Abstract
Purpose: Metastatic pancreatic adenocarcinoma has a very poor prognosis. Although irinotecan, oxaliplatin and leucovorin-modulated fluorouracil (FOLFIRINOX) significantly increases survival in advanced pancreatic cancer, compared to employing only gemcitabine (GEM), toxicities have tempered enthusiasm for its use.
Methods: This study retrospectively analyses the real-world clinical practice with full and attenuated doses of FOLFIRINOX in unselected patients with locally advanced unresectable or metastatic pancreatic cancer, treated at an Italian general hospital. Efficacy, tolerability, and toxicity were evaluated, and overall survival (OS) and progression-free survival (PFS) were estimated by Kaplan–Meier method.
Results: Fifty consecutive patients with advanced (13) or metastatic (37) pancreatic adenocarcinomas were treated with FOLFIRINOX at the Medical Oncology Unit, Piacenza General Hospital, North Italy. The first enrolled consecutive 18 patients (36%) of this series started the treatment with a full dose of the regimen, while the subsequent 32 (64%) consecutive patients received dose attenuation (−20% bolus fluorouracil and −25% irinotecan). In the entire group, the response rate, median OS, and median PFS were 30%, 10.1 months, and 5.6 months, respectively, with no differences in objective response in the 32 patients that received an attenuated dose compared with the 18 patients receiving a full dose of chemotherapy. However, neutropenia, anemia, fatigue, and vomiting were statistically increased in the 18 patients receiving a full dose compared with the 32 patients receiving an attenuated dose of FOLFIRINOX (p<0.05).
Conclusion: This study demonstrates the efficacy and tolerability of modified FOLFIRINOX in advanced and metastatic pancreatic cancer.
Keywords: metastatic pancreatic cancer, locally advanced pancreatic cancer, FOLFIRINOX, chemotherapy
Introduction
Pancreatic cancer is a major cause of cancer-related mortality in western countries and it is projected to emerge as the second leading cause of cancer-related deaths in the United States by 2030.1 Its prognosis is extremely poor, with a 5-year survival rate for all patients with pancreatic adenocarcinoma of approximately 6–8%.2–5 The vast majority of patients (80–90%) present with incurable metastatic or locally advanced unresectable disease.1 Gemcitabine (GEM) became the standard of care after a randomized trial that demonstrated an improvement in median overall survival (OS) compared with bolus of fluorouracil.6 The trial also evaluated the impact of GEM on “clinical benefit response” with an increase in clinical benefit responses in favor of GEM. Although well tolerated, the efficacy of single-agent GEM is marginal with a median OS of 5.65 months and a 1-year OS rate of 18% in patients with advanced pancreatic carcinoma.6 GEM has been evaluated in combination with a variety of cytotoxic and targeted agents, but no meaningful gains were observed in OS.7–14 Over the past few years, additional treatments have become available for patients with pancreatic cancer. In 2011, Conroy et al demonstrated that irinotecan, oxaliplatin, and leucovorin-modulated fluorouracil (FOLFIRINOX) was associated with a significant improvement in response rate (RR), progression-free survival (PFS), and clinically meaningful improvement in OS from 6.8 to 11.1 months when compared with GEM alone in metastatic pancreatic cancer.15 However, although patients’ quality of life improved compared with treatment with GEM alone, FOLFIRINOX was also associated with significant toxicities, such as neutropenia, diarrhea, and peripheral neuropathy. Some prospective and retrospective studies have confirmed the data of this trial in terms of OS, PFS, and safety, but the significant toxicities of FOLFIRINOX have tempered enthusiasm for a widespread use of FOLFIRINOX in community and academic practices.16–19 It must be emphasized that nanoparticle albumin-bound paclitaxel (nab-P) in combination with GEM has been approved for the treatment of advanced pancreatic cancer; this schedule is an equally valid front-line option for patients with metastatic pancreatic cancer and consists in the combination of GEM and nab-P.20 Some retrospective studies recently reported the comparison of treatment patterns, resource utilization, and cost of care in patients with metastatic pancreatic cancer treated with FOLFIRINOX or GEM plus nab-P.21,22 These studies compared, however, full-dose FOLFIRINOX and not reduced dose, and they concluded that patients treated with nab-P plus GEM vs FOLFIRINOX had similar treatment duration, but lower costs of outpatient prescriptions of treatment administration and of supportive care. Lower supportive care costs in the nab-P plus GEM cohort were mainly driven by lower utilization of pegfilgrastim and anti-emetics. Toxicity-related costs and drug acquisition costs should be considered when evaluating total cost of care. In addition, nanoliposomal irinotecan was approved by the FDA in combination with 5-fluorouracil (5-FU) and folinic acid for the treatment of patients with pancreatic cancer after disease progression following GEM-based therapy.23 The current standard of care for metastatic pancreatic cancer patients with a good performance status is either FOLFIRINOX or GEM plus nab-P.15,20,24 However, more recently, some prospective and retrospective studies evaluated the efficacy and the safety profile of modified FOLFIRINOX in advanced/metastatic pancreatic adenocarcinoma.24–32 To evaluate the real-world clinical practice with FOLFIRINOX, we conducted a retrospective study of 50 patients with advanced/metastatic pancreatic cancer; in 32 of these patients (64%) the treatment was planned with an attenuated dose, while in 18 cases (36%) the treatment was planned with full dose. This study was done at the Medical Oncology Unit, Piacenza General Hospital (North Italy). The efficacy and the toxicities of these schedules were assessed and reported.
