Abstract
Background
Pairing a bout of high intensity exercise with motor task practice can enhance motor learning beyond task practice alone, which is thought, in part, to be facilitated by an exercise-related increase in brain-derived neurotrophic factor (BDNF). The purpose of the current study was to examine the effect of different exercise intensities on BDNF levels and motor learning while controlling for exercise-related energy expenditure.
Methods
Forty-eight young, healthy participants were assigned to one of three groups: high-intensity exercise [High], low-intensity exercise [Low], or quiet rest [Rest]. The duration of the exercise bouts were individually adjusted so that each participant expended 200 kilocalories regardless of exercise intensity. BDNF was measured before and after exercise or rest. After exercise or rest, all participants practiced a 3-dimensional motor learning task, which involved reach movements made to sequentially presented targets. Retention was tested after 24-hours. BDNF genotype was determined for each participant to explore its effects on BDNF and motor learning.
Results
All participants equally improved performance, indicated by a reduction in time to complete the task. However, the kinematic profile used to control the reach movement differed by group. The Rest group travelled the shortest distance between the targets, the High group had higher reach speed (peak velocity), and the Low group had earlier peak velocities. The rise in BDNF post-exercise was not significant, regardless of exercise intensity, and the change in BDNF was not associated with motor learning. The BDNF response to exercise did not differ by genotype. However, performance differed between those with the polymorphism (Met carriers) and those without (Val/Val). Compared to the Val/Val genotype, Met carriers had faster response times throughout task practice, which was supported by higher reach speeds and earlier peak velocities.
Conclusion
Results indicated that both low and high-intensity exercise can alter the kinematic approach used to complete a reach task, and these changes appear unrelated to a change in BDNF. In addition, the BDNF genotype did not influence BDNF concentration, but it did have an effect on motor performance of a sequential target reach task.
Keywords: motor learning, exercise intensity, acute exercise, brain-derived neurotrophic factor
1. Introduction
Previous work in both animals and humans suggests that an acute bout of aerobic exercise may create a neural environment that promotes synaptic plasticity and long-term potentiation (LTP) (Cotman, Berchtold, & Christie, 2007; Gómez-Pinilla, Ying, Roy, Molteni, & Edgerton, 2002; Loprinzi & Frith, 2018). This exercise-induced facilitation at the neural level is associated with enhanced learning and memory (Knaepen, Goekint, Heyman, & Meeusen, 2010; Tomporowski, 2003; Vaynman, Ying, & Gomez‐Pinilla, 2004). Studies in humans have demonstrated greater performance of a visuomotor tracking task during acquisition (Mang, Snow, Campbell, Ross, & Boyd, 2014) and at retention (Mang et al., 2014; Roig, Skriver, Lundbye-Jensen, Kiens, & Nielsen, 2012; Skriver et al., 2014; Thomas, Beck, et al., 2016) when task practice was paired with a bout of high-intensity aerobic exercise compared to task practice alone. Studies that have examined the effects of acute aerobic exercise on both cognitive and motor performance and learning have suggested brain-derived neurotrophic factor (BDNF) as a potential exercise-related molecular mediator of enhanced learning.
Brain-derived neurotrophic factor is an activity-dependent protein that can regulate synaptic plasticity by modulating activity of the N-methyl-D-asparate (NMDA) receptor, a key glutamatergic receptor involved in learning and memory (Bath & Lee, 2006; Bramham & Messaoudi, 2005). Studies in animal models have demonstrated that inhibiting BDNF impaired LTP (Fritsch et al., 2010) and prevented the beneficial effects of exercise on learning (Vaynman et al., 2004). Importantly, an exercise-induced increase in BDNF concentration has been demonstrated in the brains of animal models (Vaynman et al., 2004) and systemically in humans (Knaepen et al., 2010; Mang et al., 2014; Skriver et al., 2014). It has been suggested that the exercise-related increase in BDNF may facilitate motor learning by providing a favorable environment for neural plasticity (Cotman & Berchtold, 2002; Cotman et al., 2007).
Several studies have identified a dose-response relationship regarding exercise intensity and its effects on BDNF and motor learning, which suggested greater effects following high-intensity exercise (Gustafsson et al., 2009; Thomas, Beck, et al., 2016; Vega et al., 2006). However, previous research has not controlled for overall energy expenditure (total work) when comparing high- and low-intensity exercise bouts, and it is therefore unclear if results were related to differences in exercise intensity or differences in total energy expenditure. This is particularly relevant given that moderate effects on BDNF concentration have been found with low-intensity exercise (Etnier et al., 2016; Ferris, Williams, & Shen, 2007; Gustafsson et al., 2009). In addition, a bout of moderate-intensity exercise enabled participants to maintain motor performance during practice better than participants who did not exercise prior to task practice (Snow et al., 2016). Research has demonstrated that BDNF mediates neural plasticity, and that acute aerobic exercise can increase BDNF concentration, but there is little evidence that supports a direct association between an exercise-induced increase of BDNF and enhanced motor learning. One study found an association between BDNF concentration immediately following acute aerobic exercise and motor skill retention (Skriver et al., 2014), while other studies found no correlation between the change in BDNF concentration and the change in motor skill performance (Helm et al., 2017; Mang et al., 2014). However, an association between increased BDNF and learning is evident in the cognitive learning literature (Berchtold, Castello, & Cotman, 2010; Piepmeier & Etnier, 2015; Winter et al., 2007), and therefore warrants continued investigation.
A common single-nucleotide polymorphism in the BDNF gene is present in approximately 30% of humans (Shimizu, Hashimoto, & Iyo, 2004), which causes a valine to methionine substitution at codon 66 (Val66Met). The effects of the polymorphism are largely unknown, but the Val/Met polymorphism has been suggested to impair the intracellular trafficking and activity-dependent release of BDNF (Egan et al., 2003). The polymorphism has also been associated with altered cortical plasticity (Cheeran et al., 2008; Kleim et al., 2006), impaired motor skill learning (McHughen et al., 2010), and diminished BDNF secretion in response to acute exercise (Leech & Hornby, 2017). However, other studies have demonstrated no effect of the polymorphism on motor task performance (Kleim et al., 2006), motor learning (Helm et al., 2017; Voti et al., 2011), or BDNF secretion in response to exercise (Helm et al., 2017). Given these noted discrepancies, the potentially moderating effects of the polymorphism on motor learning and the BDNF response to exercise need to be more intently considered.
The purpose of the current study was to determine the effects of a single bout of high- and low-intensity exercise on BDNF concentration and learning of a 3-dimensional (3D) serial target task, while controlling for overall energy expenditure of the exercise. We hypothesized that individuals in both exercise groups (low- and high-intensity) would demonstrate a greater increase in BDNF compared to a no-exercise rest group. We also expected individuals in the exercise groups to perform better on the motor task during acquisition and at retention compared to the rest group. Furthermore, we expected low- and high-intensity exercise to effect BDNF and task performance similarly, indicating the importance of energy expenditure. A second, exploratory aim was to examine the effect of the BDNF genetic polymorphism on the BDNF response to exercise and motor learning. Given the disparity in the research to date, we did not have an a priori hypothesis for the effect of the polymorphism.
2. Methods
2.1. Participants
Forty-eight non-disabled individuals between the ages of 20 and 29 years (Table 1) were recruited to participate from the local university community. To participate, individuals had to be right hand dominant (Oldfield, 1971), have no current or recent neurological symptoms, have no pain in the right upper extremity, and have no contraindications to strenuous exercise. All participants gave written informed consent prior to enrollment in the study. The Institutional Review Board at the University of South Carolina approved all study procedures, and the study was conducted in accordance with the Declaration of Helsinki. Participants received monetary compensation for their involvement in the study.
