Abstract
Reservoirs typically have elevated fish mercury (Hg) levels compared to natural lakes and rivers. A unique feature of reservoirs is water-level management which can result in sediment exposure to the air. The objective of this study is to identify how reservoir water-level fluctuations impact Hg cycling, particularly the formation of the more toxic and bioaccumulative methylmercury (MeHg). Total-Hg (THg), MeHg, stable isotopemethylation rates and several ancillary parameters were measured in reservoir sediments (including some in porewater and overlying water) that are seasonally and permanently inundated. The results showed that sediment and porewater MeHg concentrations were over 3-times higher in areas experiencing water-level fluctuations compared to permanently inundated sediments. Analysis of the data suggest that the enhanced breakdown of organic matter in sediments experiencing water-level fluctuations has a two-fold effect on stimulating Hg methylation: 1) it increases the partitioning of inorganic Hg from the solid phase into the porewater phase (lower log Kd values) where it is more bioavailable for methylation; and 2) it increases dissolved organic carbon (DOC) in the porewater which can stimulate the microbial communitythat can methylate Hg. Sulfate concentrations and cycling were enhanced in the seasonally inundated sediments and may have also contributed to increased MeHg production. Overall, our results suggest that reservoir management actions can have an impact on the sediment-porewater characteristics that affect MeHg production. Such findings are also relevant to natural water systems that experience wetting and drying cycles, such as floodplains and ombrotrophic wetlands.
Keywords: Porewater, Mercury methylation, Reservoir, Water-level, DOC
Graphical abstract
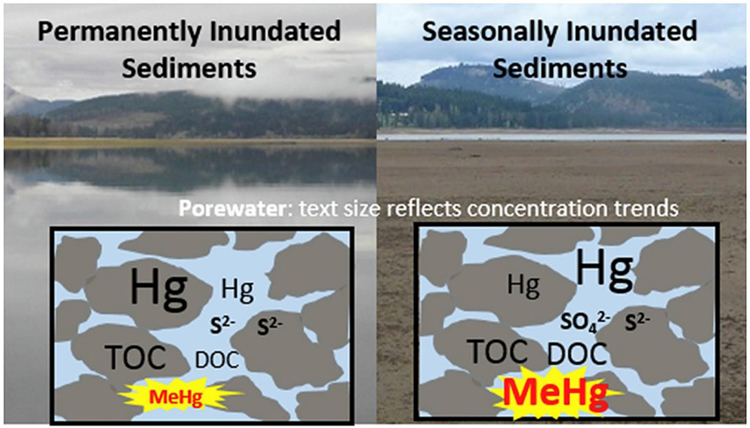
1. Introduction
Mercury (Hg) is a pollutant of global concern largely due to its ability to accumulate in fish tissue. Most anthropogenic releases of Hg to the environment are in an inorganic form; however almost all Hg in fish tissue is in an organic form—methylmercury (MeHg). Within the US, thousands of water bodies are currently under fish consumption advisories due to Hg pollution (United States Environmental Protection Agency, 2011).
Understanding the variables influencing MeHg production is key to identifying strategies that can be used to reduce MeHg levels in fish. Most Hg methylation is conducted by anaerobic microorganisms in sediments, peatlands and the hypolimnetic water of lakes (Branfireun et al., 1999, Compeau and Bartha, 1985, Eckley and Hintelmann, 2006). Sulfate reducing bacteria (SRB) have been widely reported to be important producers of MeHg (Ullrich et al., 2001); however, some iron reducing bacteria (Kerin et al., 2006) and methanogens (Hamelin et al., 2011) are also capable of Hg methylation.
The activity, abundance, and structure of the microbial community play an important role in determining MeHg production rates. As a result, MeHg concentration can be highly spatially and temporally variable across landscapes, with wetlands and peatlands typically having enhanced MeHg production (Branfireun et al., 1996, Hurley et al., 1995, Johnson et al., 2015, Stlouis et al., 1994).
The microbial community is influenced by the extent of anoxic conditions, the quantity and quality of organic carbon, and the concentration of electron acceptors such as sulfate and ferric iron (Hsu-Kim et al., 2013, Kerin et al., 2006, Ullrich et al., 2001). Microbial activity and methylation rates have also been shown to increase with temperature (King et al., 1999), which may be partially responsible for the seasonal variations in MeHg observed in many systems (Hammerschmidt and Fitzgerald, 2004). However, the extent to which Hg is methylated is also highly dependent on the bioavailability of the inorganic Hg to the methylating organisms. The bioavailable forms of inorganic Hg may include neutrally charged Hg-ligand complexes which can enter bacteria via passive diffusion (Benoit et al., 1999) and/or the active transport of Hg complexes with low molecular weight thiol ligands (Golding et al., 2002, Schaefer et al., 2011). Recent studies have also shown that dissolved organic matter can play an important role affecting the uptake of inorganic bacteria into methylating organisms (Graham et al., 2012). Only a small percent (typically ≤5%) of the total amount of inorganic Hg in aquatic systems is in a “reactive” form that is considered available for microbial uptake and methylation (Domagalski, 2001, Marvin-DiPasquale et al., 2009, Singer et al., 2016)
In sediment, bioavailable inorganic Hg is mostly associated with the porewater fraction and is related to the sediment-porewater distribution coefficient (Kd) (Buckman et al., 2015, Marvin-DiPasquale et al., 2009, Schartup et al., 2014). Compared to bulk sediment measurements, porewater Hg concentrations are also more available for benthic invertebrate uptake as well as diffusive flux into the overlying water. Sediment-porewater partitioning of Hg is controlled by the amounts of Hg binding ligands, such as sulfide and thiols, in the solid and in the dissolved phases. These ligands in turn are influenced by several factors such as sediment organic matter, redox conditionsand pH (Hammerschmidt and Fitzgerald, 2004, Schartup et al., 2014). Overall, partitioning of sediment inorganic Hg between solid and porewater phases can be an important controlling mechanism on the rate of MeHg production.
