, **p < 0.01, ***p < 0.001 (vs basal); ##p < 0.01 (LAP-CM vs PDGF); one-way ANOVA followed by Tukey. c BMDCs activate AKT and ERK signaling pathways in response SDF-1/CXCL12. Serum-starved BMDC were stimulated for 5 min with the indicated agonists and subjected to western blot analysis to assess the activation of AKT and ERK. Data represent the mean ± SEM of three independent experiments; AKT activation (pAKT Ser473): ***p < 0.001 (vs basal), one-way ANOVA followed by the Tukey test. ERK activation (pERK): **p < 0.01, ***p < 0.001 (vs basal), one-way ANOVA followed by the Tukey test. d LAP0297 lung cancer cell conditioned media (LAP-CM) and SDF-1/CXCL12 promote BMDC migration via heterotrimeric Gi proteins. BMDC cells were incubated with pertussis toxin (100 ng/mL) to address the role of Gi in LAP0297-CM and SDF-1/CXCL12-induced migration. Data represent the mean ± SEM of three independent experiments. *p < 0.05, **p < 0.01 (basal vs LAP-CM or SDF-1); ##p < 0.01 (-PTX vs + PTX); one-way ANOVA followed by Tukey. e BMDC activate AKT and ERK via Gi-dependent and independent pathways as revealed by the effect of PTX (100 ng/mL). Cells were stimulated by 5 min. Similar results were obtained in 3 independent experiments
In reciprocity, LAP0297-CM effectively stimulated protumoral BMDC which showed a sustained activation of AKT and mTOR signaling pathways and a strong, but more transient effect, on ERK signaling (Fig. 3a), and exhibited a significant migratory response (Fig. 3b). PDGF was also an effective promoter of BMDC migration (Fig. 3b). Stimulated by different GPCR- and TyrK- agonists, BMDC resulted particularly sensitive to SDF-1, which activated AKT and ERK signaling pathways (Fig. 3c, upper and lower panels, respectively), and promoted Gi-dependent BMDC migration (Fig. 3d) via a PTX-sensitive mechanism (Fig. 3e). Furthermore, AMD3100, a CXCR4 antagonist, prevented AKT activation by SDF-1 (not shown). PDGF stimulated ERK and AKT via pertussis toxin-insensitive pathways (Fig. 3e). In response to LAP0297-CM, BMDC migrated via Gi (Fig. 3d), while the effect on ERK and AKT was not sensitive to PTX (Fig. 3e).