Table 1.
Group Demographics
| High | Low | Rest | |
|---|---|---|---|
| n | 16 | 16 | 16 |
| Age | 23.19 ± 2.9 | 22.81 ± 3.3 | 24.06 ± 3.4 |
| Sex (n: Female/Male) | 9/7 | 9/7 | 13/3 |
| VO2peak (ml/kg/min) | 40.33 ± 6.2 | 41.27 ± 4.2 | 41.12 ± 8.2 |
| Fitness Level (n) | |||
| Very Poor | 1 | 0 | 0 |
| Poor | 1 | 2 | 1 |
| Fair | 2 | 2 | 1 |
| Good | 4 | 4 | 5 |
| Excellent | 3 | 3 | 4 |
| Superior | 5 | 5 | 5 |
| BDNF Genotype (n) | |||
| Val/Val | 14 | 8 | 10 |
| Val/Met | 2 | 8 | 6 |
Values represent group mean ± standard deviation.
2.2. Experimental Design
Participants completed three experimental sessions. Session one consisted of a cycle-based graded exercise test (GXT) to estimate peak aerobic capacity (VO2peak). After session one, a randomized block design separated the participants into one of three experimental conditions: high-intensity exercise (High), low-intensity exercise (Low), and rest (Rest). Session two, a minimum of 48 hours after the GXT, included either rest or exercise and practice of the 3D serial target task (STT). Session three took place twenty-four hours after session two and consisted of a retention test of the motor task.
2.3. Maximal Exercise Test Procedure
Participants were asked to refrain from strenuous physical activity for a minimum of 24 hours prior to the GXT. After the test protocol was thoroughly explained and the participant was comfortably seated on the cycle ergometer (Monark 828 E, Monark Exercise, Vansbro, Sweden), a two-minute warm-up at a resistance of 0 kiloponds (kp) was completed. Following the warm-up, resistance was increased to 2 kp and the test began. The test continued in 2-minute stages and the resistance was incrementally increased 0.5 kp at the start of each stage. Throughout the test, participants were instructed to maintain a pedaling cadence of 60 revolutions per minute (rpm), which was indicated by a metronome. At the end of each stage, heart rate (Polar Electro, Kempele, Finland) and Borg’s rating of perceived exertion (RPE) (Borg, 1982) were recorded. Termination of the test occurred if the participant was not able to maintain the cadence, the individual reached volitional exhaustion, and/or age-predicted maximal heart rate (220-age) was met. Following the test, estimated VO2peak was calculated with the following calculation (Medicine, 2013), and participants were classified by fitness level (very poor, poor, fair, good, excellent, or superior; see Table 1).
Fitness level classifications were used to evenly distribute fitness level between the experimental groups (high-intensity, low-intensity, and rest), ensuring that all groups had similar levels of fitness.
2.4. Acute Exercise Procedure
Results from the GXT were used to define the exercise prescription during session two. All participants were required to maintain a pedaling cadence of 60 rpm. Exercise intensity (the resistance of the cycle ergometer) was determined as a percentage of maximum resistance obtained during the GXT. For individuals in the High group, resistance was initially set at 80% of max until the individual expended 100 kilocalories (kcals) of energy. At this point, the resistance was reduced 0.5 kp and exercise continued until another 100 kcals of energy were expended. For the Low group, resistance was set at 40% of max until the individual expended 200 kcals. The duration of the exercise bout was individually modified so that each participant (regardless of intensity) expended 200 kcals of energy (High average duration = 16.75 min, Low average duration = 28.67 min) using the equation:
Heart rate and RPE were recorded every two minutes throughout the exercise bout. Individuals in the Rest group were required to sit quietly for 20 minutes. Use of electronic devices and sleeping were prohibited. For all groups, blood lactate was assessed with a portable lactate analyzer (Lactate Plus, Nova Biomedical, Waltham, MA) immediately before and after either rest or exercise.
2.5. Blood Sample Collection and BDNF Analysis
Immediately before and after exercise or rest, 10 ml of blood was obtained from an antecubital vein into Vacutainer tubes containing EDTA. All samples were centrifuged immediately after collection and kept on wet ice until the end of the experimental session. At this time plasma was aliquoted and stored at −80°C for further analysis. Plasma BDNF concentrations were later analyzed in duplicate using sandwich ELISA kits (PromoCell, Heidelberg, Germany) per the manufacturer’s instructions. An intra-assay coefficient of variation (CV) was calculated between duplicate samples to assess the relative variability between the two measurements. The average CV across all BDNF assays was 8.36%.
2.6. Serial Target Task
Although many of the previous studies that investigated exercise-enhanced motor learning used a continuous tracking task (Mang et al., 2014; Roig et al., 2012; Skriver et al., 2014; Thomas, Beck, et al., 2016; Thomas, Johnsen, et al., 2016), the benefits of high-intensity acute aerobic exercise has also been demonstrated with a discrete sequence-specific implicit motor learning task (Mang, Snow, Wadden, Campbell, & Boyd, 2016). The serial targeting task used in the aforementioned study (Mang et al., 2016) was similar to the traditionally used serial reaction time task (Nissen & Bullemer, 1987), and involved movements of a computer mouse to accurately capture targets. We recently transformed this 2-dimensional (2D) task into 3D space, which necessitated the use of whole-arm movements to capture targets (Baird & Stewart, 2017). This 3D version of the STT involves the same motor learning principles as the 2D version, but the additional motor demands of moving unsupported in 3D space more closely represents movements performed in the real world.
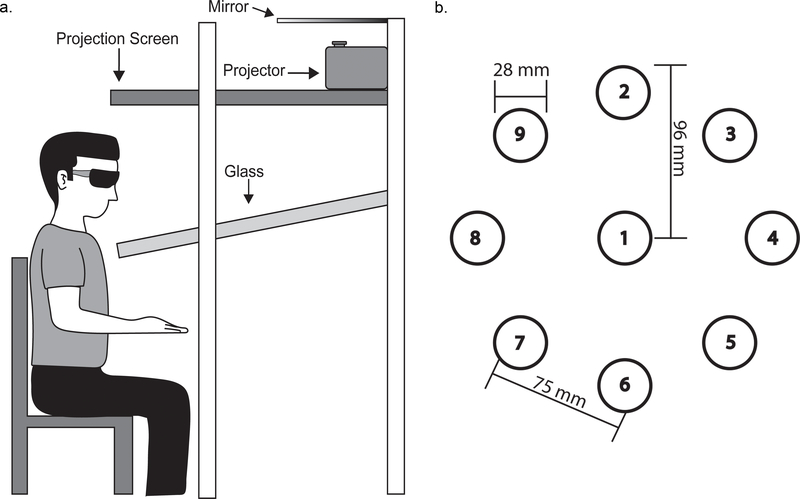
Task Setup and Design: Participants sat facing a virtual display (Innovative Sports Training Inc., Chicago, IL), and the task was projected down into the workspace in front of them (Fig. 1A). Specialized glasses were worn to provide 3D visualization of the targets. Eight targets, represented as red spheres (28 mm in diameter), were positioned equidistant in a circle (96 mm radius) with an additional target in the center (nine total target placements, Fig. 1B). An electromagnetic marker was secured to the right index finger. Finger position was indicated by a white sphere (25 mm diameter) providing a visual representation of the movement and position data during reaching. One target was presented at a time, and participants were required to move the white sphere (cursor) through 3D space to capture the target. For a target to be considered “hit”, the center of the cursor was required to be within 5 mm of the center of the target for 500 msec. Targets were presented in eight-target sequences under two sequence conditions: random and repeated. The two sequence types provide a distinction between improvements in generalized motor control (random sequences) and changes associated with implicit motor learning (repeated sequences). Users were not made aware of the repeated sequence, and were instructed to move to all targets as quickly and accurately as possible. The two sequence types were matched for difficulty using Fitts’ law (Fitts & Peterson, 1964). The distance between the targets was the same regardless of sequence type (total distance = 93.8 cm; see (Baird & Stewart, 2017) for a detailed description).
