Abstract
Aortitis is an extremely rare condition, and it may mimic febrile neutropenia during cancer chemotherapy. A 55-year-old female diagnosed with T2N1M1 stage IV breast carcinoma received chemotherapy with EC (epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks). At the eleventh day after the first administration of EC, she developed a high-grade fever of 38 °C and stomatitis. We started the intravenous administration of antibiotics at hospitalization, because the laboratory data showed normal white blood cell values 3800/μl but a severe inflammatory reaction (CRP 25.13 mg/dl). The fever and high CRP value continued, and the WBC rose to 14,500/μl at 20th day. However, her condition was stable. On the 25th day after the administration of EC, she complained of back pain, so we performed computed tomography (CT) again and observed thickening of the rind surrounding the descending aorta and bilateral pleural effusion, indicating acute periaortitis. We performed examinations concerning vasculitis and connective tissue disease, but the values were all within normal ranges showing no relationship with other diseases. We stopped the administration of antibiotics on the 20th day, and while we did not administer corticosteroids, however, the fever was resolved and her WBC decreased. She was discharged on day 33, and the other chemotherapy was restarted with pertuzumab, trastuzumab and docetaxel. The patient has remained well without inflammatory reactions. CT at 90 days after EC therapy showed the resolution of the thickened rind of the descending aorta and no aneurysmic changes.
Keywords: Periaortitis, Breast cancer, Chemotherapy
Introduction
The development of a fever during cancer chemotherapy is mainly caused by febrile neutropenia, which is related to commonly localized infections (pharyngitis, skin infection, pneumonia, ano-rectal infection and urinary tract infections) [1]. However, fevers can sometimes be caused by non-infectious diseases such as drug allergies, connective tissue disease and malignant disease [2]. A few reports have further suggested a relationship with aortitis or periaortitis. However, only three cases of periaortitis induced by bevacizumab combination therapy [3] and granulocyte colony-stimulating factor [4, 5] have been reported.
We herein report a case of periaortitis with a high-grade fever during chemotherapy with epirubicin and cyclophosphamide for breast cancer.
Case report
A 55-year-old female had undergone breast screening at our hospital every year due to multiple bilateral breast cysts for several years. She had a history of three gravidas and three parities and no personal history of other cancers or diseases.
A physical examination revealed a left breast lump and swelling of the left axillary lymph nodes at the most recent screening. Mammography showed a tumor density with architectural distortion. Magnetic resonance imaging (MRI) revealed a left breast tumor measuring 3 cm in diameter and computed tomography (CT) showed multiple liver metastases.
We performed a core needle biopsy which confirmed the histological diagnosis of mucinous carcinoma. An Immuno-histochemical analysis indicated ER (−) PR (−) HER2 (3+) and a Ki67 labelling index of 50%. Given these findings, she was diagnosed with T2N1M1 stage IV breast carcinoma and started on chemotherapy with EC (epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks) therapy. Other drugs concomitantly administered are shown in Table 1.
Table 1.