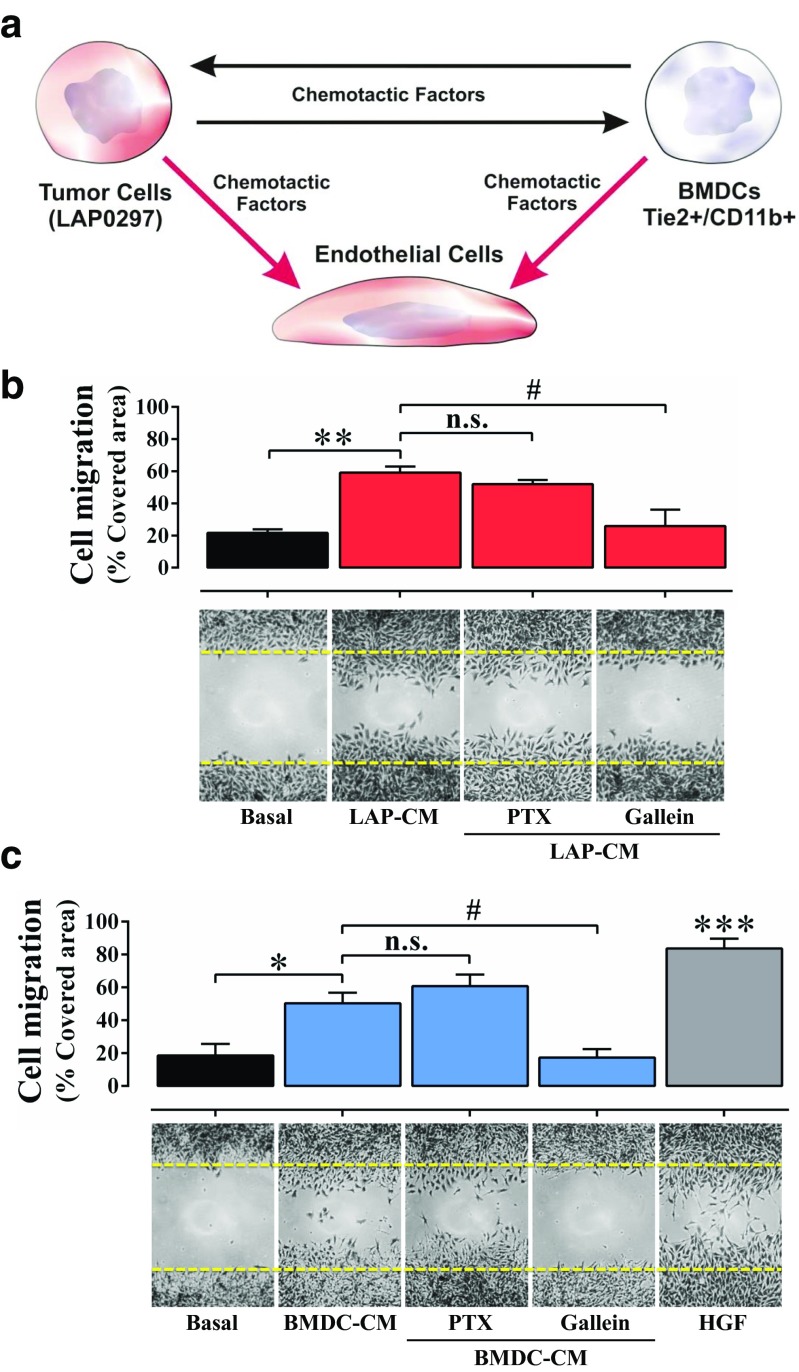
BMDC-derived and lung cancer cell conditioned media promote endothelial cell migration via GPCR-dependent signaling pathways
Endothelial cells are critical players within the tumor microenvironment. Thus, we wanted to address whether cultured protumoral BMDC, as well as lung cancer cells, promoted endothelial cell migration via GPCR-dependent signaling pathways (Fig. 4a). Cell migration assays revealed that conditioned media obtained from lung cancer cells (Fig. 4b) and BMDC (Fig. 4c) stimulated endothelial cells to migrate via Gβγ-dependent signaling pathways, as revealed by the inhibitory effect of gallein. Pertussis toxin did not interfere with endothelial cell migration indicating that heterotrimeric G proteins, other that Gi, were the likely source of Gβγ involved in their migratory response to cancer and BMDC-secreted factors. As positive control, migration in response to HGF was demonstrated (Fig. 4c).
Fig. 4.
Gβγ-dependent endothelial cell migration is promoted by conditioned media from lung cancer cells and BMDC. a Hypothetical axis of communication between lung cancer cells and BMDC with endothelial cells. b Gβγ-dependent endothelial cell migration. Confluent endothelial cell cultures were starved for 14 h, scratched with a pipette tip, washed, and stimulated with LAP0297-CM. Cells were allowed to migrate for 18 h. To address the participation of Gi and Gβγ-dependent pathways, cells were treated with PTX (100 ng/mL) and gallein (10 μM Gal) for two hours before and throughout the migration period. Data represent the mean ± SEM of three independent experiments. **p < 0.01 (versus Basal); #p < 0.05 (LAP-CM versus gallein), n.s. no significance; one-way ANOVA followed by Tukey. c BMDC-conditioned media stimulate endothelial cell migration via Gβγ. Endothelial cell migration induced by BMDC-CM in the absence or presence of PTX and gallein was tested by cell migration assays, HGF (10 ng/mL) served as positive control. Data represent the mean ± SEM of three independent experiments. *p < 0.05, ***p < 0.001 (vs basal); #p < 0.05 (BMDC-CM versus gallein), n.s. no significance; one-way ANOVA followed by Tukey