Figure 1.
Sequential Target Task (STT) setup. A. Side view of a participant sitting at the virtual display. Stereoscopic glasses provided a 3-dimensional view of the virtual environment. Virtual targets were sent from the projector, reflected off the mirror, and presented in the area below the glass. B. Representation of the nine possible target locations. Each target was 28 mm in diameter. Targets were presented in a circular array with a radius of 96 mm and a tangent distance between any adjacent targets of 75 mm. The repeated sequence consisted of targets 1, 8, 6, 5, 9, 4, 8, 2.
Throughout the task, the position of the electromagnetic marker was sampled at a rate of 120Hz, and data were analyzed with a custom MATLAB script (Mathworks, Inc.; Natick, MA). Response time, the total time to complete an eight-target sequence, was the primary measure of task performance (Baird & Stewart, 2017; Brodie, Borich, & Boyd, 2014; Brodie, Meehan, Borich, & Boyd, 2014; Mang et al., 2016). Other kinematic variables (total distance travelled to complete a sequence, mean peak velocity during a sequence, and mean time to peak velocity) were assessed to define the kinematic profile of the reach, and assess how the kinematics changed over time by group. Distance travelled: The total distance of the hand path when completing an 8-target sequence was measured to assess how exercise specifically affected a spatial component of reach performance. Peak Velocity: The highest measured velocity (cm/s) for each reach made during a sequence was found and averaged across an 8-target sequence. Time to Peak Velocity: For each reach to a target, the time it took to obtain peak velocity was analyzed and averaged for each 8-target sequence. An earlier time-to-peak velocity indicates a greater reliance on feedforward motor control, an important characteristic of sequence-specific motor learning (Sainburg & Schaefer, 2004; Schmidt, 1975). Peak velocity and time-to-peak velocity are both temporal components of reach performance.
Baseline: Prior to exercise or rest, all participants completed one trial of the random sequence, which served as an introduction to the task and a baseline measurement of task performance. Acquisition: The acquisition period immediately followed the second blood draw after exercise or rest. Task procedures were the same for all groups. Sequence presentation alternated between the two sequence types throughout task practice. A total of 144 sequences (72 random alternating with 72 repeated) were completed. Total time to complete the acquisition period was approximately 40 minutes. Retention: All participants returned 24 hours (± 2 hours) after experimental session two for a retention test, where an additional 72 sequences (36 random alternating with 36 repeated) were completed. All other STT procedures were identical to task practice the previous day. Explicit Awareness Testing: After completion of the retention period, explicit awareness of the repeated sequence was assessed. All participants completed six explicit awareness tests. The participants viewed three different sequences during each test. The participants were then asked if they recognized the presence of the repeated sequence. Three of the six tests contained the repeated sequence.
2.7. Saliva Collection and BDNF genotyping
After the completion of explicit awareness testing, 2 ml of saliva was collected with an Oragene collection kit (DNA Genotek, Ottawa, Ontario, Canada) from each participant to determine BDNF genotype. Genetic analysis was carried out at AKESOgen genomics lab (Norcross, GA) with a TaqMan genotype assay (c_11592758_10) per manufacturer’s instructions.
2.8. Data Analysis
Data from each sequence type (random or repeated) were combined and averaged into blocks of nine trials for analysis (acquisition = eight blocks of nine trials, retention = four blocks of nine trials). Assumptions of normality of the distribution for these variables were explored through histograms and assessed with the Shapiro-Wilk’s test for normality. A reciprocal transformation was applied to any non-normal data. Statistical analyses were completed using SPSS software (SPSS 24.0, IBM Corporation, Armonk, NY) and significance was set at p < 0.05 for all tests. Fisher’s least significant difference was used to further investigate any significant differences.
2.8.1. Motor Task Performance
Baseline: A one-way analysis of variance (ANOVA) was performed to determine if there were any group differences in performance (response time) at baseline. Acquisition: A univariate generalized linear model (GLM) was used to assess the between group differences for response time and all kinematic variables (distance travelled, peak velocity, and time to peak velocity). Fixed factors for group (High, Low, Rest), sequence type (random, repeated), and time (blocks 1 to 8) were included in the model. Partial eta squared (η2) was used to estimate the effect size of any differences in task performance (η2 of 0.01–0.059 = small effect; 0.06–0.139 = medium effect; ≥0.14 = large effect) (Cohen, 1988). Retention: Retention was defined as the degree of forgetting between the end of the acquisition phase (Block 8) and the beginning of the retention phase (Block 9). A non-significant main effect of time indicated performance was maintained at retention. A GLM that included fixed factors for group, sequence type, and time (Block 8 to 9) were included in the model to assess if response time, distance travelled, peak velocity, or time to peak velocity changed over time. Explicit Awareness: Awareness of the repeated sequence was assessed as a measure of specificity (correctly identifying 2 of the 3 negative tests) and sensitivity (correctly identifying 2 of the 3 positive tests). A subsequent repeated measures ANOVA was performed to compare response times between individuals who were deemed to be aware of the repeated sequence and individuals who were not aware of the repeated sequence.
2.8.2. BDNF Response
The BDNF response was assessed as the percent change from measurement time point 1 (before exercise or rest) to measurement time point 2 (after exercise or rest) to account for varying BDNF concentrations at baseline. A one-way ANOVA for BDNF percent change was performed to determine the effect of exercise intensity on BDNF concentration. To identify specific characteristics that may be related to a large BDNF response, we performed Pearson’s correlations to compare VO2peak, body mass index, and percent change of blood lactate to the change in BDNF across all groups.
2.8.3. BDNF and Motor Learning
A series of bivariate comparisons (Pearson’s correlations) were conducted to compare the change in BDNF to the change in motor task performance. The percent change in BDNF was compared to the percent change of response time from the baseline trial to the first trial of acquisition, from the baseline trial to the last trial of acquisition, and the baseline trial to the first trial of retention. Similarly, the percent change in BDNF was compared to the percent change of response time from the last trial of the acquisition period to the first trial of the retention period.
2.8.4. BDNF Genotype
To determine if the BDNF polymorphism affected the BDNF response to exercise, participants in the exercise groups (High and Low) were re-grouped by genotype (Val/Val or Val/Met) regardless of their original experimental condition. An independent samples t-test was performed comparing the percent change in BDNF between the genotype groups. We also investigated if BDNF genotype influenced motor task performance. All participants (High, Low, and Rest) were re-grouped by genotype regardless of their original experimental condition. A GLM with fixed factors for time (Block 1 to 8), sequence type (random, repeated), and genotype (Val/Val, Val/Met) was used to analyze the effects of the polymorphism on response time, distance traveled, peak velocity, and time to peak velocity.