Material and methods
We retrospectively analyzed the medical records of all patients with locally advanced or metastatic pancreatic cancer treated with FOLFIRINOX as first-line chemotherapy between July 2013 and July 2017 at the Medical Oncology Department, Piacenza General Hospital (North Italy). Patients were considered eligible if they have received at least one cycle of FOLFIRINOX. This study was a retrospective cohort, single-institution analysis. Its objectives were to evaluate the efficacy and safety of FOLFIRINOX in unselected patients with advanced/metastatic pancreatic cancer in a general hospital. The study was approved by the Institutional Review Board of Piacenza hospital and conducted according to the Helsinki Declaration. Written informed consent was obtained from each patient that underwent both full and attenuated doses of FOLFIRINOX. In addition, all the patients of this study signed written informed consent allowing research use of their clinical data. Patient characteristics, toxicities, RR, PFS, and OS were evaluated. The patients were not randomized. Standard practice for the first 18 patients was to start treatment using full-dose FOLFIRINOX, consisting of 85 mg of oxaliplatin per square meter given as a 2 hrs intravenous infusion, immediately followed by leucovorin at dose of 400 mg per square meter, delivered as a 2-hr intravenous infusion, with the addition, after 30 mins, of irinotecan at a dose of 180 mg per square meter, given as a 90-min intravenous infusion. This treatment was immediately followed by fluorouracil at a dose of 400 mg per square meter administered by intravenous bolus, followed by a continuous intravenous infusion of 2,400 mg per square meter over a 46-hr period every 2 weeks. The premedication regimen consisted of intravenous ondansetron and dexamethasone and prophylactic treatment with atropine was given to prevent cholinergic syndrome. Granulocyte-Colony Stimulating Factor (G-CSF) was used as a secondary prophylaxis, when required. Cycles were planned for 6 months (12 cycles), and were stopped before the 12 planned cycles, for tumor progression or occurrence of unacceptable toxicity. After the first 18 consecutive patients started with full dose of this regimen, a dose reduction was made with this percentage: bolus-fluorouracil reduced by 20% and irinotecan reduced by 25%. The regimen was changed on the basis of previous report suggesting that modest dose attenuation was associated with good tolerability and with efficacy comparable with results reported by Conroy T et al.15,32
All the patients were evaluated for toxicities with history, clinical examination, performance status, complete blood count, and metabolic panel with liver and kidney tests. Response assessment with imaging ultrasound and computerized tomography (CT) was obtained every 4–6 cycles of treatment using the Response Evaluation Criteria in Solid Tumors guidelines (RECIST, version 1.1).33 Patients who received chemotherapy for locally advanced/non resectable, but non-metastatic cancer underwent CT scan for response assessment. They were evaluated by a multidisciplinary team with surgeons and radiologists to establish the possibility of the surgical resection. All patients receiving the two schedules of FOLFIRINOX were eligible for toxicity analysis; patients were followed for PFS and OS.