Reservoirs have been shown to have elevated MeHg concentrations in water and fish compared to natural lakes and is related to the degree of water-level fluctuations (Brigham et al., 2002, Kamman et al., 2005, Larson et al., 2014, Montgomery et al., 2000, Selch et al., 2007, Sorensen et al., 2005). Newly created reservoirs have been shown to have higher MeHg production due to increased organic material available after flooding terrestrial landscapes (Hall et al., 2005, St Louis et al., 2004); however even decades after the initial impoundment reservoirs can continue to have elevated MeHg levels due to ongoing seasonal water-level fluctuations (Anderson et al., 1995, Bodaly et al., 2007) and/or changes in the foodweb structure (lotic to lentic). Water-level fluctuations are believed to promote the recycling of sulfide in sediment to sulfate when exposed to the air, which can enhance microbial methylation when sediments are re-wetted (Eckley et al., 2015, Evers et al., 2007). In addition, this process may enhance partitioning of sediment-bound Hg into the porewater/aqueous phase where it is more available for microbial uptake; however to our knowledge the influence of this mechanism has not been previously assessed in reservoirs. Despite increased MeHg in reservoirs, Hg dynamics in these systems have been much less studied compared to natural lakes. Because reservoirs are waterbodies that are actively managed, understanding how water level management actions influence Hg cycling provides an opportunity to improve environmental conditions. Many natural systems such as river floodplains and ombrotrophic wetlands also experience seasonal wetting and drying cycles that can impact Hg cycling.
The overarching objective of our study is to identify areas of elevated MeHg production within reservoir sediment and to determine the variables that drive these trends. Specifically, we will test the hypothesis that water-level fluctuations result in an increase in MeHg production within a reservoir and that this is influenced by enhanced sulfate cycling and increased partitioning of inorganic Hg to porewater. A previous study conducted at this site (Cottage Grove Reservoir) identified a subtle, yet statistically significant, increase in sediment MeHg concentrations in the seasonally inundated mudflats of the reservoir compared to the permanently inundated sediments (Eckley et al., 2015). In our current study, we expand on these findings by including the areas along the reservoir shoreline that are seasonally inundated wetlands and by adding porewater measurements and isotopic Hg methylation assays to further explore the mechanisms driving MeHg production. In addition, we assess the potential for sediment Hg concentrations to influence the overlying water concentrations.
2. Methods
2.1. Site description
The Cottage Grove Reservoir in western Oregon (43.69403; −123.07328; Fig. 1) is a flood control reservoir constructed in 1942. The reservoir’s watershed includes the historical Black Butte mine (cinnabar ore) approximately 15 km upstream. During its operation from the 1890s to the 1960s, the mine produced approximately 635,000 kg of Hg and over 200,000 m3 of mine tailings (Ecology and Environment, 1998). Sediment core data suggests that Hg at the historical mine site continues to be transported downstream to the reservoir (Ambers and Hygelund, 2001, Curtis et al., 2013).
Fig. 1.