3. Results
3.1. Participant Randomization and Acute Exercise Procedure
The distribution of fitness level between the groups was not significantly different (χ2 = 3.45, p = 0.969). During the exercise intervention in Session two, individuals in the High group attained a significantly higher exercise heart rate (t = 7.40, p < 0.001) and RPE (t = 8.59, p < 0.001) compared to the Low group (Table 2). Blood lactate concentration was significantly increased for the High group compared to the Low and Rest groups (F(2,44) = 46.18, p < 0.001). Taken together, the data indicate that a high level of exercise intensity was achieved in the High group, a low level of exercise intensity was maintained in the Low group, and no change in physical activity level was observed in the Rest group.
Table 2.
Exercise Response Characteristics
| High | Low | Rest | |
|---|---|---|---|
| Exercise HR | 168.14 ± 15.1* | 132.27 ± 10.7 | - |
| Exercise RPE | 17.25 ± 1.7* | 11.19 ± 2.2 | - |
| Change in Lactate (mmol/l) | 5.09 ± 0.6* | 0.63 ± 0.3 | −0.06 ± .3 |
| Percent Change BDNF | 164.53 ± 465.56 | 152.76 ± 324.75 | 37.8 ± 195.65 |
Values represent group mean ± standard deviation.
p < 0.05 for difference between groups.
3.2. Motor Task Performance
Baseline: Response time did not significantly differ by group (F(2,45) = 1.78, p = 0.18) prior to exercise or rest.
Acquisition: All groups significantly reduced response time during the acquisition period (F(7,734) = 32.158, p < 0.001, η2 = 0.24; Fig. 2). Changes in performance occurred quickly; significantly faster times were evident as early as the second block of practice (mean difference = 2.35 sec). Furthermore, all groups completed the repeated sequence faster than the random sequence throughout acquisition (F(1, 734) = 6.68, p = 0.01, η2 = 0.01, difference between sequences evident at trial 8). While all groups improved performance over time as expected, there were no between group differences in performance (F(2, 734) = 2.287, p = 0.10), and no group by time interaction (F(2, 734) = 0.20, p = 0.99). Group differences remained non-significant after adjusting for sex, BDNF genotype, and fitness level (F(2, 754) = 1.995, p = 0.137). However, group differences were evident when examining the kinematic profiles of the reach movements (Fig. 3). The Rest group completed the sequences with a significantly shorter distance travelled than the High (mean difference = 7.45 cm) and Low (mean difference = 6.9 cm) groups (F(2, 734) = 7.99, p < 0.001, η2 = 0.02). Conversely, the High group reached with a higher peak velocity. A significant group effect (F(2,734) = 8.85, p < 0.001, η2 = 0.02) showed that the High group had faster reach speed than both the Low (mean difference = 2.55 cm/s) and Rest (mean difference = 3.23 cm/s) groups. Time to peak velocity occurred earlier for the Low group (F(2,734) = 22.78, p < 0.001, η2 = 0.06) compared to the High (mean difference = 20 msec) and the Rest (mean difference = 20 msec) groups. All results remained significant after adjusting for sex, BDNF genotype, and fitness level (Distance: F(2,754) = 9.97, p < 0.001; Peak Velocity: F(2,754) = 31.35, p < 0.001; Time to Peak Velocity: F(2,754) = 12.44, p < 0.001).
Figure 2.
Response Time. A. Response time (sec) to complete a sequence across the acquisition phase and the retention phase for all groups. Each data point consists of an average of nine sequences. Error bars represent standard error. Error bars ascend from the marker for the random sequences and descend from the marker for the repeated sequences. No group differences in response time were evident. B. Response time for the High-intensity group. C. Response for the Low-intensity group. D. Response time for the Rest group.
Figure 3.
Kinematic Variables. Distance of the hand path (A), peak velocity (B), and time to peak velocity (C) across the acquisition phase and the retention phase for all groups. Each data point consists of an average of nine sequences. Error bars represent standard error. Error bars ascend from the marker for the random sequences and descend from the marker for the repeated sequences. A. The Rest group travelled the shortest distance when completing a sequence compared to the High and Low groups (p < 0.001). B. The High group had the highest peak velocity compared to the Rest and Low groups (p < 0.001). C. The Low group had the earliest time to peak velocity compared to the Rest and High groups (p < 0.001).
Retention: Response time was maintained at retention (F(1,180) = 0.65, p = 0.80), and did not change differently by group from the end of acquisition to the start of retention (F(2,180) = 0.15, p = 0.86). Similar results were found for all kinematic variables. For each kinematic variable, performance was maintained at retention (distance: F(1,180) = 0.05, p = 0.82; peak velocity: F(1,180) = 0.16, p = 0.69; time to peak velocity: F(1,180) = 0.02, p = 0.90), and there were no group by time interactions (distance: F(2,180) = 0.58, p = 0.56; peak velocity: F(2,180) = 0.02, p = 0.98; time to peak velocity: F(2,180) = 0.33, p = 0.72), which indicated that exercise did not affect how performance changed between the end of acquisition and the start of retention. Further, all results remained non-significant after adjusting for sex, BDNF genotype, and fitness level (Response Time: F(2,184) = 0.074, p =0.79; Distance: F(2,184) = 0.062, p =0.80; Peak Velocity: F(2,184) = 0.196, p = 0.66; Time to Peak Velocity: F(2,184) = 0.017, p = 0.90).
Explicit Awareness: No participant could recall the repeated sequence from memory. A total of seven participants (14.58%) were deemed to have awareness of the repeated sequence, however, there was no significant difference in performance between those who were aware of the sequence and those who were not aware (F(1,46) = 2.587, p = 0.12).
3.3. BDNF Response
We were unable to obtain blood samples for three participants (two in the Low group and one in the Rest group), and therefore BDNF data was not available for those individuals. Although both exercise groups had a relatively large increase in BDNF concentration compared to the Rest group (Table 2), there was no significant difference between the groups (F(2,42) = 0.60, p = 0.55). Lack of significance is likely due to the high variability within each group. Individuals in the High group had a BDNF response that ranged from −87.04% to 1740.25%. In the Low group, the BDNF response ranged from −84.57% to 986.77%. Similarly, a range of −95.51% to 685.29% was found in the Rest group. Given the large inter-individual variability within each group, a secondary analysis was conducted that assessed the variability (standard deviation) of the exercise groups (High and Low) normalized to the variability of the Rest group, which is assumed to represent the natural variability of a biological sample (Atkinson & Batterham, 2015). After accounting for the natural variability of BDNF, standard deviations for both exercise groups were still large (High = ± 422.46%, Low = ± 259.19%), indicating that the BDNF response to a bout of aerobic exercise had high inter-individual variability. Furthermore, no significant correlations were found between the percent change in BDNF and the change in VO2peak, body mass index, and percent change of blood lactate (VO2peak: r = −0.02, p = 0.90; body mass index: r = −0.005, p = 0.97; change in lactate: r = −0.097, p = 0.53).
3.4. BDNF and Motor Learning
No relationship was found between the percent change of BDNF concentration and motor learning, measured as the percent change of response time from baseline (baseline to first trial of acquisition: r = −0.091, p = 0.56; baseline to last trial of acquisition: r = −0.198, p = 0.20; baseline to first trial of retention: r = −0.213, p = 0.17). There was no relationship between the change in BDNF and the degree of forgetting for the random sequence (r = 0.185, p = 0.22), but there was a significant correlation between the change in BDNF and the degree of forgetting for the repeated sequence (r = 0.309, p = 0.04). There was no relationship between the percent change in lactate and motor learning, measured as the percent change of response time from baseline to the end of acquisition (r = 0.121, p = 0.43).