OS was calculated from the start of the treatment to the date of death. PFS was calculated from the start of FOLFIRINOX to the date of radiographic progression (local or metastatic), or death. PFS and OS estimates were obtained using Kaplan–Meier method. Both OS and PFS were calculated for entire group and for the subgroups: 1) locally advanced; 2) metastatic pancreatic cancer; 3) patients that received full dose; and 4) patients that received attenuated dose of chemotherapy.
Results
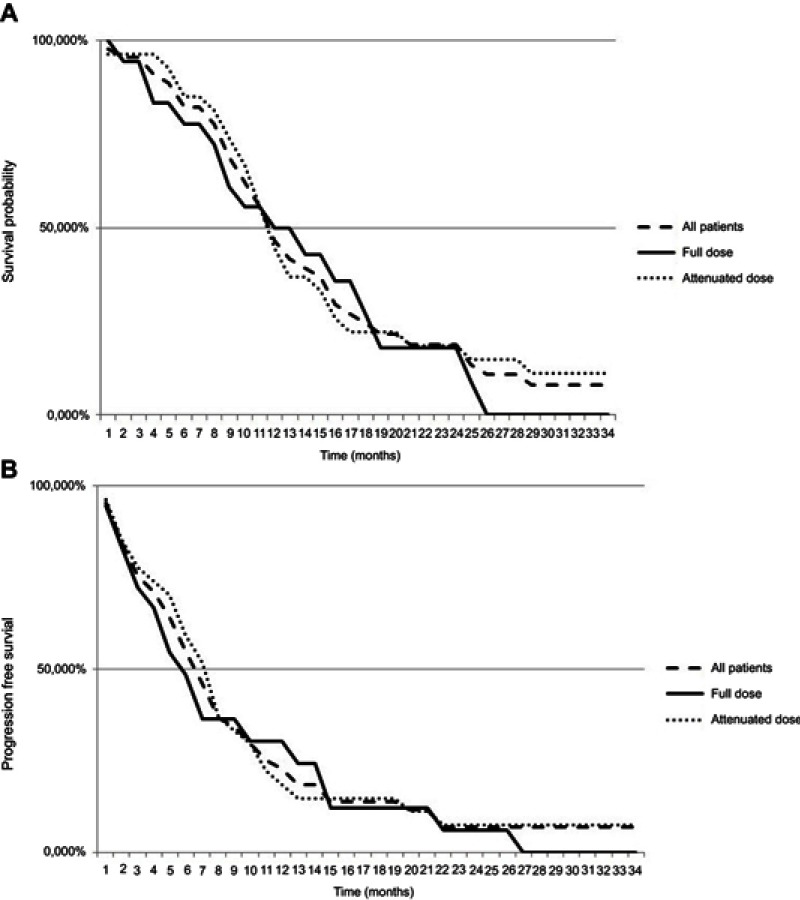
Between July 2013 and July 2017, 50 patients with advanced/metastatic pancreatic adenocarcinoma were treated with FOLFIRINOX at the Medical Oncology Unit, Piacenza General Hospital (North Italy). There were 13 patients with locally advanced unresectable disease and 37 with metastatic disease. All the patients were pathologically diagnosed with percutaneous (37 patients) or endoscopic (13 patients) ultrasound-guided biopsy. The median age was 65 years (range, 40–76), 24 patients were female (48%) and 26 (52%) men. The patient and disease characteristics are shown in Table 1. Eighteen patients received a full-dose FOLFIRINOX and 32 an attenuated dose; performance status was 0–1 in 42 cases (84%) and 2 in the remaining 8 patients (16%). Pancreatic tumor location was in the head in 27 patients (54%), in the body in 17 cases (34%), and in the tail in 6 cases (11.1%). The disease status (metastatic/locally advanced, Eastern Cooperative Oncology Group performance status, tumor location, site of metastases, age, and sex) did not differ between the two groups (Table1). The entire group of the 50 patients received a median of 8 cycles of FOLFIRINOX (range 1–12). The mean number of cycles performed was 9 (range 3–12) in the group of modified and 7 (range 1–12) in the group of standard FOLFIRINOX. Nine patients, 4 with modified and 5 with standard therapy received <4 cycles. One of the 18 patients receiving a full dose suspended the FOLFIRINOX chemotherapy after the first cycle because of a prolonged pancytopenia with febrile neutropenia. RR in locally advanced and metastatic disease are shown in Table 2. In the whole cohort, no patient showed a complete response and 15 patients (30%) had a partial response with an overall RR of 30%, while a further 8 patients (16%) showed stable disease. No differences in RR were registered between the two groups of patients receiving attenuated dose or full dose of chemotherapy (Table 2). The OS and PFS are shown in Table 3 and Figure 1A and B. For the entire cohort of patients, the median OS was 10.07 months and the median PFS was 5.63 months; in patients with locally advanced disease, the median OS was 11.47 months while median PFS was 6.27 months. There were no significant differences in OS (10.52 vs 10.07 months) and PFS (5.7 vs 6.13 months) in the 32 patients who received attenuated dose chemotherapy compared with the 18 patients that received a full dose.