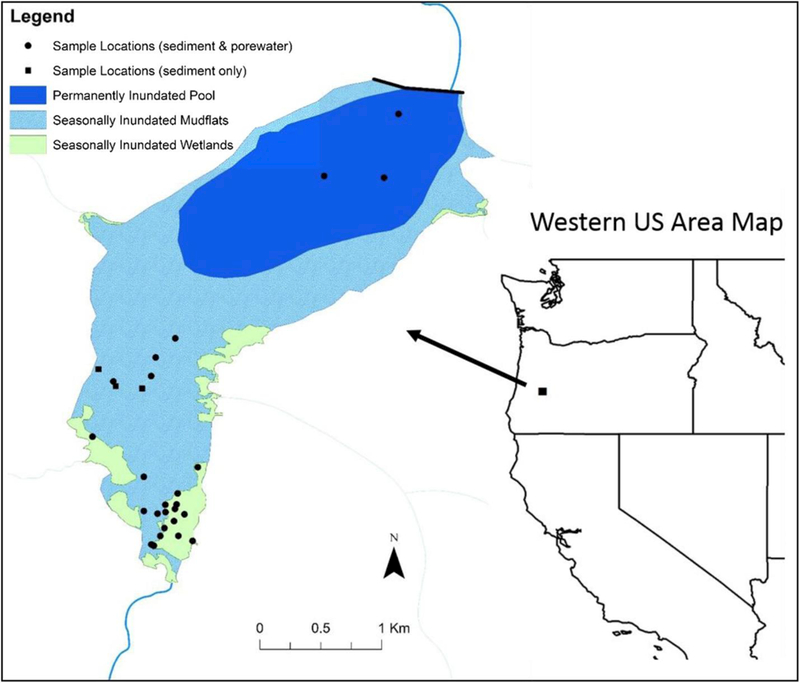
Map showing the sample locations in Cottage Grove Reservoir. Note: the Black Butte mine is located 15 km upstream of the reservoir and is outside the scale of this areas kept low during much of the year that experiences higher rainfall (September through April). During the low pool conditions, over 60% of the reservoir area is exposed sediment, which are seasonally inundated during the summer months. When the water levels are raised to full-pool conditions, vegetated wetland areas that occur mostly near the inlet are inundated with reservoir water. The wetlands are fairly extensive (∼36 ha) and cover approximately 8% of the total surface area of the reservoir.
In this study, we’re defining the wetlands as the areas that have continuous year-round vegetation that is emergent during full-pool conditions. The vegetation is dominated by reed canary grass and cattails. In comparison the mudflat areas do not have any emergent vegetation above the water surface during full-pool conditions; however during the ∼6-months of the year that they are exposed to the air some areas become colonized by short grasses or forbs.
2.2. Field sampling
Sediment, porewater and surface water samples were collected in three distinct areas of the reservoir: the permanently inundated area; the seasonally inundated sediments without vegetation; and the seasonally inundated wetland areas (Fig. 1). Due to the important role of wetlands in MeHg production, this area was sampled the most intensively. Samples were collected during four seasonal conditions: winter low-pool (March 2014; 2015); spring full-pool (June 2014; May 2015); summer full-pool (August 2014); and fall low-pool following drawdown (November 2014) (see Fig. S1 in the Supporting Information (SI)).
Sediment samples were collected using a large bore gravity sediment corer (Aquatic Research Instruments) with polycarbonate core tubes. The samples were collected with ∼10 cm of overlying water above the sediment. The sediment cores were capped, and stored in a cooler at 4 °C during transport to the mobile laboratory. Within several hours after collection, the top 8 cm of the sediment cores were extruded in an anoxic glove box filled with ultra-high purity nitrogen gas. For solid phase analysis, cores were sectioned into 2 cm increments in triplicate and were composited and frozen in glass jars until analysis. For porewater analysis, sediments cores were sectioned in 4 cm increments in quadruplicate and composited to obtain sufficient volume of porewater for analysis.
In the anoxic glove box, sediment used for porewater analysis was placed in centrifuge tubes (Corning), capped and covered with Parafilm®. Samples were centrifuged at 3500 rpm for 30 min to separate the porewater from the sediment. The centrifuge tubes were transferred to a separate, clean anoxic glove box (i.e. not used for sediment core processing) for vacuum filtration and preservation within 12 h of the field sample collection. The single-use disposable filter apparatus (Fisher Scientific) were rinsed with 10% HCl and ultrapure water immediately prior to filtration.
Clean hands/dirty hands techniques were followed during water sample collection and processing (United States Geological Survey, 2006). Samples for aqueous THg and MeHg were collected in acid cleaned Teflon bottles following the procedures outlined by Lewis and Brigham (2004); all other samples were collected in new glass or polyethylene bottles. Whole and filtered (0.45 μm) water samples were obtained using a peristaltic pump with field replicates collected at a 10% frequency. The filters and sample line were acid-cleaned with 10% HCl and rinsed with ultrapure water prior to use. Samples were preserved immediately after collection in the field (whole and filtered THg/MeHg: ultra-pure HCl to pH < 2; sulfide: Zn acetate; DOC: sulfuric acid; Anions: 4 °C). Reduced iron (Fe2+) was analyzed colorimetrically (HACH DR/890 Colorimeter using Phenanthroline following HACH method 8146 (1, 10 Phenanthroline Method)).
During the March and May 2015 sampling events, an additional set of sediment core samples were collected for stable Hg isotope addition methylation assays following similar techniques as described elsewhere (Mitchell and Gilmour, 2008). The samples were collected in small diameter (5 cm) core tubes with a minimum of 10 cm of overlying water and capped. The isotope spike solution was created 1 h prior to the injections by mixing filtered lake water with a concentrated spike stock solution for a final concentration of 0.25 mg/L. Within a few hours after collection, the top 4 cm of the core were spiked with 1 ml of 198Hg isotope solution which were applied using a syringe through small holes in the core tube that were drilled 1 cm apart and sealed with silicone. Each 1 cm section of the core received 0.25 μg of 198Hg isotope for a total of 1 μg added within the top 4 cm of the core. The methylation rates are presented as the % of added inorganic Hg tracer that was methylated per unit time.