All the concominant drugs in EC therapy
| Drug | Dose | Administration | |
|---|---|---|---|
| Route | Date | ||
| Epirubicin | 90 mg/m2 | i.v. | day 1 |
| Cyclophosphamide | 600 mg/m2 | i.v. | day 1 |
| Palonosetron | 0.75 mg | i.v. | day 1 |
| Dexamethasone | 13.2 mg | i.v. | day 1 |
| 8 mg | Oral | day 2–4 | |
| Aprepitant | 125 mg | Oral | day 1 |
| 85 mg | Oral | day 2–3 | |
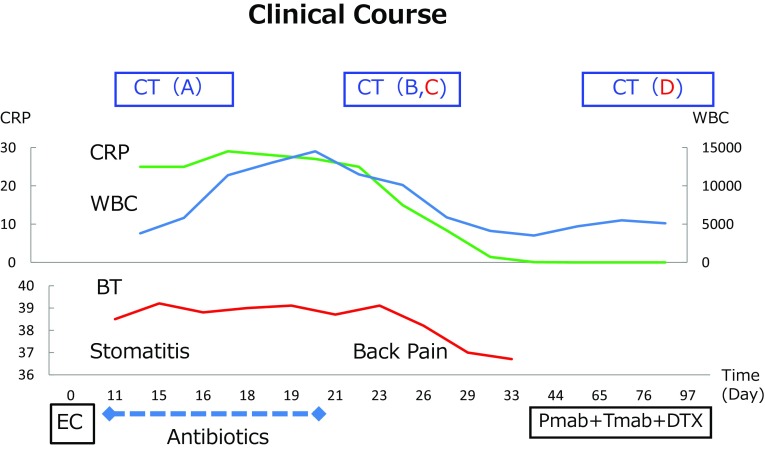
On the 11th day after the first administration of EC, she developed a high-grade fever of 38 °C and stomatitis (Fig. 2). We started the intravenous administration of antibiotics (CFPM 4 g/day × 3 days followed by MEPM 1 g/day × 2 days) at hospitalization, because the laboratory data showed normal white blood cell values 3800/μl but a severe inflammatory reaction (CRP 25.13 mg/dl). The change of laboratory data was shown in Table 2. The change of white blood picture indicates granulocytosis and no information for eosinophil or basophils, while moderate liver dysfunction was seen in the clinical course.
Fig. 2.
Clinical course; the change of body temperature (BT), CRP and WBC counts were indicated
Table 2.
Laboratory data from the time of EC therapy (Day 0) to dischage (Day 33)
| Normal range | Day 0 | Day 14 | Day 20 | Day 25 | Day 33 | |
|---|---|---|---|---|---|---|
| WBC (×103/μl) | 3.5–8.7 | 4.1 | 3.8 | 14.5 | 10.1 | 4.1 |
| Basophils (%) | 0.0–3.0 | 0.8 | 1.5 | |||
| Eosinophils (%) | 0.0–5.0 | 1.7 | 1.5 | |||
| Neutrophils (%) | 29.0–72.0 | 66.2 | 47 | 64 | 77 | 73.7 |
| Lymphocytes (%) | 18.0–56.0 | 21.1 | 7 | 5 | 4 | 14.7 |
| Monocytes (%) | 0.0–11.0 | 10.2 | 33 | 13 | 8 | 8.6 |
| RBC (×104/μl) | 374–495 | 435 | 387 | 329 | 324 | 342 |
| Hb (g/dl) | 11.0–15.1 | 14.1 | 12.4 | 10.4 | 10.4 | 11 |
| Platelets (×104/μl) | 13.9–38.3 | 23.6 | 33.9 | 41.8 | 55.2 | 61.9 |
| BUN (mg/dl) | 8.0–20.0 | 11.9 | 8.6 | 4.7 | 7.2 | 11.2 |
| Cr (mg/dl) | 0.46–0.79 | 0.84 | 1.02 | 0.69 | 0.64 | 0.74 |
| AST (U/l) | 13–30 | 33 | 207 | 61 | 60 | 39 |
| ALT (U/l) | 7–23 | 28 | 176 | 133 | 119 | 67 |
| LDH (U/l) | 124–222 | 195 | 302 | 256 | 211 | 150 |
| ALP (U/l) | 106–322 | 393 | 588 | 1608 | 1522 | 1055 |
| γGTP (U/l) | 9–32 | 67 | 214 | 378 | 311 | 214 |
| CRP (mg/dl) | 0.00–0.14 | 25.13 | 27.22 | 15.27 | 0.09 |
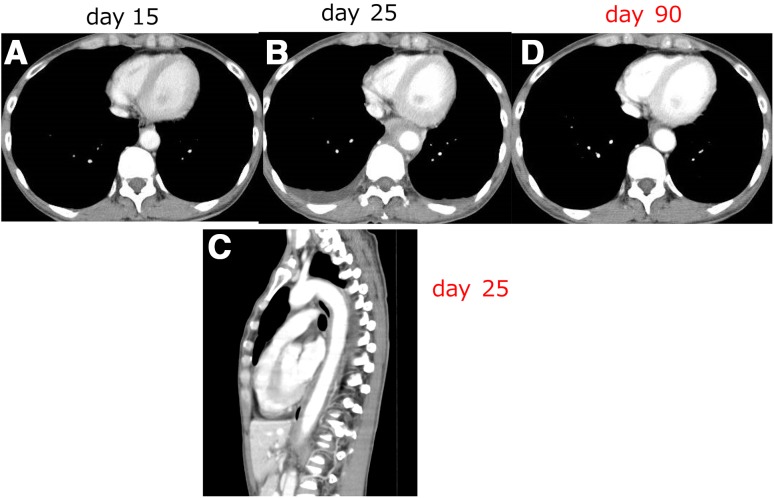
CT on the 14th day showed no abnormalities suggestive of the fever’s origin (Fig. 1a), and a blood culture showed no bacterial infection. The fever and high CRP value continued and WBC rose to 14,500/μl at day 20. However, her condition was stable.