Table 1.
Characteristics of the 50 patients with locally advanced/metastatic pancreatic cancer, 18 patients receiving a full dose compared with the 32 patients receiving an attenuated dose
| Characteristics | Patients n (%) | Full-dose patients (%) | Attenuated dose patients (%) | p-valuea | |||
|---|---|---|---|---|---|---|---|
| n=50 | (100) | n=18 | (36) | n=32 | (64) | ||
| Age, median (range) | 65 (40–76) | 65 (40–76) | 65 (41–75) | ||||
| Sex Male Female |
24 26 |
(48) (52) |
8 10 |
(44.4) (55.6) |
16 16 |
(50) (50) |
0.77 |
| Stage Metastatic cancer Advanced unresectable cancer |
37 13 |
(74) (26) |
14 4 |
(77.8) (22.2) |
23 9 |
(71.9) (28.1) |
0.75 |
| ECOG performance status | |||||||
| 0 | 25 | (50) | 8 | (44.4) | 17 | (53.1) | 0.77 |
| 1 | 17 | (34) | 7 | (38.9) | 10 | (31.3) | 0.76 |
| 2 | 8 | (16) | 3 | (16.7) | 5 | (15.6) | 1 |
| Tumor location | |||||||
| Head | 27 | (54) | 9 | (50) | 18 | (56.3) | 0.77 |
| Body | 17 | (34) | 7 | (38.9) | 10 | (31.2) | 0.76 |
| Tail | 6 | (12) | 2 | (11.1) | 4 | (12.5) | 1 |
| Sites of metastasis | |||||||
| Liver | 28 | (56) | 12 | (66.7) | 16 | (50) | |
| Limphnode | 18 | (36) | 8 | (44.4) | 10 | (31.6) | |
| Lung | 17 | (34) | 7 | (38.9) | 10 | (31.6) | |
| Peritoneum | 20 | (40) | 8 | (44.4) | 12 | (37.5) | |
| Biliary stent | 16 | (32) | 6 | (33.3) | 10 | (31.6) | |
Note: ap-value calculated using Fisher’s exact test.
Table 2.
Objective response in the 50 patients with locally advanced/metastatic pancreatic cancer, 18 patients receiving a full dose compared with the 32 patients receiving an attenuated dose
| Objective response | Patients n (%)(total=50) | p-valuea |
|---|---|---|
| CR (All patients=50) Patients receiving an attenuated doses (n=32) Patients receiving a full doses (n=18) |
0 (0) 0 (0) 0 (0) |
1 |
| PR (All patients=50) Patients receiving an attenuated doses (n=32) Patients receiving a full doses (n=18) |
15 (30) 11 (34.4) 4 (22.2) |
0.52 |
| SD (All patients=50) Patients receiving an attenuated doses (n=32) Patients receiving a full doses (n=18) |
8 (16) 6 (18.8) 2 (11.1) |
0.69 |
| PD (All patients=50) Patients receiving an attenuated doses (n=32) Patients receiving a full doses (n=18) |
20 (40) 13 (40.6) 7 (38.9) |
1 |
Note: ap-value calculated using Fisher’s exact test.
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Table 3.