3.5. BDNF Genotype
BDNF Response: The distribution of BDNF genotype did not deviate from Hardy-Weinberg equilibrium (χ2 = 1.92, p = 0.17), as results from genetic testing revealed the Met allele was present in 33% of the study population (Val/Met n = 16, Val/Val n = 32, no participants had genotype Met/Met; see Table 1 for group distribution). There was no difference in BDNF concentration at baseline between the two genotype groups (Val/Val = 419.43 ± 381.06 pg/ml, Val/Met = 334.29 ± 244.05 pg/ml, t = 0.75, p = 0.46). A large mean difference was evident for the BDNF response to exercise between the genotype groups (Val/Val = 211.17% ± 454.04, Val/Met = 15.65% ± 99.83), but this difference was not statistically significant (t = 1.18, p = 0.25). The BDNF response to exercise was highly variable regardless of genotype or exercise group (Fig. 4).
Figure 4.
BDNF exercise response by BDNF genotype. The Low-intensity group is represented by the gray bars and the High-intensity group is represented by the black bars. Each bar represents an individual participant. The BDNF response was not significantly different by BDNF genotype.
Motor Learning: A significant difference for response time was found between the two genotype groups (F(1,750) = 14.40, p < 0.001, η2 = 0.02). Individuals with the polymorphism (Val/Met) completed the task faster than individuals without the polymorphism (Val/Val) (mean difference = 0.84 seconds; Fig. 5A). Group differences were also found when examining the kinematic variables that control reach movements. Val/Val participants had a significantly shorter hand path when completing a sequence compared to the Val/Met participants (F(1,750) = 26.09, p < 0.001, η2 = 0.03, mean difference = 7.68 cm; Fig. 5B). Conversely, the Val/Met group demonstrated an advantage for the temporal components of performance. Met carriers had significantly higher peak velocities compared to the Val/Val group (F(1,750) = 73.77, p < 0.001, η2 = 0.09, mean difference = 5.10 cm/s; Fig. 5C), as well as earlier peak velocities (F(1,750) = 20.43, p < 0.001, η2 = 0.03, mean difference = 0.014 sec; Fig. 5D). All results remained significant after adjusting for sex, experimental condition, and fitness level (Response Time: F(2,754) = 41.67, p < 0.001, Distance: F(2,754) = 18.42, p < 0.001; Peak Velocity: F(2,754) = 136.03, p < 0.001; Time to Peak Velocity: F(2,754) = 43.36, p < 0.001).
Figure 5.
Response time and kinematic variables of performance by BDNF genotype. Response time, distance of the hand path, peak velocity, and time to peak velocity across the acquisition phase and the retention phase for both genotype groups. Error bars represent standard error. Error bars ascend from the marker for the random sequences and descend from the marker for the repeated sequences. A. The Val/Met genotype had significantly lower response times for both sequence types compared to the Val/Val genotype (p = 0.002). B. The Val/Val genotype had a significantly shorter distance when completing a sequence compared to the Val/Met genotype (p < 0.001). C. The Val/Met genotype had higher peak velocities when reaching to the targets compared to the Val/Val genotype (p < 0001). D. No difference in time to peak velocity was present between the genotypes (p = 0.05).
4.0. Discussion
The purpose of the current study was to examine the effects of high- and low-intensity acute aerobic exercise on BDNF concentration and motor learning when exercise bouts were matched for overall energy expenditure. While an acute bout of exercise prior to practice was not associated with changes in response time, exercise affected the kinematic approach that controlled reach performance. Individuals in the Rest group increased spatial accuracy to improve response time. Conversely, individuals in the exercise groups altered temporal components of performance to improve response time. The High group completed reaches with higher peak velocities, and the Low group had earlier peak velocities. Changes in performance were not associated with changes in BDNF concentration. Additionally, while BDNF genotype did not have an effect on the BDNF response with acute exercise, it did have an effect on motor task performance.
4.1. Motor Task Performance and Learning
Our results indicated that an acute bout of exercise, whether at a high- or low-intensity, did not enhance motor learning or motor performance of a 3D sequential target task. These results conflict with findings from previous studies that have demonstrated an association between high-intensity exercise and motor learning (Mang et al., 2014; Mang et al., 2016; Roig et al., 2012; Skriver et al., 2014; Thomas, Johnsen, et al., 2016). These disparate findings may be related to the demands of the motor task examined. An effect of exercise on learning was not evident in a recent study that included a motorically demanding task (walking on a split-belt treadmill) (Helm et al., 2017). However, when task demands have been less motorically challenging (small movements of the thumb or wrist), an effect of exercise has been demonstrated (Mang et al., 2014; Roig et al., 2012; Skriver et al., 2014; Thomas, Johnsen, et al., 2016). The less motorically demanding tasks may enable more neural resources to be allocated to the cognitive components of the task. Therefore, exercise may have enhanced the cognitive aspects of performance rather than the motor aspects, which nonetheless resulted in improved performance. This is supported by research that has demonstrated a robust relationship between acute exercise and cognitive function (Ferris et al., 2007; Tomporowski, 2003; Winter et al., 2007).
Differences in the results of the current study compared to others that have demonstrated a positive effect of exercise on motor learning may also be related to the type of motor task. In the current study, the motor task involved a sequence of discrete movements; many previous studies utilized motor tasks that involved continuous movements. Discrete versus continuous movement tasks rely on different principles of learning, and discrete movements may have additional motor control demands involving the timing of the transition between movement onset and offset (Huys, Studenka, Rheaume, Zelaznik, & Jirsa, 2008). It is possible that exercise may differentially influence motor learning based on the type of movements involved in the task (Chambaron, Berberian, Delbecque, Ginhac, & Cleeremans, 2009). Further, the brain regions that primarily support discrete sequence learning (basal ganglia) are distinct from those that support learning of sequences involving continuous movements (cerebellum) (Doya, 2000), suggesting that acute exercise may preferentially affect the cerebellum compared to other brain regions.
One additional explanation for the difference in our results compared to previous studies relates to the timing of the exercise bout in relation to task practice. Whereas exercise was completed prior to task practice in the current study, other studies have demonstrated an effect of acute exercise on motor memory when exercise occurred after task practice (Roig et al., 2012; Skriver et al., 2014; Thomas, Beck, et al., 2016). Exercise prior to task practice may prime the nervous system for learning (Mang et al., 2014), whereas exercise after task practice is thought to enhance memory consolidation possibly through an exercise-induced increase in neurochemicals such as norepinephrine or BDNF (McGaugh, 2006; Roig et al., 2012). Our choice to complete exercise before task practice may explain why there was an effect of exercise during acquisition (i.e. the kinematic approach used to complete the task), but no effect on retention as measured by forgetting. This suggests that exercise might influence skill acquisition when completed before task practice, but may have a greater effect on motor learning when completed after task practice.