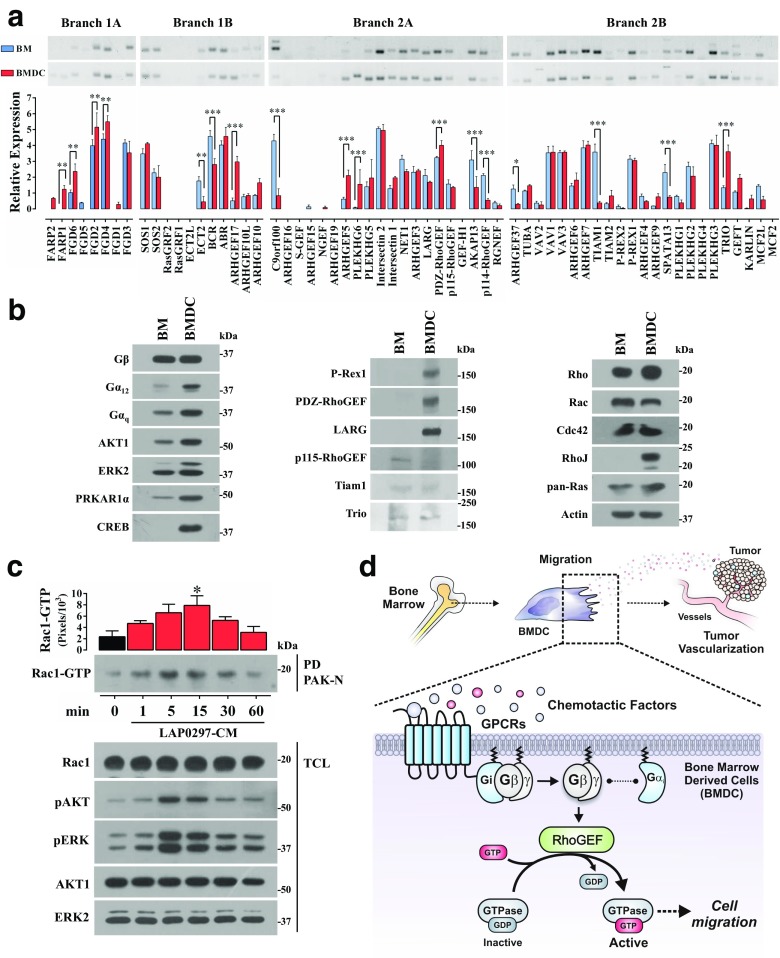
RhoGEFs expressed in BMDC
In order to systematically identify in BMDC the signaling effectors intrinsically linked to the activation of Rho GTPases, known to be involved in cell migration, we defined their profile of RhoGEFs. We compared BMDC and fresh bone marrow cells addressing, by RT-PCR, the expression of all murine DH-RhoGEFs. As in our previous analysis of RhoGEFs in tumor endothelial cells, data were presented according to their phylogenetic links which, based on the common DH domains, define 4 main phylogenetic branches (Hernandez-Garcia et al. 2015). As shown in Fig. 5a, both cell populations showed a complex pattern of RhoGEFs. Some of them, such as FARP1, FGD6, FGD2, FGD4, ARHGEF17, ARHGEF5, PLEKHG6, PDZ-RhoGEF and Trio were significantly more abundant in BMDC; while others such as Ect2, BCR, C9orf100, AKAP13, ARHGEF37, Tiam1 and Spata13 were decreased. At the protein level, among the RhoGEFs known as downstream effectors of GPCR-dependent signaling pathways (Fukuhara et al. 1999, 2000; Hart et al. 1998; Welch et al. 2002), P-Rex1, PDZ-RhoGEF and LARG were enriched in BMDC compared to fresh bone marrow cells, while p115-RhoGEF, Tiam1 and Trio showed similar expression levels (Fig. 5b). Among the small GTPases, RhoJ, a GTPase previously highlighted by its relevance in tumor angiogenesis (Kim et al. 2014), was highly expressed in BMDC (Fig. 5b, right panel), while other small GTPases as Rac, Rho, Cdc42 and Ras had similar expression levels between the cells evaluated (Fig. 5b). All the blots shown in Fig. 5b were done with the same total cell lysates; expression of actin, used as loading control, is shown at the bottom of the right panel. Activation of these pathways in BMDC stimulated with lung cancer cell conditioned media was demonstrated by pull-down analysis of active Rac, which was activated in a time-dependent manner in parallel to the phosphorylation of AKT and ERK; expression of total Rac, AKT and ERK served as loading controls (Fig. 5c).
Fig. 5.