2.3. Laboratory analysis
Whole water samples were analyzed for THg, MeHg, and total suspended solids (TSS). Filtered water samples were analyzed for major anions, DOC, sulfide, and reduced iron (Fe2+). Sediment samples were analyzed for THg, MeHg, sulfide, and total organic carbon (TOC). Water and sediment samples were analyzed at the Pacific Northwest National Laboratory (PNNL) Marine Sciences Laboratory (Sequim, WA) for MeHg and THg (non-isotopic specific) following EPA draft method 1630 and method 1631. TSS was analyzed at Manchester Environmental Laboratory (Port Orchard, WA) following USGS Method I-3765; DOC and TOC were analyzed at ALS-Columbia (Kelso, WA) following EPA Method 415.3 and EPA SW-846 9060; and sulfate and sulfide were analyzed at Alpha Analytical (Sparks, NV) following EPA Method 300.0, Standard Method 14500S2D and EPA Method 9030B. The THg and MeHg stable isotope samples were analyzed at the USGS WDML (DeWild et al., 2002, DeWild et al., 2004, Olund et al., 2004). All data met the requirements outlined in each of the Methods and was reviewed and validated to the Stage 2B level by laboratory Quality Assurance Officers (United States Environmental Protection Agency, 2009).
The mean ± standard deviation of whole (n = 4) and filtered (n = 10) water field blanks for THg were <0.22 ± 0.24 ng/L and <0.40 ± 0.35 ng/L and for MeHg were <0.03 ± 0.01 ng/L and <0.02 ± 0.01 ng/L respectively. The blank concentrations are reported as “less than” because several of the blanks samples were below the method detection limit, which was used in the calculation of the averages. The blanks of all other water quality parameters were below their method reporting limits. The relative percent difference (RPD) of the field replicate water samples for whole surface water and filtered surface and porewater THg were 4.2 ± 0.0% and 19 ± 20%; and for MeHg were 16 ± 16% and 24 ± 18%.
Generalized linear model (GLM) analysis using Statgraphics Centurion software was performed using covariates, randomized, and nested factors to determine differences between parameters. The GLM approach allows us to identify if there are significant differences in a dependent variable (e.g. MeHg concentrations) as a function of continuous (e.g. carbon, sulfide, sulfate concentrations, etc) and categorical predictor variables (seasonally versus permanently inundated conditions, seasons, location, etc). Data showing skewed and wide distributions were log transformed prior to statistical analysis to avoid a small number of samples disproportionately influencing the results.
3. Results and discussion
3.1. Sediment THg and MeHg—spatial variability and influential variables
At a given location, sediments showed similar THg least square mean (LSM) concentrations with depth within the core (p = 0.98), but MeHg concentrations were significantly higher in the top 2 cm of sediment and decreased with depth (p < 0.001; n = 155; GLM R2 = 0.65, with %TOC as a covariate, season as a random effect, and depth nested within location; Fig. S2 in the SI). These results are consistent with other studies that have also shown that MeHg concentrations and production are highest in the uppermost sediments just below the sediment-water interface (He et al., 2007, Hollweg et al., 2009, Schafer et al., 2010). There were not significant differences in the THg and MeHg concentrations at 0–4 and 4–8 composite porewater samples (Fig. S2 in SI); partially due to the larger variability in the porewater concentrations, but also because porewater samples were composited over larger depth intervals (i.e. 0–4 cm and 4–8 cm) which would have diluted any elevated MeHg concentrations in just the top 2 cm.
The mean surface sediment THg concentrations were significantly higher in the permanently inundated area of the reservoir compared to the seasonally inundated areas (GLM R2 = 0.37; p < 0.001, df = 67; with %TOC and season as covariates; Fig. 2). The highest THg occurred along the central channel of the reservoir and are believed to represent the depositional zone for Hg transported by the Coast Fork Willamette (CFW) River which drains the watershed that includes the historical Black Butte Hg Mine (Fig. S3 in SI). The MeHg concentrations showed the opposite trends as THg, with the areas experiencing seasonal variations in the water levels having significantly higher concentrations compared to the permanently inundated area (GLM with %TOC and THg as covariates, R2 = 0.41, p < 0.001, df = 67; Fig. 2A). In addition to seasonal water level variations (p < 0.001), the sediment THg (p < 0.001) and to a lesser extent %TOC (p = 0.05) were also factors that significantly contributed to the measured sediment MeHg concentrations. The significance of %TOC also increases when looking at the %MeHg instead of the MeHg concentration (p = 0.002). The %MeHg is the ratio of MeHg/THg expressed as a percent and has been used in several studies as a measure of a system’s net methylation efficiency normalized to the amount of inorganic Hg available (Benoit et al., 2003, Kehrig et al., 2002, Mitchell et al., 2008). Overall, these results show that water level fluctuations are apparently an important factor driving sediment MeHg concentrations (permanently inundated: 0.71 ± 0.43 ng g−1; seasonally inundated: 2.70 ± 0.17 ng g−1; Fig. 2B) and the %MeHg (permanently inundated: 0.18± 0.12%; seasonally inundated 0.55± 0.5%).