While there were no group differences in STT performance (response time) or learning, exercise, at both a high- and low-intensity, altered the kinematic approach that controlled reach performance. The Rest group had the shortest distance of the hand path compared to both exercise groups (Fig. 3A), and the change in hand path distance occurred in parallel with response time; this finding is consistent with previous work from our lab using the same task (Baird & Stewart, 2017). Conversely, individuals in the exercise groups altered temporal components of performance to reduce response time. Throughout acquisition and retention, the High group had higher peak velocities (faster movement execution) compared to the Rest and Low groups (Fig. 3B). During a reach movement, velocity of the hand is encoded by the motor cortex (M1), and greater cortical activity is associated with a higher velocity (Moran & Schwartz, 1999; Wang, Chan, Heldman, & Moran, 2007). A bout of high-intensity exercise has been shown to enhance intracortical M1 excitability and plasticity (Mang et al., 2014; Singh, Neva, & Staines, 2014), which in turn may facilitate faster movement execution. The Low group had significantly earlier peak velocities while completing the motor task compared to the High and Rest groups (Fig. 3C). An earlier time to peak velocity indicates increased reliance on feedforward control, which is characterized by movements that are less dependent on sensory feedback and instead rely more on preplanning of the action (Adams, 1971; Sainburg & Schaefer, 2004; Seidler-Dobrin & Stelmach, 1998). Brain regions associated with feedforward motor control include M1, premotor cortex, and basal ganglia (Seidler, Noll, & Thiers, 2004). These regions are also involved in motor planning and programming of sequential motor patterns (Halsband, Ito, Tanji, & Freund, 1993; Hikosaka, Nakamura, Sakai, & Nakahara, 2002; Houk & Wise, 1995; Mitz, Godschalk, & Wise, 1991). A bout of low-moderate intensity exercise has been shown to result in disinhibition within M1 (McDonnell, Buckley, Opie, Ridding, & Semmler, 2013; Smith, Goldsworthy, Garside, Wood, & Ridding, 2014). Less inhibitory influences in motor regions may promote greater network communication between brain regions facilitating motor planning and feedforward control. Future studies that investigate the effect of exercise on performance and learning may further distinguish the effects of differing exercise intensities on specific aspects of motor performance (spatial vs. temporal, planning vs. execution), and identify the neural correlates associated with behavioral change.
4.2. BDNF Response
Although the average change in BDNF following exercise was large for both the High and Low groups, the BDNF response was not statistically significantly different from the Rest group. Regardless of group, the change in BDNF concentration was highly variable between individuals. After using the variability in the Rest group to account for the natural variability of BDNF over time, large inter-individual differences were still present in both exercise groups, indicating that the BDNF response to exercise also varies greatly by person. Inter-individual variability of BDNF concentration, both at rest and in response to exercise, has yet to be fully examined, and is often overlooked in favor of reporting average group differences. In addition, some studies have identified individuals with a large BDNF response as outliers and removed them from data (Skriver et al., 2014), which may mask the true variability of the change in BDNF concentration. Distinguishing specific characteristics that identify individuals as “responders” versus “non-responders” would help elucidate the mechanisms related to the activity-dependent release of BDNF in humans.
4.3. BDNF and Motor Learning
An exercise related increase in BDNF has been suggested to facilitate neuroplasticity associated with changes in motor behavior following exercise (Cotman & Berchtold, 2002). In the current study, the change in motor performance over time was not associated with changes in BDNF, with the exception of the degree of forgetting for the repeated sequence wherein a greater change in BDNF was related to greater levels of forgetting. The significant relationship between the change in BDNF and the degree of forgetting for the repeated sequence suggests BDNF may have a greater influence on memory consolidation compared to its effect on skill acquisition. This is supported by evidence that has demonstrated a greater effect of acute exercise on motor skill retention when exercise occurred after as opposed to before motor task practice (Roig et al., 2012; Skriver et al., 2014; Thomas, Beck, et al., 2016).
The lack of a correlation between the change in BDNF and motor learning corresponds to results of others who also demonstrated no relationship between BDNF concentration and motor learning (Helm et al., 2017; Mang et al., 2014). However, the lack of a BDNF-motor learning relationship stands in contrast to the relationship between BDNF and cognitive learning (Tomporowski, 2003). This may indicate that BDNF preferentially enhances the neural processes associated with cognitive learning as opposed to the neural processes that support motor learning. Work in animal models has also demonstrated a robust association between BDNF and learning (Gómez-Pinilla et al., 2002; Van Praag, Shubert, Zhao, & Gage, 2005; Vaynman et al., 2004). However, an important distinction between research in animal models and humans is the method of measuring BDNF. In humans, directly assessing central levels of BDNF is not feasible, and researchers must rely on peripheral measurements. While BDNF is thought to cross the blood brain barrier bidirectionally (Pan, Banks, Fasold, Bluth, & Kastin, 1998; Pan & Kastin, 2004), it is unclear whether peripheral measurement provides a true representation of the BDNF concentration centrally. For example, when BDNF concentrations were simultaneously measured from the internal jugular vein and the radial artery during exercise, there was a difference of 84% between the two locations (Rasmussen et al., 2009). These results indicate that peripheral measurements of BDNF may not accurately capture the exercise-related increase in central BDNF, and the potential facilitatory effects of BDNF should not automatically be discounted if a change in peripheral concentration is not found.
While continued investigation of the relationship between BDNF and motor learning in humans is necessary, other potential mechanisms should also be considered. For example, neurotransmitters such as norepinephrine, epinephrine, and dopamine increase after high-intensity exercise and have been associated with enhanced learning (Skriver et al., 2014; Winter et al., 2007), yet these substrates have not been heavily examined, and their mechanistic properties are still largely unknown. Dopamine may be of particular relevance to exercise-enhanced motor learning as the presence of the dopamine D2 receptor polymorphism has been suggested to be involved in the effect of acute exercise on motor learning (Mang et al., 2017). In addition, changes in intracortical excitability and plasticity are evident post-exercise (Mang et al., 2014; McDonnell et al., 2013; Singh, Duncan, Neva, & Staines, 2014; Smith et al., 2014), but the neural mechanisms that support these changes are undetermined. Establishing a causal mechanism linking exercise and changes in cortical excitability could provide valuable insight into exercise-enhanced motor learning.
4.4. BDNF Genotype
The BDNF polymorphism has been associated with a diminished BDNF response with exercise (Leech & Hornby, 2017). However, the variability observed in the BDNF response in the current study could not be explained by the presence of the Met allele. While individuals in the Val/Val group had a larger average change in BDNF compared to the Val/Met group, there was no statistically significant difference between groups (Fig. 4); the high variability between individuals within each group may have contributed to this finding. Furthermore, the small number of participants in the Val/Met group that completed an acute bout of exercise may have also limited statistical power to find differences. However, the findings of the current study, along with previous work (Helm et al., 2017), suggest the effect of the BDNF polymorphism on activity-dependent release of BDNF secretion in humans may be limited. Identifying and accounting for the source of variability in the BDNF response to exercise beyond the presence of the BDNF polymorphism is needed.
While there was no difference in the BDNF response between genotype groups, a difference in motor task performance was present. Individuals with the Met allele completed the task faster (faster response times) than Val/Val homozygotes. This was an unexpected finding as previous research has either shown no effect of the Met allele on motor learning (Helm et al., 2017; McHughen, Pearson-Fuhrhop, Ngo, & Cramer, 2011), or individuals with the Met allele have demonstrated impaired motor learning compared to those without the Met allele (Fritsch et al., 2010; McHughen et al., 2010). It is important to note that while Val/Met individuals completed the task faster, the overall change in performance was similar between the genotype groups, which indicated group differences in motor performance rather than motor learning.
The kinematic profiles that controlled reach performance also differed by genotype. The Val/Val group had a significantly shorter hand path than the Val/Met group, while the Val/Met group had higher and earlier peak velocities than the Val/Val group (Fig. 5). Greater performance in the spatial domain for the Val/Val genotype has been shown in previous work (McHughen et al., 2010), however, to our knowledge, this is the first study to demonstrate an advantage in the temporal domain of motor performance for the Val/Met genotype. Previously, an advantage for individuals with the Met allele had only been observed in a few select cognitive tasks (Gajewski, Hengstler, Golka, Falkenstein, & Beste, 2011, 2012; Getzmann, Gajewski, Hengstler, Falkenstein, & Beste, 2013). The basis for these differences in motor performance is unknown, but may be related to dissimilarities in brain structure between the genotype groups (Bueller et al., 2006; Pezawas et al., 2004), which may result in functional differences. For example, individuals with the polymorphism modulated activation in a broad network of sensorimotor brain regions differently than those without the polymorphism in response to motor training (McHughen et al., 2010). These findings suggest that structural and functional differences between the genotype groups may alter motor behavior in a way that can be both favorable (temporal advantage) and unfavorable (spatial disadvantage).