Expression profile of RhoGEFs and selected signaling proteins in protumoral cultured bone marrow derived cells (BMDC) and freshly isolated bone marrow cells (BM). a The whole group of DH-RhoGEFs encoded by the mouse genome was considered to analyze, by RT-PCR, their expression in BMDC and BM as previously described (Hernandez-Garcia et al. 2015). RhoGEFs are grouped according to their phylogenetic links (Hernandez-Garcia et al. 2015). Data represent the mean ± SEM of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, two-way ANOVA followed by Tukey test. b Western blot of selected signaling proteins, RhoGEFs and GTPases in cultured BMDC compared to freshly isolated BM cells. The same total cell lysates were used for the blots presented in all the panels in the figure. Equal amounts of total BMDC and fresh BM cell proteins were adjusted using actin as loading control, which is shown at the bottom of the right panel. Blots are representatives of three independent experiments. c GTPase Rac activation in BMDC stimulated lung cancer cell conditioned media (LAP0297-CM). BMDC from FVB-Tie2-GFP mice were incubated overnight in serum free EBM-2 media and stimulated with LAP0297-CM for the indicated times. Rac-GTP pulldown was performed with GST-PAK-CRIB beads. Activation of AKT and ERK was tested in parallel. Data represent the mean ± SEM of three independent experiments. *p < 0.05, one-way ANOVA followed by Dunnett test. d Model highlighting the putative role of GPCR-regulated RhoGEFs in BMDC migration induced by tumor-derived chemotactic factors with a particular contribution of Gβγ-dependent signaling pathway. The overall contribution of this signaling heterodimer in chemotactic cell migration has been previously reviewed (Vazquez-Prado et al. 2016)
Discussion
The intriguing role of cells from the bone marrow that migrate to promote tumor growth and metastasis has been the focus of intense research leading to the identification of diverse cell populations and demonstration of their unequivocal contribution to cancer microenvironment. However, the signaling effectors involved in protumoral BMDC migration remain elusive. Protumoral cells from the bone marrow include progenitor cells and immune precursors such as progenitor endothelial cells (EPCs), pro-angiogenic monocytes/macrophages and suppressor leukocytes (Cooks et al. 2018; De Palma et al. 2005; Gao et al. 2008; Giannoni et al. 2013). These bone marrow-derived cell populations pave the way to metastatic niches (Gao et al. 2008), but also provide resistance to therapies as anti-angiogenic drugs. Cells in the tumor microenvironment support cancer progression via non-cell-autonomous mechanisms based on their ability to secrete chemokines, cytokines, growth factors and proteins of the extracellular matrix (Cooks et al. 2018; Smith and Kang 2013; Vincent and Postovit 2018). Here we investigated the crosstalk between a cultured population of protumoral BMDC and lung cancer cells which reciprocally promoted cell migration. Our results show that conditioned media from these cells stimulate cell migration via GPCR-dependent mechanisms acting through Gβγ. Furthermore, we showed that both cell populations promoted endothelial cell migration also via Gβγ-dependent pathways, as revealed by the inhibitory effect of gallein. Importantly, the Gβγ/PI3Kβ pathway has been targeted in vitro in preclinical models of cancer cells, demonstrating that its inhibition prevents cell migration and invasive capacity (Dbouk et al. 2012).
To start addressing the molecular mechanisms of BMDC migration we wanted to reveal their profile of RhoGEFs which in general terms are the signaling effectors that activate Rho GTPases, the molecular switches that specify the spatiotemporal dynamics of the actin cytoskeleton during cell migration (Lawson and Ridley 2018; Vazquez-Prado et al. 2016). We cultured a heterogeneous population of Tie2+/CD45+/CD11b + BMDC that promoted tumor growth (in contrast to the inhibitory effect of fresh bone marrow cells). Expression of RhoGEFs demonstrated to be complex. Compared to fresh bone marrow cells, protumoral BMDC exhibited a higher expression of various, still poorly characterized, RhoGEFs (Fig. 5a). One of them, ARHGEF17 which exhibited a threefold increase, was originally described as a tumor endothelial marker (TEM4) in colon cancer (St Croix et al. 2000).