OS and PFS in the 50 patients with locally advanced/metastatic pancreatic cancer, 18 patients receiving a full dose compared with the 32 patients receiving an attenuated dose
| Patients | Median OS (range) (months) |
Median PFS (range) (months) |
|---|---|---|
| All patients (n=50) | 10.07 (0.53-28.2) | 5.63 (0.4-21.93) |
| Receiving an attenuated doses (n=32) | 10.52 (0.53-28.2) | 5.70 (0.4-21.93) |
| Receiving a full doses (n=18) | 10.07 (1.0-24.3) | 6.13(0.77-21.5) |
| Metastatic (n=37) | 10.07 (0.53-28.2) | 5.63 (0.4-21.93) |
| Receiving an attenuated doses (n=23) | 10.52 (0.53-28.2) | 5.70 (0.4-21.93) |
| Receiving a full doses (n=14) | 10.90 (1.0-18.7) | 6.13 (0.77-14.43) |
| Locally advanced (n=13) | 11.47 (4.6-24.3) | 6.27 (1.3-21.5) |
| Receiving an attenuated doses (n=9) | 11.47 (4.6-15.6) | 5.98 (1.3-12.87) |
| Receiving a full doses (n=4) | 12.83 (15.9-24.3) | 7.10 (7.3-21.5) |
Abbreviations: OS, overall survival; PFS, progression-free survival.
Figure 1.
(A) Kaplan–Meier, overall survival. (B) Kaplan–Meier, progression-free survival.
Treatment-related toxicities are summarized in Table 4. Any-grade neutropenia was recorded in 28 patients (56%); in 16 (32%) it was grade 3/4, with febrile neutropenia in 3 patients (6%). Grade 3/4 thrombocytopenia was registered in 3 patients (6%). Grade 3/4 anemia was registered in 6 patients (12%). Non-hematologic toxicities were: fatigue in 30 patients (60%) and with grade 3/4 in 11 patients (22%); diarrhoea grade 1/2 in 13 (26%) and 3 cases with grade 3/4 (6%), vomiting in 32 cases (64%), with grade 3/4 in 4 patients (8%), and peripheral sensory neuropathy in 10 patients (20%), with grade 3/4 in 4 patients (8%). There were no deaths related to chemotherapy toxicities. The incidence of grade 3 or 4 neutropenia (55.6%vs 18.8%, p=0.01), anemia (27.8% vs 3.1%, p=0.02), fatigue (47.1%% vs 9.4, p=0.01), and vomiting (23.5% vs 0%, p=0.01) was significantly increased in the 18 patients receiving a full dose compared with the 32 patients treated with an attenuated dose of FOLFIRINOX. Fifteen patients (33.3%) had biliary stents in situ at the time of treatment with FOLFIRINOX and three cases of cholangitis were registered. G-CSF (Filgrastim) was given a secondary prophylaxis in the 16 patients (32%) who developed grade 3 or 4 neutropenia after the first or subsequent cycles. The G-CSF was given in 9 of the 18 patients (50%) treated with a full dose of the regimen and in 7 of the 32 patients treated with an attenuated dose (21.9%). The difference was not statistically significant (p=0.06). Only one (7.7%) of the 13 patients with locally advanced pancreatic cancer underwent surgical exploration with curative intent. However, peritoneal micrometastasis prevented surgical treatment.
Table 4.
Treatment-related toxicities in the 50 patients with locally advanced/metastatic pancreatic cancer, 18 patients receiving a full dose compared with the 32 patients receiving an attenuated dose
| Toxicity | Any grade n (%) |
Grade ¾ n (%) |
p-valuea |
|---|---|---|---|
| Neutropenia (All patients=50) | 28 (56) | 16 (32) | 0.01 |
| Receiving an attenuated dose (n=32) | 15 (46.9) | 6 (18.8) | |
| Receiving a full dose (n=18) | 13 (72.2) | 10 (55.6) | |
| Febrile neutropenia (all patients=50) | 3 (6) | 3 (6) | 1 |
| Receiving an attenuated dose (n=32) | 2 (6.3) | 2 (6.3) | |
| Receiving full dose (n=18) | 1 (5.6) | 1 (5.6) | |
| Thrombocytopenia (all patients=50) | 17 (34) | 3 (6) | 0.29 |
| Receiving an attenuated dose (n=32) | 11 (34.4) | 1 (3.1) | |
| Receiving a full dose (n=18) | 6 (39.3) | 2 (11.1) | |
| Anemia (all patients=50) | 29 (58) | 6 (12) | 0.02 |
| Receiving an attenuated dose (n=32) | 13 (40.6) | 1 (3.1) | |
| Receiving a full dose (n=18) | 16 (88.9) | 5 (27.8) | |
| Fatigue (all patients=50) | 30 (60) | 11 (22) | 0.01 |
| Receiving an attenuated dose (n=32) | 14 (43.8) | 3 (9.4) | |
| Receiving a full dose (n=18) | 16 (88.9) | 8 (47.1) | |
| Diarrhoea (all patients=50) | 13 (26) | 3 (6) | 0.29 |
| Receiving an attenuated dose (n=32) | 8 (25) | 1 (3.1) | |
| Receiving a full dose (n=18) | 5 (27.8) | 2 (12.5) | |
| Vomiting (all patients=50) | 32 (64) | 4 (8) | 0.01 |
| Receiving an attenuated dose (n=32) | 15 (46.9) | 0 (0) | |
| Receiving a full dose (n=18) | 17 (94.4) | 4 (23.5) | |
| Peripheral sensory neuropathy (all patients=50) | 10 (20) | 4 (8) | 0.13 |
| Receiving an attenuated dose (n=32) | 5 (15.6) | 1 (3.1) | |
| Receiving a full dose (n=18) | 5 (29.4) | 3 (17.6) |
Note: ap-value calculated using Fisher’s exact test.