4.5. Limitations
The between-subjects design of the current study presents an inherent amount of variability that may have been prevented with a within-subjects design. Particularly, the variability that surrounds the baseline levels of BDNF and the BDNF response to exercise might have been limited if all participants completed each of the experimental conditions. However, a within-subjects design would introduce a practice effect on the motor task that would have prevented an accurate measure of motor learning. The chosen time course for the retention test may have also limited our ability to find an effect of exercise on learning. Other studies have shown that the effects of exercise on motor learning may not be apparent until at least seven days after the initial learning phase (Roig et al., 2012; Skriver et al., 2014; Thomas, Beck, et al., 2016; Thomas, Johnsen, et al., 2016). It is possible that a retention test at a later time point might have shown an exercise-induced effect on learning. The timing of the exercise bout may also influence the effect of acute exercise on motor learning. Exercising after motor task practice may enhance consolidation processes thereby improving motor learning (Roig et al., 2012). Furthermore, while we attempted to keep energy expenditure constant between the exercise groups, work levels were estimated rather than directly measured through calorimetry, and thus small differences in energy expenditure may have existed. Finally, we only examined a single neural substrate (BDNF). Future research should consider investigating the effects of other neurochemicals that may have exercise-induced effects on the CNS (e.g. dopamine).
5.0. Conclusions
The current study indicated that a session of acute aerobic exercise at a specific energy expenditure does not influence peripheral BDNF concentration or motor learning. However, exercise at both a high- and low-intensity modified the kinematic approach that controls reach movements. High-intensity exercise was associated with higher peak velocities of reach movements, and low-intensity exercise facilitated earlier peak velocities. Given the high inter-individual variability of the BDNF response, other mechanisms may support the underlying neural processes related to the changes in behavior, but more investigation is needed to identify the source of the variability. Furthermore, despite not having an effect on BDNF concentration, the BDNF genetic polymorphism influenced motor performance and needs to be considered in future investigations on the effect of exercise on motor learning. The results of the current study could have implications for intervention protocols being developed that aim to improve motor performance or motor learning through BDNF based pathways.
Acknowledgments
Funding: This work was supported by grant 15SDG24970011 from the American Heart Association and by a SPARC Graduate Research Grant from the Office of the Vice President for Research at the University of South Carolina.
Footnotes
Declarations of Interest: none
References
- Adams JA (1971). A closed-loop theory of motor learning. Journal of motor behavior, 3(2), 111–150. [DOI] [PubMed] [Google Scholar]
- Atkinson G, & Batterham AM (2015). True and false interindividual differences in the physiological response to an intervention. Experimental physiology, 100(6), 577–588. [DOI] [PubMed] [Google Scholar]
- Baird J, & Stewart JC (2017). Sequence-specific implicit motor learning using whole-arm three-dimensional reach movements. Experimental Brain Research, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, & Lee FS (2006). Variant BDNF (Val66Met) impact on brain structure and function. Cognitive, Affective, & Behavioral Neuroscience, 6(1), 79–85. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Castello N, & Cotman CW (2010). Exercise and time-dependent benefits to learning and memory. Neuroscience, 167(3), 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg GA (1982). Psychophysical bases of perceived exertion. Med sci sports exerc, 14(5), 377–381. [PubMed] [Google Scholar]
- Bramham CR, & Messaoudi E (2005). BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Progress in neurobiology, 76(2), 99–125. [DOI] [PubMed] [Google Scholar]
- Brodie SM, Borich MR, & Boyd LA (2014). Impact of 5‐Hz rTMS over the primary sensory cortex is related to white matter volume in individuals with chronic stroke. European Journal of Neuroscience, 40(9), 3405–3412. [DOI] [PubMed] [Google Scholar]
- Brodie SM, Meehan SK, Borich M, & Boyd LA (2014). 5 Hz repetitive transcranial magnetic stimulation over the ipsilesional sensory cortex enhances motor learning after stroke. Frontiers in human neuroscience, 8, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, & Zubieta J-K (2006). BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biological psychiatry, 59(9), 812–815. [DOI] [PubMed] [Google Scholar]
- Chambaron S, Berberian B, Delbecque L, Ginhac D, & Cleeremans A (2009). Implicit motor learning in discrete and continuous tasks: Toward a possible explanation of discrepant results. In: Nova Science. [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, … Rothwell JC (2008). A common polymorphism in the brain‐derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. The Journal of physiology, 586(23), 5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. 2nd In: Hillsdale, NJ: erlbaum. [Google Scholar]
- Cotman CW, & Berchtold NC (2002). Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in neurosciences, 25(6), 295–301. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, & Christie L-A (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in neurosciences, 30(9), 464–472. [DOI] [PubMed] [Google Scholar]
- Doya K (2000). Complementary roles of basal ganglia and cerebellum in learning and motor control. Current opinion in neurobiology, 10(6), 732–739. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, … Dean M (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell, 112(2), 257–269. [DOI] [PubMed] [Google Scholar]
- Etnier JL, Wideman L, Labban JD, Piepmeier AT, Pendleton DM, Dvorak KK, & Becofsky K (2016). The effects of acute exercise on memory and brain-derived neurotrophic factor (BDNF). Journal of Sport and Exercise Psychology, 38(4), 331–340. [DOI] [PubMed] [Google Scholar]
- Ferris LT, Williams JS, & Shen C-L (2007). The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine & Science in Sports & Exercise(39), 728–734. [DOI] [PubMed] [Google Scholar]
- Fitts PM, & Peterson JR (1964). Information capacity of discrete motor responses. Journal of experimental psychology, 67(2), 103. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, & Lu B (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron, 66(2), 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, & Beste C (2011). The Met-allele of the BDNF Val66Met polymorphism enhances task switching in elderly. Neurobiology of Aging, 32(12), 2327. e2327–2327. e2319. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, & Beste C (2012). The Met-genotype of the BDNF Val66Met polymorphism is associated with reduced Stroop interference in elderly. Neuropsychologia, 50(14), 3554–3563. [DOI] [PubMed] [Google Scholar]
- Getzmann S, Gajewski PD, Hengstler JG, Falkenstein M, & Beste C (2013). BDNF Val66Met polymorphism and goal-directed behavior in healthy elderly—evidence from auditory distraction. Neuroimage, 64, 290–298. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, & Edgerton VR (2002). Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. Journal of neurophysiology, 88(5), 2187–2195. [DOI] [PubMed] [Google Scholar]
- Gustafsson G, Lira CM, Johansson J, Wisén A, Wohlfart B, Ekman R, & Westrin Å (2009). The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry research, 169(3), 244–248. [DOI] [PubMed] [Google Scholar]
- Halsband U, Ito N, Tanji J, & Freund H-J (1993). The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain, 116(1), 243–266. [DOI] [PubMed] [Google Scholar]
- Helm EE, Matt KS, Kirschner KF, Pohlig RT, Kohl D, & Reisman DS (2017). The influence of high intensity exercise and the Val66Met polymorphism on circulating BDNF and locomotor learning. Neurobiology of learning and memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, & Nakahara H (2002). Central mechanisms of motor skill learning. Current opinion in neurobiology, 12(2), 217–222. [DOI] [PubMed] [Google Scholar]