GPCR-regulated RhoGEFs such as P-Rex1 and RGS-RhoGEFs were enriched, at the protein level, in protumoral BMDC (Fig. 5b). P-Rex1, a Rac-GEF known as a Gβγ effector that activates Rac and cell migration in response to SDF-1 (Carretero-Ortega et al. 2010), has been found overexpressed in breast cancer and its expression correlated with a bad prognostic (Sosa et al. 2010). PDZ-RhoGEF and LARG, two of three known RGS-RhoGEFs, are direct effectors of Gα13 (Fukuhara et al. 2001). Interestingly, Gα13, which is essential for vascular development (Ruppel et al. 2005), plays a positive role in the contribution of the bone marrow to tumor angiogenesis (Chen et al. 2009). Altogether these findings are consistent with a potential role of G protein coupled RhoGEFs in the response of BMDC to agonists of tumor origin and set the basis to study their contribution to tumor growth. Based on the prominent role of Gβγ in chemotactic signaling by Gi-coupled GPCRs (Cervantes-Villagrana et al. 2018; Neptune and Bourne 1997; Neptune et al. 1999), the response to BMDC to SDF-1 and the activation of Rac, AKT, and mTOR by lung cancer cell conditioned media; we postulate that the signaling axis including Gi → Gβγ/PI3K/P-Rex1 is putatively involved in the response of BMDC to chemotactic factors secreted by lung cancer cells. Consistent with this possibility, we recently demonstrated that Gαq and Gα13 subunits maintain inhibitory interactions with Gβγ, indicating that although chemotactic factors frequently stimulate receptors known to be coupled to multiple G proteins, activation of Gβγ-regulated effectors linked to cell migration mainly depends on Gi, particularly when pertussis-toxin, a specific inhibitor of Gi heterotrimers, impedes chemotactic migration (Cervantes-Villagrana et al. 2018). Alternatively, GPCR-dependent and pertussis toxin-resistant chemotactic migration can be inhibited by gallein (the Gβγ inhibitor), indicating that Gs may also be a good provider of this signaling heterodimer. This possibility is also supported by our recent demonstration that GTPase-deficient Gαq and Gα13, but no Gαi or Gαs, maintain stable interactions with Gβγ indicating that Gi and Gs heterotrimers fully dissociate upon activation of their cognate receptors (Cervantes-Villagrana et al. 2018). Moreover, Gs-coupled EP2 receptors promote endothelial cell migration via P-Rex1 (Adame-Garcia et al. 2018), which integrates diverse signaling inputs and serves as a novel effector of protein kinase A regulatory subunit (Adame-Garcia et al. 2018; Vazquez-Prado et al. 2016). Besides P-Rex1, as the main Gβγ-dependent chemotactic effector identified in BMDC, other RhoGEFs detected might also be targets of Gβγ signaling; among them, PLEKHG2 and ARHGEF5 have been reported as Gβγ targets (Runne and Chen 2013; Ueda et al. 2008; Vazquez-Prado et al. 2016). Interestingly, our results show a differential expression of ARHGEF5 in protumoral BMDC compared to fresh bone marrow cells, further suggesting a potential role of this GEF in BMDC migration. In contrast to P-Rex1, specific for Rac, ARHGEF5 activates Rho (Wang et al. 2009), we speculate that these GEFs increase the potential spatiotemporal effects of Gβγ on cell migration, since it is well accepted that Rac and Rho are activated at the leading and trailing edges of migrating cells (Lawson and Ridley 2018). Based on our results, we propose a model shown in Fig. 5d in which Gβγ-regulated RhoGEFs promote BMDC cell migration in response to lung cancer cell-secreted factors which exacerbate the tumor microenvironment also contributing to stimulate endothelial cells.
In conclusion, cultured protumoral Tie2+/CD45+/CD11b + BMDC and lung cancer cells stimulate each other to migrate via Gi-coupled receptors acting through Gβγ-dependent signaling pathways. BMDC express a complex profile of RhoGEFs including some known to be regulated by G proteins such as P-Rex1, PDZ-RhoGEF and LARG, which are differentially expressed at the protein level respect to fresh bone marrow cells. In addition, based on their differentially expression in protumoral BMDC, ARHGEF5, ARHGEF17 and PLEKHG6, which have been poorly studied, emerge as interesting candidates for future studies aimed to understand their potential role in the protumoral activities of BMDC. The signaling properties and molecular profiling of RhoGEFs in protumoral bone marrow-derived cells set the basis to address their contribution to cancer progression. Based on the paramount role of Gβγ in chemotactic signaling, Gβγ-regulated RhoGEFs qualify as potential targets to prevent the contribution of BMDC to cancer progression.