At the time of last follow-up, 24 patients (48%) had received one or more further lines of palliative chemotherapy. Of these 24 patients, 16 patients (66.7%) received GEM/nab-P, 4 patients (16.7%) received capecitabine, and 4 patients (16.7%) received GEM. After completing treatment with FOLFIRINOX, 2 patients received consolidation radiotherapy (54 Gy in 30 fractions) with concomitant capecitabine.
Discussion
The purpose of this study was to evaluate the efficacy and safety of a FOLFIRINOX regimen in advanced/metastatic pancreatic cancer in an Italian general hospital. Despite the majority of the patients treated at our institution receiving an attenuated dose of FOLFIRINOX, our findings suggest that the efficacy was not compromised by this reduction, with a significant improvement in toxicity. In a recently reported prospective Phase II study, irinotecan and bolus fluorouracil were reduced by 25% and a decrease in adverse events (AEs) without negative impact on efficacy was registered.31 According to these results, some authors reported a modified regimen of FOLFIRINOX, resulting in lower hematological and non-hematological toxicity rates, with comparable efficacy outcomes.25–32 It has been demonstrated that in a modified FOLFIRINOX regimen, to preserve optimal RR and disease control rate in advanced/metastatic pancreatic cancer, the recommended values of cumulative relative dose intensity (CRDI) are for FOLFIRINOX >70% and 55%, respectively, and if a CRDI is >80%, primary G-CSF prophylaxis is recommended.26 The RR of 30% in our patients was not significantly different from the RR of 31.8% in the historical FOLFIRINOX-treated patients reported by Conroy et al, and was comparable with the data reported by other retrospective and prospective series.15,25–32 Survival outcomes, with an OS of 10.1 months and a PFS of 5.6 months in patients with metastatic pancreatic cancer, were also comparable with the data reported in the prospective randomized trial.15 Our results support the assumption that these outcomes can be achieved outside of a clinical study, also in a general hospital in the real-world practice. As expected, the outcomes for patients with locally advanced pancreatic cancer were better than those for patients with metastatic disease, with a median OS of 11.47 months and a median PFS of 6.3 months. Again, this was comparable with previously published retrospective and prospective series.25–32
However, although FOLFIRINOX is associated with a significant increase in RR and OS in metastatic pancreatic cancer compared to GEM alone, we note that the toxicities of FOLFIRINOX have tempered enthusiasm for a full-dose use in community and academic centers.31 In the present study, the majority of patients with advanced/metastatic pancreatic cancer were treated with an attenuated dose of FOLFIRINOX; this choice was based also on the results of previous studies which demonstrated lower toxicity rate, with comparable efficacy outcomes with full-dose FOLFIRINOX.25,30,32 Our experience suggests that modest dose attenuations, in conjunction with the use of growth factor support as a secondary prophylaxis, are associated with less toxicity than observed in the FOLFIRINOX-treated patients reported by Conroy et al.15 Although 22% of our patients had biliary stents for obstruction, we observed only three cases of cholangitis which recovered after antibiotic therapy. Chemotherapy remains the mainstay of treatment for patients with metastatic pancreatic cancer. The alternative sequence of the active drugs would be interesting as recently reported in metastatic pancreatic cancer.34,35 FOLFIRINOX and Gem/nab-P are both approved for the first-line treatment of this cancer; however, present different median OS (11.1 vs 8.5 months), PFS (6.6 vs 3.3 months), and objective RR (32% vs 33%) in favor of FOLFIRINOX, they present moreover different toxicities and Gem/Nab-P shows a better tolerance.15,20 It must be emphasized that the results of a Markov analytical decision model showed FOLFIRINOX to be cost-effective, especially with the reduced cost of using generic drugs, as performed in our series of patients in which generic drugs were used.36
Conclusion
In conclusion, with the limitations of a retrospective study our results demonstrate that attenuated dose of FOLFIRINOX is feasible in outpatients with metastatic/advanced pancreatic cancer in a general hospital, in the real-world clinical practice, the dose reduction as performed in our patients did not compromise efficacy, but reduced the number of AE.