Fig. 2.
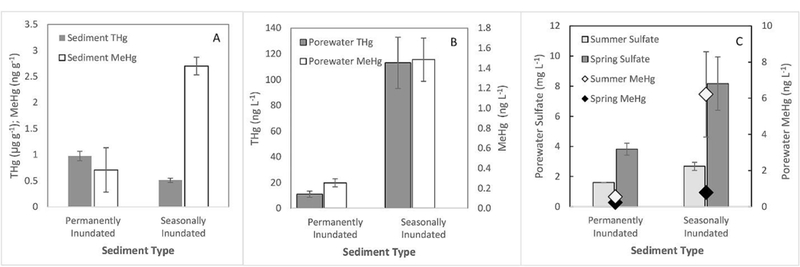
Comparison of sediment and porewater parameters between sediments that were permanently inundated and seasonally inundated. Comparison of mean ± SE sediment (A) and porewater (B) THg and MeHg values for the top 2 cm of sediment grouped by whether the sediment experiences seasonal water level fluctuations or is permanently inundated year-round. All of the parameters showed significant differences (p < 0.05) between the two sediment types. Note: graph A has THg and MeHg plotted with different units on the same Y axis; graph B has THg and MeHg plotted on separate Y axes. Graph C shows comparison of seasonal changes in porewater sulfate and MeHg concentrations.
3.2. Porewater THg and MeHg concentrations—spatial variability and influential variables
After the reservoir water level is raised in the spring, the porewater sulfate concentrations were significantly higher in the sediments that were previously exposed to the air (8.2 ± 1.8 mg L−1) compared to the permanently inundated areas (3.8 ± 0.4 mg L−1; t-test p = 0.02, df = 9; Fig. 2C). When the same locations were measured during the-late summer, there was a decrease in porewater sulfate concentrations; with the magnitude of change being significantly larger in the sediments experiencing seasonal water level fluctuations (Δ 5.5 ± 1.6 mg L−1) compared to the permanently inundated areas (Δ 2.2 ± 0.4 mg L-1; t-test p = 0.04, df = 9; Fig. 2C). These results support the hypothesis that the elevated MeHg levels in reservoirs are influenced by enhanced sulfate recycling of sulfide to sulfate due to water level fluctuations.
Porewater THg concentrations showed significant spatial variability within the reservoir (ANOVA p = 0.007, df = 45); with the porewater THg concentrations being significantly higher in the areas experiencing water level fluctuations (116 ± 29 ng L−1) compared to the permanently inundated sediments (10.9 ± 2.4 ng L−1; ANOVA p = 0.01, df = 44; Fig. 2B). This was opposite of what was observed in the bulk sediment samples, where THg was highest in the permanently inundated areas. These results suggest that the water level variations may increase the partitioning of THg to the porewater from the sediment upon rewetting of the sediments seasonally. This is reflected in the significantly lower sediment-porewater distribution coefficients (log Kd values) for the seasonally inundated areas (THg: 3.9 ± 0.1) compared to the permanently inundated areas (THg: 5.2 ± 0.1; t-test p < 0.001, df = 67).
Sediment TOC can play an important role in THg and MeHg dynamics in sediments (Schartup et al., 2014). The sediment TOC content in the wetland areas along the shoreline that experience water level fluctuations are significantly higher than the mudflat and permanently inundated areas (wetlands: 21 ± 2.1%; 5.8 ± 0.5%; ANOVA p < 0.001, df = 65). Much of the high organic carbon associated with the wetland areas is due to the samples being collected in densely vegetated areas and the sediments containing plant roots. Variations in the sediment carbon levels are a contributing factor to the observed differences in THg and MeHg between the seasonally and permanently inundated areas. Because grasses and forbs colonize the sediment during the periods of water drawdown, the impact of water level fluctuations and sediment carbon levels are linked and requires multivariate statistical tests such as GLM analysis that can be used to distinguish the unique contributions from these variables (described below). While there were significant differences in the sediment TOC levels between seasonally and permanently inundates sediments, there was not a significant difference when looking at the porewater DOC concentrations between these areas (61 ± 5.5 mg L−1; ANOVA p = 0.22, df = 32).
The porewater MeHg concentrations were significantly higher in the seasonally inundated areas (1.5 ng L−1) compared to the permanently inundated areas (0.26 ng L−1; ANOVA p = 0.006, df = 43; Fig. 2B). GLM analysis showed that the following variables were all significant predictors of the porewater MeHg concentrations: season, water-level fluctuations, porewater DOC, THg and THg log Kd(R2 = 0.76, p < 0.001, df = 41). However, porewater sulfate, reduced iron, and pH were not significantly correlated with the porewater MeHg concentrations (p > 0.20). The lack of a direct correlation between spatial and temporal MeHg and sulfate concentrations is not surprising since it is the microbial sulfate reduction to sulfide that is often associated with MeHg production.