Acknowledgments
Technical assistance provided by Estanislao Escobar-Islas, Margarita Valadez, David Pérez, and Jaime Estrada-Trejo is acknowledged. We thank Victor Hugo Rosales-García (Central Laboratories of Cinvestav) for technical assistance in cytometry; and Ricardo Gaxiola-Centeno and Benjamín Emmanuel Chavez-Álvarez (UPEAL-Cinvestav) for breeding and maintaining mice colonies. This work was supported by CONACyT (Consejo Nacional de Ciencia y Tecnología, Mexico) Grants 286274 (to J. V.-P.) and 240119 (to G. R.-C.). R.D.C.-V., and V.M.C.-A were supported by fellowships from CONACyT.
Abbreviations
- VEGF
Vascular Endothelial Growth Factor
- SDF-1
Stromal Cell-Derived Factor 1
- S1P1R
Sphingosine-1-Phosphate Receptors
- S1P
Sphingosine-1-phosphate
- IL-8
Interleukin-8
- RhoGEFs
Rho guanine exchange factors
- P-Rex1
Phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 protein
- PH
Pleckstrin homology domain
- PAE
Porcine Aortic Endothelial
- mTOR
mammalian target of rapamycin
- LPA
Lysophosphatidic acid
- LLC
Lewis lung carcinoma
- HGF
Hepatocyte Growth Factor
- EPC
Endothelial Progenitor Cell
- EGF
Epidermal Growth Factor
- FGF
Fibroblast Growth Factor
- DH
Dbl-Homology domain
- BMDC
Bone Marrow-Derived Cells
- BM
Bone Marrow
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adame-Garcia SR, Cervantes-Villagrana RD, Orduna-Castillo LB, Del Rio JC, Gutkind JS, Reyes-Cruz G, Taylor SS, Vazquez-Prado J (2018) cAMP-dependent activation of the Rac guanine exchange factor P-REX1 by type I protein kinase a (PKA) regulatory subunits. J Biol Chem:jbc.RA118.006691. 10.1074/jbc.RA118.006691 [DOI] [PMC free article] [PubMed]
- Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515(7525):130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- Bonig H, Priestley GV, Nilsson LM, Jiang Y, Papayannopoulou T. PTX-sensitive signals in bone marrow homing of fetal and adult hematopoietic progenitor cells. Blood. 2004;104(8):2299–2306. doi: 10.1182/blood-2004-04-1605. [DOI] [PubMed] [Google Scholar]
- Carretero-Ortega J, Walsh CT, Hernandez-Garcia R, Reyes-Cruz G, Brown JH, Vazquez-Prado J. Phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 (P-rex-1), a guanine nucleotide exchange factor for Rac, mediates angiogenic responses to stromal cell-derived factor-1/chemokine stromal cell derived factor-1 (SDF-1/CXCL-12) linked to Rac activation, endothelial cell migration, and in vitro angiogenesis. Mol Pharmacol. 2010;77(3):435–442. doi: 10.1124/mol.109.060400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Villagrana RD, Adame-Garcia SR, Garcia-Jimenez I, Color-Aparicio VM, Beltran-Navarro YM, Konig GM, Kostenis E, Reyes-Cruz G, Gutkind JS, Vazquez-Prado J (2018) Gbetagamma signaling to the chemotactic effector P-REX1 and mammalian cell migration is directly regulated by Galphaq and Galpha13 proteins. J Biol Chem:jbc.RA118.006254. 10.1074/jbc.RA118.006254 [DOI] [PMC free article] [PubMed]
- Chavez-Vargas L, Adame-Garcia SR, Cervantes-Villagrana RD, Castillo-Kauil A, Bruystens JG, Fukuhara S, Taylor SS, Mochizuki N, Reyes-Cruz G, Vazquez-Prado J. Protein kinase a (PKA) type I interacts with P-Rex1, a Rac guanine nucleotide exchange factor: EFFECT ON PKA LOCALIZATION AND P-Rex1 SIGNALING. J Biol Chem. 2016;291(12):6182–6199. doi: 10.1074/jbc.M115.712216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang JJ, Rafii S, Huang XY. Suppression of tumor angiogenesis by Galpha(13) haploinsufficiency. J Biol Chem. 2009;284(40):27409–27415. doi: 10.1074/jbc.M109.025460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG, Harris CC. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9(1):771. doi: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, Khalil BD, Barrett MO, Waldo GL, Surve C, Hsueh C, Perisic O, Harteneck C, Shepherd PR, Harden TK, Smrcka AV, Taussig R, Bresnick AR, Nurnberg B, Williams RL, Backer JM. G protein-coupled receptor-mediated activation of p110beta by Gbetagamma is required for cellular transformation and invasiveness. Sci Signal. 