It must be emphasized, however, that a rising incidence of pancreatic cancer is projected to become the second leading cause of cancer-related deaths, so there is a great need to develop more effective therapies, and the management of this disease will require prioritizing clinical trial enrolment whenever possible, rather than prioritizing standard of care treatment.29
Disclosure
LC declares consulting and advisory roles with Astrazeneca and Merck, and travel, accommodations and expenses from Pfizer, Ipsen, and Celegene. The other authors report no conflicts of interest in this work.
References
- 1.Lambert A, Gavoille C, Conroy T. Current status on the place of FOLFIRINOX in metastatic pancreatic cancer and future directions. Terap Adv Gastroenterol. 2017;10(8):631–645. doi: 10.1177/1756283X17713879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics. Cancen J Clin. 2017;67:7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer. 2009;45:931–939. doi: 10.1016/j.ejca.2008.11.018 [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre AC, Maurel J, Boutreux S, et al. Pancreatic cancer: incidence, treatment and survival trends-1175 cases in Calvados (France) from 1978 to 20002. Gastroenterol Clin Biol. 2009;33:1045–1051. doi: 10.1016/j.gcb.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 5.Philip PA. Locally advanced pancreatic cancer: where should we go from here? J Clin Oncol. 2011;29:4066–4068. doi: 10.1200/JCO.2011.37.2532 [DOI] [PubMed] [Google Scholar]
- 6.Burris HA, Moore MJ, Andersen J, et al. Improvement in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403 [DOI] [PubMed] [Google Scholar]
- 7.Conroy T, Mitry E. Chimiothérapie de l’adénocarcinome du pancréas métastatique: defis et espoirs. Bull Cancer. 2011;98:1438–1446. doi: 10.1684/bdc.2011.1494 [DOI] [PubMed] [Google Scholar]
- 8.Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB 3rd. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: eastern cooperative oncology group trial E2297. J Clin Oncol. 2002;20(15):3270–3275. doi: 10.1200/JCO.2002.11.149 [DOI] [PubMed] [Google Scholar]
- 9.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multi center, phase III trial of the swiss group for clinical cancer research and the central European cooperative oncology group. J Clin Oncol. 2007;25(16):2212–2217. doi: 10.1200/JCO.2006.09.0886 [DOI] [PubMed] [Google Scholar]
- 10.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23(15):3509–3516. doi: 10.1200/JCO.2005.06.023 [DOI] [PubMed] [Google Scholar]
- 11.Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increate tumor response rate. J Clin Oncol. 2004;22(18):3776–3783. doi: 10.1200/JCO.2004.12.082 [DOI] [PubMed] [Google Scholar]
- 12.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the cancer and leukemia group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–3622. doi: 10.1200/JCO.2010.28.1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: southwest oncology group-directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–3610. doi: 10.1200/JCO.2009.25.7550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore MJ, Gldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525 [DOI] [PubMed] [Google Scholar]
- 15.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 16.Chllmma MK, Cook N, Dhani NC, et al. FOLFIRINOX for advanced pancreatic cancer: the princess margaret cancer centre experience. Br F Cancer. 2016;115:649–654. doi: 10.1038/bjc.2016.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Valentin T, Biagi J, Bourque S, et al. Eastern canadian colorectal cancer consensus conference: standards of care for the treatment of patients with rectal, pancreatic, and gastrointestinal stromal tumours and pancreatic neuroendocrine tumours. Curr Oncol. 2013;20:e455–e464. doi: 10.3747/co.20.1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moorcraft SY, Khan K, Peckitt C, et al. FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: the Royal Marsden experience. Clin Colorectal Cancer. 