There are two distinct relationships between sediment %TOC and the THg log Kdvalues from the sediments that were permanently inundated with water versus those that experienced seasonal wetting and drying (Fig. 3). For the permanently inundated sediments there was a significant positive relationship between %TOC and THg log Kd values, which is consistent with the findings from several other studies on systems without water-level fluctuations (Bloom et al., 1999, Hammerschmidt and Fitzgerald, 2004, Marvin-DiPasquale et al., 2009). For these permanently inundated sediments, the sediment organic carbon appears to promote the binding of THg to the solid phase. In contrast, the seasonally inundated sediments showed a significant negative relationship between sediment TOC and porewater THg log Kd values (Fig. 3). One factor that may be affecting these relationships is the differences in the %TOC between the permanently inundated sediments (4.5± 0.4%) and seasonally inundated sediments (11.4± 1.4%). Dissolved ligands may have a larger influence on Hg partitioning into the porewater phase as the amount of solid phase TOC increases; however these relationships can be highly variable depending on the organic carbon quality and degree of humification. An assessment of the carbon quality was not included in our study and is an area that is in need of further investigation to better understand its role on sediment-porewater Hg dynamics in response to water level fluctuations.
Fig. 3.
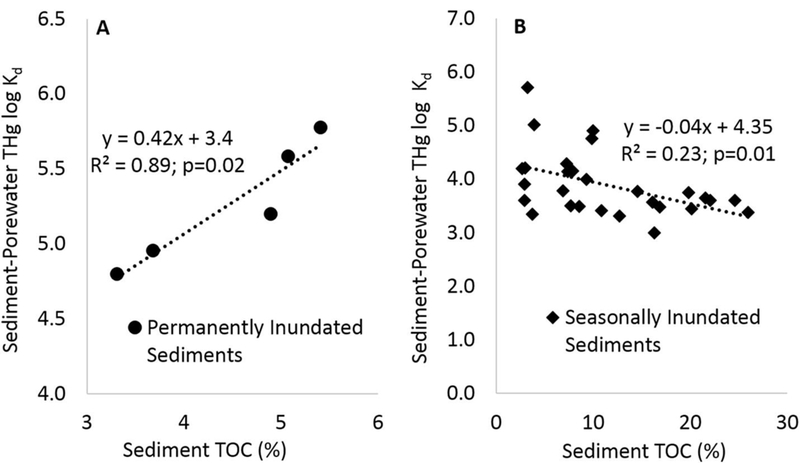
Regression analysis of sediment %TOC verus porewater THg log Kd for permanently inundated sediments (panel A) and seasonally inundated sediments (panal B). All data is from the upper-sediment (0–4 cm) samples. Note, if the data from the seasonally inundated sediments is compared over the same range of sediment %TOC values as occurs in the permanently inundated sediments (i.e. <6% TOC), then the relationship is no longer significant.
The sediments experiencing water level fluctuations could have enhanced breakdown of solid-phase organic matter when the sediments are exposed to the air, which could facilitate the partitioning of THg into the porewater phase. This increased partitioning of THg into the porewater-phase raises its bioavailability and may contribute to the higher MeHg concentrations in sediments experiencing water-level fluctuations. This is supported by the positive relationship between sediment %TOC and porewater DOC (Fig. 4A), suggesting that the lower THg log Kd values at higher %TOC may result from higher DOC levels facilitating the partitioning of THg into the porewater phase. If assessed directly, the regression slope between THg log Kd and DOC is also negative, however due to the high variability in the dataset and the competing influence of sediment sulfide levels, this slope is not significant (p = 0.1). The THg log Kd values showed a positive relationship with the sediment sulfide concentrations (Fig. 4B) suggesting that Hg binding to solid-phase sulfides in the sediment reduce the partitioning of Hg into the porewater phase. The degree to which wetting and drying cycles affect the conditions conducive to methylation is expected to be dependent on both the frequency and duration of the inundation cycles (Singer et al., 2016).
Fig. 4.
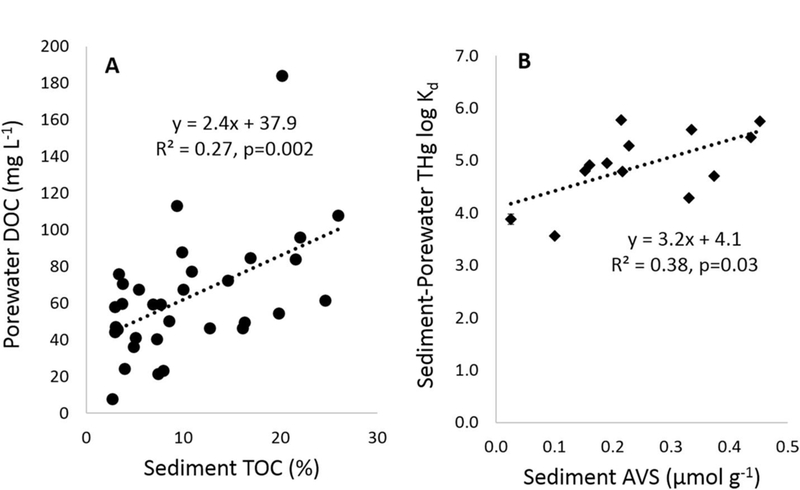
Regression of sediment and porewater constituents. Graph A shows sediment %TOC versus porewater DOC for all the upper sediment (0–4 cm) samples during full-pool conditions. Graph B shows the sediment sulfide (AVS) concentration versus porewater THg log Kd values. Sediment sulfide concentrations were below detection in 82% of the sediment samples collected; in this graph the correlations is based on only the samples were sulfide was detected with the addition of a single mean value (±SE) representing all of the values less than the 0.025 μg g−1reporting limit.