2012;5(253):ra89. doi: 10.1126/scisignal.2003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Fricker SP, Anastassov V, Cox J, Darkes MC, Grujic O, Idzan SR, Labrecque J, Lau G, Mosi RM, Nelson KL, Qin L, Santucci Z, Wong RS. Characterization of the molecular pharmacology of AMD3100: a specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol. 2006;72(5):588–596. doi: 10.1016/j.bcp.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem. 1999;274(9):5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Chikumi H, Gutkind JS. Leukemia-associated rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to rho. FEBS Lett. 2000;485(2–3):183–188. doi: 10.1016/S0014-5793(00)02224-9. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Chikumi H, Gutkind JS. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene. 2001;20(13):1661–1668. doi: 10.1038/sj.onc.1204182. [DOI] [PubMed] [Google Scholar]
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- Giannoni E, Taddei ML, Parri M, Bianchini F, Santosuosso M, Grifantini R, Fibbi G, Mazzanti B, Calorini L, Chiarugi P. EphA2-mediated mesenchymal-amoeboid transition induced by endothelial progenitor cells enhances metastatic spread due to cancer-associated fibroblasts. J Mol Med. 2013;91(1):103–115. doi: 10.1007/s00109-012-0941-9. [DOI] [PubMed] [Google Scholar]
- Guzman-Hernandez ML, Vazquez-Macias A, Carretero-Ortega J, Hernandez-Garcia R, Garcia-Regalado A, Hernandez-Negrete I, Reyes-Cruz G, Gutkind JS, Vazquez-Prado J. Differential inhibitor of Gbetagamma signaling to AKT and ERK derived from phosducin-like protein: effect on sphingosine 1-phosphate-induced endothelial cell migration and in vitro angiogenesis. J Biol Chem. 2009;284(27):18334–18346. doi: 10.1074/jbc.M109.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280(5372):2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garcia R, Iruela-Arispe ML, Reyes-Cruz G, Vazquez-Prado J. Endothelial RhoGEFs: a systematic analysis of their expression profiles in VEGF-stimulated and tumor endothelial cells. Vasc Pharmacol. 2015;74:60–72. doi: 10.1016/j.vph.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Duda DG, Jain RK, Fukumura D. Histopathologic findings and establishment of novel tumor lines from spontaneous tumors in FVB/N mice. Comp Med. 2008;58(3):253–263. [PMC free article] [PubMed] [Google Scholar]
- Jahangiri B, Khalaj-Kondori M, Asadollahi E and Sadeghizadeh M (2018) Cancer-associated fibroblasts enhance cell proliferation and metastasis of colorectal cancer SW480 cells by provoking long noncoding RNA UCA1. J Cell Commun Signal [DOI] [PMC free article] [PubMed]
- Kaur S, Elkahloun AG, Singh SP, Arakelyan A, Roberts DD. A function-blocking CD47 antibody modulates extracellular vesicle-mediated intercellular signaling between breast carcinoma cells and endothelial cells. J Cell Commun Signal. 2018;12(1):157–170. doi: 10.1007/s12079-017-0428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazerounian S, Lawler J. Integration of pro- and anti-angiogenic signals by endothelial cells. J Cell Commun Signal. 2018;12(1):171–179. doi: 10.1007/s12079-017-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, Park I, Jung J, Kataoka H, Lee D, Heo WD, Kim I, Jon S, Adams RH, Nishikawa S, Uemura A, Koh GY. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Cancer Cell. 2014;25(1):102–117. doi: 10.1016/j.ccr.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15(2):73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson CD, Ridley AJ. Rho GTPase signaling complexes in cell migration and invasion. J Cell Biol. 2018;217(2):447–457. doi: 10.1083/jcb.201612069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol. 2008;73(2):410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7(11):1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Madlambayan GJ, Butler JM, Hosaka K, Jorgensen M, Fu D, Guthrie SM, Shenoy AK, Brank A, Russell KJ, Otero J, Siemann DW, Scott EW, Cogle CR. Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood. 2009;114(19):4310–4319. doi: 10.1182/blood-2009-03-211342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci U S A. 1997;94(26):14489–14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Iiri T, Bourne HR. Galphai is not required for chemotaxis mediated by Gi-coupled receptors. J Biol Chem. 1999;274(5):2824–2828. doi: 10.1074/jbc.274.5.2824. [DOI] [PubMed] [Google Scholar]