- Houk JC, & Wise SP (1995). Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cerebral Cortex, 5(2), 95–110. [DOI] [PubMed] [Google Scholar]
- Huys R, Studenka BE, Rheaume NL, Zelaznik HN, & Jirsa VK (2008). Distinct timing mechanisms produce discrete and continuous movements. PLoS Computational Biology, 4(4), e1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, & Cramer SC (2006). BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature neuroscience, 9(6), 735–737. [DOI] [PubMed] [Google Scholar]
- Knaepen K, Goekint M, Heyman EM, & Meeusen R (2010). Neuroplasticity—Exercise-Induced Response of Peripheral Brain-Derived Neurotrophic Factor. Sports Medicine, 40(9), 765–801. [DOI] [PubMed] [Google Scholar]
- Leech KA, & Hornby TG (2017). High-intensity locomotor exercise increases brain-derived neurotrophic factor in individuals with incomplete spinal cord injury. Journal of neurotrauma, 34(6), 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi PD, & Frith E (2018). A brief primer on the mediational role of BDNF in the exercise‐memory link. Clinical physiology and functional imaging. [DOI] [PubMed] [Google Scholar]
- Mang CS, McEwen LM, MacIsaac JL, Snow NJ, Campbell KL, Kobor MS, … Boyd LA (2017). Exploring genetic influences underlying acute aerobic exercise effects on motor learning. Scientific reports, 7(1), 12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Snow NJ, Campbell KL, Ross CJ, & Boyd LA (2014). A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. Journal of Applied Physiology, 117(11), 1325–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Snow NJ, Wadden KP, Campbell KL, & Boyd LA (2016). High-Intensity Aerobic Exercise Enhances Motor Memory Retrieval. Medicine and science in sports and exercise, 48(12), 2477–2486. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Buckley JD, Opie GM, Ridding MC, & Semmler JG (2013). A single bout of aerobic exercise promotes motor cortical neuroplasticity. Journal of Applied Physiology, 114(9), 1174–1182. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2006). Make mild moments memorable: add a little arousal. Trends in cognitive sciences, 10(8), 345–347. [DOI] [PubMed] [Google Scholar]
- McHughen SA, Pearson-Fuhrhop K, Ngo VK, & Cramer SC (2011). Intense training overcomes effects of the val66met BDNF polymorphism on short-term plasticity. Experimental brain research, 213(4), 415–422. [DOI] [PubMed] [Google Scholar]
- McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Crespo LM, Procaccio V, & Cramer SC (2010). BDNF val66met polymorphism influences motor system function in the human brain. Cerebral Cortex, 20(5), 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine, A. C. o. S. (2013). ACSM’s guidelines for exercise testing and prescription: Lippincott Williams & Wilkins. [Google Scholar]
- Mitz AR, Godschalk M, & Wise SP (1991). Learning-dependent neuronal activity in the premotor cortex: activity during the acquisition of conditional motor associations. Journal of Neuroscience, 11(6), 1855–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran DW, & Schwartz AB (1999). Motor cortical representation of speed and direction during reaching. Journal of neurophysiology, 82(5), 2676–2692. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, & Bullemer P (1987). Attentional requirements of learning: Evidence from performance measures. Cognitive psychology, 19(1), 1–32. [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, & Kastin AJ (1998). Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology, 37(12), 1553–1561. [DOI] [PubMed] [Google Scholar]
- Pan W, & Kastin AJ (2004). Polypeptide delivery across the blood-brain barrier. Current Drug Targets-CNS & Neurological Disorders, 3(2), 131–136. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, … Weinberger DR (2004). The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. The Journal of Neuroscience, 24(45), 10099–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepmeier AT, & Etnier JL (2015). Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. Journal of Sport and Health Science, 4(1), 14–23. [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, … Pilegaard H (2009). Evidence for a release of brain‐derived neurotrophic factor from the brain during exercise. Experimental physiology, 94(10), 1062–1069. [DOI] [PubMed] [Google Scholar]
- Roig M, Skriver K, Lundbye-Jensen J, Kiens B, & Nielsen JB (2012). A single bout of exercise improves motor memory. PloS one, 7(9), e44594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, & Schaefer SY (2004). Interlimb differences in control of movement extent. Journal of neurophysiology, 92(3), 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA (1975). A schema theory of discrete motor skill learning. Psychological review, 82(4), 225. [Google Scholar]
- Seidler-Dobrin RD, & Stelmach G (1998). Persistence in visual feedback control by the elderly. Experimental brain research, 119(4), 467–474. [DOI] [PubMed] [Google Scholar]
- Seidler R, Noll D, & Thiers G (2004). Feedforward and feedback processes in motor control. Neuroimage, 22(4), 1775–1783. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, & Iyo M (2004). Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 126(1), 122–123. [DOI] [PubMed] [Google Scholar]
- Singh AM, Duncan RE, Neva JL, & Staines WR (2014). Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC sports science, medicine and rehabilitation, 6(1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AM, Neva JL, & Staines WR (2014). Acute exercise enhances the response to paired associative stimulation-induced plasticity in the primary motor cortex. Experimental brain research, 232(11), 3675–3685. [DOI] [PubMed] [Google Scholar]
- Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JW, Kiens B, & Nielsen JB (2014). Acute exercise improves motor memory: exploring potential biomarkers. Neurobiology of learning and memory, 116, 46–58. [DOI] [PubMed] [Google Scholar]
- Smith AE, Goldsworthy MR, Garside T, Wood FM, & Ridding MC (2014). The influence of a single bout of aerobic exercise on short-interval intracortical excitability. Experimental brain research, 232(6), 1875–1882. [DOI] [PubMed] [Google Scholar]
- Snow NJ, Mang CS, Roig M, McDonnell MN, Campbell KL, & Boyd LA (2016). The effect of an acute bout of moderate-intensity aerobic exercise on motor learning of a continuous tracking task. PloS one, 11(2), e0150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Beck MM, Lind RR, Korsgaard Johnsen L, Geertsen SS, Christiansen L, … Lundbye-Jensen J (2016). Acute exercise and motor memory consolidation: the role of exercise timing. Neural plasticity, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Johnsen LK, Geertsen SS, Christiansen L, Ritz C, Roig M, & Lundbye-Jensen J (2016). Acute exercise and motor memory consolidation: the role of exercise intensity. PloS one, 11(7), e0159589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomporowski PD (2003). Effects of acute bouts of exercise on cognition. Acta psychologica, 112(3), 297–324. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Shubert T, Zhao C, & Gage FH (2005). Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience, 25(38), 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, & Gomez‐Pinilla F (2004). Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience, 20(10), 2580–2590. [DOI] [PubMed] [Google Scholar]
- Vega SR, Strüder HK, Wahrmann BV, Schmidt A, Bloch W, & Hollmann W (2006). Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain research, 1121(1), 59–65. [DOI] [PubMed] [Google Scholar]
- Voti PL, Conte A, Suppa A, Iezzi E, Bologna M, Aniello M, … Berardelli A (2011). Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Experimental brain research, 212(1), 91–99. [DOI] [PubMed] [Google Scholar]
- Wang W, Chan SS, Heldman DA, & Moran DW (2007). Motor cortical representation of position and velocity during reaching. Journal of neurophysiology, 97(6), 4258–4270. [DOI] [PubMed] [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, … Floel A (2007). High impact running improves learning. Neurobiology of learning and memory, 87(4), 597–609. [DOI] [PubMed] [Google Scholar]