2014;13:232–238. doi: 10.1016/j.clcc.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 19.Rombousts SJ, Mungroop TH, Heilmann MN, et al. FOLFIRINOX in locally advanced and metastatic pancreatic cancer: a single centre cohort study. J Cancer. 2016;7:1861–1866. doi: 10.7150/jca.16279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim GP, Parisi MF, Patel BM, Pelletier CL, Belk KW. Comparison of treatment patterns, resource utilization, and cost of care in patients with metastatic pancreatic cancer treated with first-line nab-paclitaxel plus gemcitabine or FOLFIRINOX. Expert Rev Clin Pharmacol. 2017;10(5):559–565. doi: 10.1080/17512433.2017.1302330 [DOI] [PubMed] [Google Scholar]
- 22.McBride A, Bonafede M, Cai Q, et al. Comparison of treatment patterns and economic outcomes among metastatic pancreatic cancer patients initiated on nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Expert Rev Clin Pharmacol. 2017;1–8. doi: 10.1080/17512433.2017.1365598 [DOI] [PubMed] [Google Scholar]
- 23.Rahman FAU, Ali S, Saif MW. Update on the role of nanoliposomal irinotecan in the treatment of metastatic pancreatic cancer. Ther Adv Gastroenterol. 2017;10(7):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducreux M, Seufferlein T, Van Laethem JL, et al. Systemic treatment of pancreatic cancer revisited. Semin Oncol. 2019;46(1):28–38. [DOI] [PubMed] [Google Scholar]
- 25.Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42:1311–1315. doi: 10.1097/MPA.0b013e31829e2006 [DOI] [PubMed] [Google Scholar]
- 26.Lee JC, Kim JW, Ahn S, et al. Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: using cumulative relative dose intensity. Eur J Cancer. 2017;76:125–133. doi: 10.1016/j.ejca.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 27.Li X, Mao T, Zhang Q, et al. Modified-FOLFIRINOX in metastatic pancreatic cancer: A prospective study in Chinese population. Cancer Lett. 2017;10(406):22–26. doi: 10.1016/j.canlet.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto M, Hikichi T, Suzuki T, et al. Successful chemotherapy with modified FOLFIRINOX for pancreatic acinar cell carcinoma. Clin J Gastroenterol. 2017;10(6):564–569. doi: 10.1007/s12328-017-0785-5 [DOI] [PubMed] [Google Scholar]
- 29.Yoshida K, Iwashita T, Uemura S, et al. A multicenter prospective phase II study of first-line modified FOLFIRINOX for unresectable advanced pancreatic cancer. Oncotarget. 2017;8(67):111346–111355. doi: 10.18632/oncotarget.22795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghorani E, Wong HH, Hewitt C, Calder J, Corrie P, Safety BB. Efficacy of modified FOLFIRINOX for advanced pancreatic adenocarcinoma: a UK single-centre experience. Oncology. 2015;89(5):281–287. doi: 10.1159/000439171 [DOI] [PubMed] [Google Scholar]
- 31.Stein SM, James ES, Deng Y, et al. Final Analysis of phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. BJC. 2016;114:737–743. doi: 10.1038/bjc.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunturu KS, Yao X, Cong X, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol. 2013;30(1):361. doi: 10.1007/s12032-012-0361-2 [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 34.Taieb J, Rinaldi Y, Pointet A-L, et al. Gemcitabine plus nab-paclitaxel until progression or given sequentially with 5-fluorouracile plus irinotecan (FOLFIRI.3) for first-line treatment of metastatic pancreatic ductal adenocarcinoma (mPDAC): a randomized phase II study (PRODIGE 37-FIRGEMAX). J Clin Oncol. 2018;36:4107. doi: 10.1200/JCO.2018.36.15_suppl.4107 [DOI] [Google Scholar]
- 35.Assenat E, Fouchardiere CDL, Mollevi C, et al. Sequential treatment with Nab-paclitaxel plus gemcitabine and folfirinox in metastatic pancreatic adenocarcinoma: GABRINOX phase II results. J Clin Oncol. 2018;36(15 suppl):4109. [Google Scholar]
- 36.Tam VC, Ko YJ, Mittmann N, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20:90–106. doi: 10.3747/co.20.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]