Fig. 1.
CT findings on day 15 (a), on day 25 (b, c) and on day 90 after the administration of EC. The thickened rind of the descending aorta developed on day 25 (b), sagittal view shows the sickness of descending aorta at 4–10th vertebra (c) and recovered on day 90 (d)
On the 25th day after the administration of EC, she complained of back pain, so we performed CT again and observed thickening of the rind surrounding the descending aorta and bilateral pleural effusion (Fig. 1b, c) indicating acute periaortitis. We performed examinations concerning the vasculitis and connective tissue disease, but the values were all within normal ranges and showed no relationship with other diseases, PR3-ANCA 0.4 U/ml, MPO-ANCA 0.1 U/ml, IgG4 27.1 mg/dl, rheumatoid factor < 5 IU/ml, antinuclear antigen not detected, IgG 1529 mg/dl, IgA 201 mg/dl IgM 32 mg/dl. We stopped the administration of antibiotics on the 20th day, and while we did not administer corticosteroids, her fever resolved and her WBC decreased. She was discharged on day 33, and chemotherapy was restarted with pertuzumab, trastuzumab and docetaxel. The patient has remained well with no inflammatory reactions. CT at 90 days after the EC therapy showed the resolution of thickened rind of the descending aorta and shows no aneurysmic changes (Fig. 1d).
Discussion
A fever in cancer patients under the treatment of anti-cancer drugs is a common adverse event associated with neutropenia. It is critical to recognize neutropenic fever early and start empiric systemic antibacterial therapy promptly, in order to avoid progression to a septic status and possible death [6].
However, fevers in cancer patients can sometimes be caused by non-infectious disease, such as tumor necrosis, drug allergies, connective tissue disease and unknown reasons. A few reports have further suggested a relationship with aortitis or periaortitis. Cases of periaortitis induced by bevacizumab combination therapy [3] and granulocyte colony-stimulating factor [4] have been reported (Fig. 2).
The etiology of aortitis is varied. Takayasu’s disease and giant cell artritis cause chronic inflammation of the large vessels [7]. Infectious disease, aneurysmic changes and connective tissue disease such as rheumatoid arthritis and systemic lupus arthritis are also related to aortitis [8]. Periaortitis induced by a drug allergy has additionally been reported, with some cases diagnosed as anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis [9].
Periaortitis is also recognized as a clinical type of retroperitoneal fibrosis [10], sometimes being caused by IgG4-related disease [11]. However, no laboratory data in the present study suggested any relation to these diseases.
The acute inflammation that occurred in this case might have been caused by a drug allergy. Drug-induced periaorititis is rare, but some reports can be seen [12, 13].
Concerning the diagnosis of periaortitis, the most common clinical manifestations for the diagnosis are reported to be musculo-skeletal pain and lower limb pain, back pain and a fever [14]. 2-18F-Fluorine-2 deoxy-D glucose positron emission tomography (FDG-PET) has proven useful for diagnosing aortitis and assessing the patient’s response to therapy [15].