- Ohle SJ, Anandaiah A, Fabian AJ, Fine A, Kotton DN. Maintenance and repair of the lung endothelium does not involve contributions from marrow-derived endothelial precursor cells. Am J Respir Cell Mol Biol. 2012;47(1):11–19. doi: 10.1165/rcmb.2011-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9(6):702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Ridge SM, Bhattacharyya D, Dervan E, Naicker SD, Burke AJ, Murphy JM, O'Leary K, Greene J, Ryan AE, Sullivan FJ, Glynn SA. Secreted factors from metastatic prostate cancer cells stimulate mesenchymal stem cell transition to a pro-tumourigenic 'activated' state that enhances prostate cancer cell migration. Int J Cancer. 2018;142(10):2056–2067. doi: 10.1002/ijc.31226. [DOI] [PubMed] [Google Scholar]
- Runne C, Chen S. PLEKHG2 promotes heterotrimeric G protein betagamma-stimulated lymphocyte migration via Rac and Cdc42 activation and actin polymerization. Mol Cell Biol. 2013;33(21):4294–4307. doi: 10.1128/MCB.00879-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppel KM, Willison D, Kataoka H, Wang A, Zheng YW, Cornelissen I, Yin L, Xu SM, Coughlin SR. Essential role for Galpha13 in endothelial cells during embryonic development. Proc Natl Acad Sci U S A. 2005;102(23):8281–8286. doi: 10.1073/pnas.0503326102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi H, Ii M, Jujo K, Yokoyama A, Hagiwara N, Asahara T. Improved culture-based isolation of differentiating endothelial progenitor cells from mouse bone marrow mononuclear cells. PLoS One. 2011;6(12):e28639. doi: 10.1371/journal.pone.0028639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JP, Basch R, Shamamian P. Hematopoietic stem cells and endothelial cell precursors express Tie-2, CD31 and CD45. Blood Cells Mol Dis. 2004;32(1):168–175. doi: 10.1016/j.bcmd.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013;91(4):411–429. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM, Luo J, Benovic JL, Klein-Szanto A, Yagi H, Gutkind JS, Parsons RE, Kazanietz MG. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell. 2010;40(6):877–892. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Ueda H, Nagae R, Kozawa M, Morishita R, Kimura S, Nagase T, Ohara O, Yoshida S, Asano T. Heterotrimeric G protein betagamma subunits stimulate FLJ00018, a guanine nucleotide exchange factor for Rac1 and Cdc42. J Biol Chem. 2008;283(4):1946–1953. doi: 10.1074/jbc.M707037200. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prado J, Bracho-Valdes I, Cervantes-Villagrana RD, Reyes-Cruz G. Gbetagamma Pathways in Cell Polarity and Migration Linked to Oncogenic GPCR Signaling: Potential Relevance in Tumor Microenvironment. Mol Pharmacol. 2016;90(5):573–586. doi: 10.1124/mol.116.105338. [DOI] [PubMed] [Google Scholar]
- Vincent KM, Postovit LM. Matricellular proteins in cancer: a focus on secreted frizzled-related proteins. J Cell Commun Signal. 2018;12(1):103–112. doi: 10.1007/s12079-017-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kumamoto Y, Wang P, Gan X, Lehmann D, Smrcka AV, Cohn L, Iwasaki A, Li L, Wu D. Regulation of immature dendritic cell migration by RhoA guanine nucleotide exchange factor Arhgef5. J Biol Chem. 2009;284(42):28599–28606. doi: 10.1074/jbc.M109.047282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108(6):809–821. doi: 10.1016/S0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]