Within the seasonally inundated sediments, the THg concentrations that were <1 μg g−1 showed a linear relationship with sediment MeHg (Fig. 5A). However, at higher sediment THg concentrations (>1 μg g−1) the sediment MeHg concentrations did not continue to increase. In the sediments with the higher THg concentrations, the partitioning of THg between the sediment and porewater also decreased (Fig. 5B). Because Hg in the porewater phase is more likely to be methylated than Hg bound in the solid matrix (Hsu-Kim et al., 2013), the lower partitioning of THg into the porewater phase of the most highly contaminated sediments appears to limit MeHg production. The sediment with elevated THg concentrations (i.e ∼>1 μg g−1) are about an order of magnitude higher than background concentrations for this area and likely consists of some highly insoluble cinnabar eroded from the historical Black Butte Hg Mine upstream (Curtis et al., 2013). These sediments with the highest THg concentrations appear to be in a form that is less soluble and less bioavailable for methylation than some of the sediments with lower THg concentrations. However, a significant portion of the THg in sediments that are <1 μg g−1 fall within the linear region of sediment-THg: MeHg relationship are still above typical background concentrations in this region (<0.2 μg g−1) and also likely have origins associated with upstream mining activities. The differences in bioavailability/partitioning of the THg associated with these sediments may reflect THg originating from variations in ore processing techniques throughout the life of the mine (Ecology and Environment, 1998) and/or temporal variations in material weathering. The importance of THg in the porewater phase being associated with MeHg production is highlighted by the linear relationship between these variables over the full range in concentrations (Fig. 5C).
Fig. 5.
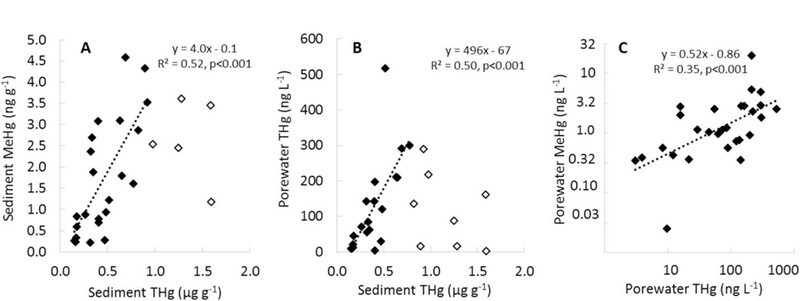
The regression lines and equations are based on data points shown as black-filled diamond; data points not included in the regression analysis are shown as white-filled diamonds. Graph A shows the regression of sediment THg versus sediment MeHg; Graph B shows the regression of sediment THg versus porewater THg, and Graph C shows porewater THg versus porewater MeHg. Data are shown for all seasonally inundated sediments measured during full-pool conditions in the spring and summer.
3.3. The influence of sediment dynamics on water-column THg and MeHg concentrations
The water-column THg and MeHg concentrations in the Cottage Grove Reservoir appear to be influenced in part by processes occurring in the sediment. For example, both the porewater THg and MeHg concentrations were significantly correlated with the THg and MeHg concentrations measured in water overlying the sediments (excluding two locations near the inflow of the CFW River; Fig. 6). For the MeHg correlation, the relationship was only significant when using log-transformed data and including only samples from the summer months (R2 = 0.44, p = 0.05, df = 8; Fig. 6B)when MeHg production tends to be the highest (Korthals and Winfrey, 1987, Watras et al., 2005) and inflow of MeHg from the CFW River is the lowest (Eckley et al., 2015). When comparing bulk sediment concentrations to the overlying water, the MeHg concentrations showed a significant correlation (R2 = 0.52, p = 0.02, df = 8), while the THg concentrations did not (p > 0.05 whether correlation was performed on THg-W, THg-F, or THg-P concentrations). The connection between sediment MeHg production and the MeHg concentrations in overlying water highlights the importance of sediment processes to affect biota with both benthic as well as pelagic-based food webs.
Fig. 6.