In the presented case, because of the development of the pain, we performed repeated CT, which allowed us to arrive at the diagnosis of periaortitis based on the finding of a thickened rind of the descending aorta. We were unable to perform FDG-PET. The symptoms of back pain and fever recovered within 2 weeks. Given the clinical course of this patient, we believe that some cases may be misdiagnosed as other diseases or a fever of unknown origin. Therefore, the true incidence of aortitis during cancer therapy may be difficult to determine.
Aortitis is an extremely rare condition; however, we should keep in mind this disease, when confronted with patients with a fever of unknown origin who complaints back or lower limb pain.
References
- 1.Sickles EA, Greene WH, Wiernik PH. Clinical presentation of infection in granulocytopenic patients. Arch Intern Med. 1975;135:715–719. doi: 10.1001/archinte.1975.00330050089015. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz HW. Fever of unknown origin or fever of too many origins? N Engl J Med. 2013;368:197–199. doi: 10.1056/NEJMp1212725. [DOI] [PubMed] [Google Scholar]
- 3.Murakami S, Saito H, Ohe M, et al. Periaortitis associated with anti-neutrophil cytoplasmic antibodies induced by bevacizumab combination therapy. Intern Med. 2013;52:589–591. doi: 10.2169/internalmedicine.52.6632. [DOI] [PubMed] [Google Scholar]
- 4.Adiga GU, Elkadi D, Malik SK, et al. Abdominal aortitis after use of granulocyte colony-stimulating factor. Clin Drug Investig. 2009;29:821–825. doi: 10.2165/11530790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Darie C, Boutalba S, Fichter P, et al. Aortitis after G-CSF injections. Rev Med Interne. 2004;25:225–229. doi: 10.1016/j.revmed.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 7.Caspary L. Inflammatory diseases of the aorta. Vasa. 2016;45:17–29. doi: 10.1024/0301-1526/a000491. [DOI] [PubMed] [Google Scholar]
- 8.Scheel AK, Meller J, Vosshenrich R, et al. Diagnosis and follow up of aortitis in the elderly. Ann Rheum Dis. 2004;63:1507–1510. doi: 10.1136/ard.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitahara T, Hiromura K, Maezawa A, et al. Case of propylthiouracil-induced vasculitis associated with anti-neutrophil cytoplasmic antibody (ANCA); review of literature. Clin Nephrol. 1997;47:336–340. [PubMed] [Google Scholar]
- 10.Zeina AR, Slobodin G, Naschitz JE, et al. Isolated periaortitis: clinical and imaging characteristics. Vasc Health Risk Manag. 2007;3:1083–1086. [PMC free article] [PubMed] [Google Scholar]
- 11.Mizushima I, Inoue D, Yamamoto M, et al. Clinical course after corticosteroid therapy in IgG4-related aortitis/periaortitis and periarteritis: a retrospective multicenter study. Arthritis Res Ther. 2014;16:R156. doi: 10.1186/ar4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiyohara H, Hisamatsu T, Matsuoka K, et al. Crohn’s disease in which the patient developed aortitis during treatment with Adalimumab. Intern Med. 2015;54:1725–1732. doi: 10.2169/internalmedicine.54.3853. [DOI] [PubMed] [Google Scholar]
- 13.Hamasaki H, Hakoshima M, Yanai H. Periaortitis induced by metformin. Diabetes Metab. 2015;41:344–345. doi: 10.1016/j.diabet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Loricera J, Blanco R, Hernandez JL, et al. Non-infectious aortitis: a report of 32 cases from a single tertiary centre in a 4-year period and literature review. Clin Exp Rheumatol. 2015;33:S-19-31. [PubMed] [Google Scholar]
- 15.Rozin AP, Bar-Shalom R, Strizevsky A, et al. Fever due to aortitis. Clin Rheumatol. 2007;26:265–267. doi: 10.1007/s10067-005-0134-9. [DOI] [PubMed] [Google Scholar]