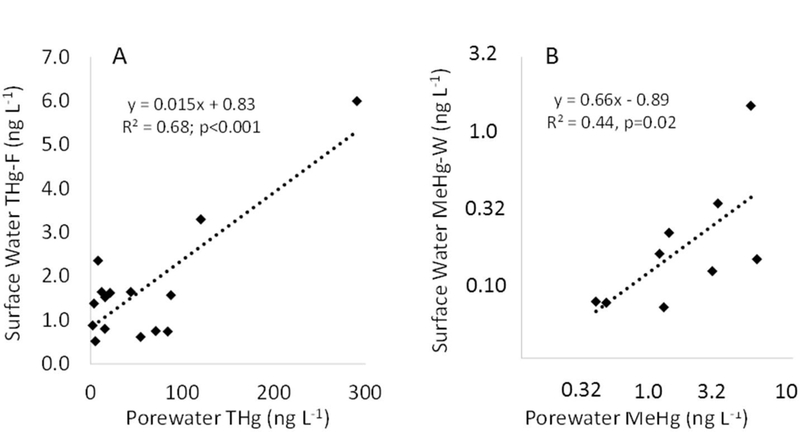
Graph A showing the sediment porewater THg concentrations versus filtered THg concentrations in the overlying water at the different sample locations throughout the reservoir. Two sample locations that were both located near the inflow of the CFW River were excluded from the analysis since the overlying water was more reflective of watershed sources than sediment fluxes. Graph B shows the sediment porewater MeHg concentrations versus whole-water MeHg concentrations in the overlying water at the different sample locations throughout the reservoir plotted on log-scales. Only data collected during the summer sampling events were included in the MeHg regression.
3.4. Stable isotope addition sediment methylation assays
Sediment methylation rates were measured using stable isotope additions in March and May during relatively similar environmental conditions (e.g. water temp: 8.5–10.8 °C; porewater sulfate: 3.4 ± 0.7 and 3.0 ± 0.3 mg L−1; t-test p = 0.59, df = 5). Comparison of the methylation rates at paired locations showed there were no significant temporal differences between the two sampling events (1.8 ± 0.3 and 1.1 ± 0.3% d−1; paired t-test p = 0.16, df = 6). Within the areas with water level fluctuations the amount of MeHg produced was higher in the wetlands compared to the mudflat areas, however, due to the small sample size from the mudflats (n = 3), the differences were not significantly different (t-test p = 0.08, df = 9). Grouping the methylation rates from the seasonally inundated sediments (2.3 ± 0.4% d−1) showed that they were significantly higher than the rates from the permanently inundated pool (1.0 ± 0.1% d−1; t-test p = 0.007, df = 12). These results indicate that given the same spike of inorganic Hg, the sediments subjected to water level fluctuations have higher methylation rates. These findings are consistent with the conclusions based upon the ambient MeHg measurements, which also showed higher concentrations in the seasonally inundated sediments.
Fluctuating water-levels was the most significant variable influencing the methylation rates (p < 0.001) followed by the sediment %TOC (p = 0.001), and porewater sulfate concentration (p = 0.02) (GLM R2 = 0.87, p < 0.001, df = 14). The relationship between methylation rates and sediment %TOC was negative, suggesting that organic carbon reduced the availability of the added Hg tracer through greater partitioning to the sediment phase. Porewater DOC concentrations, which would be more reflective of the carbon source available to the microbial community, were not significantly correlated with methylation rates (p = 0.63), which may result from relatively high DOC concentrations in all of the sediments used in the methylation assays (29–102 mg L−1).
The isotope Hg spike in the methylation assays was added in an aqueous solutionand is presumed to have a higher bioavailability to methylating organism than ambient sediment-bound THg. As such, the methylation rate obtained from isotopic spikes can be compared with the sediment ambient %MeHg to provide information about the bioavailability of the ambient Hg (Lehnherr et al., 2012). In the sediments of Cottage Grove Reservoir, there was a significant correlation between the spatial distribution of methylation rates of the isotopic tracer and the ambient %MeHg (Fig. S4 in SI). These results suggest that the ambient sediment THg is fairly bioavailable within the reservoir such that the ambient Hg is being methylated in similar proportions to the added spiked inorganic Hg. It should be noted that the majority (75%) of the sediment samples where the methylation assays were performed had ambient THg concentrations <1 μg g−1 and were within the range where a linear relationship with sediment ambient MeHg was observed (Fig. 6A). Had the isotopic methylation assays been performed on sediments mostly with higher ambient THg concentrations (i.e. >1 μg g−1) we may not have observed a good correlation between the behavior of the spike isotopic Hg and the ambient Hg.
The location with the lowest %TOC of any of the samples (2% compared to the mean from the other sites of 7± 2%) was the only data point to fall outside of the regression equation’s 95% prediction intervals (Fig. S4 in SI; 0.36 %MeHg; 4.1% d−1). This is consistent with findings described earlier in the text where there was a negative correlation between methylation rates and sediment %TOC, presumably due to enhanced partitioning of the added spike from the aqueous phase to the solid phase in sediments with higher %TOC.
4. Conclusions
Overall, the results of the sediment and porewater data suggest that MeHg production is enhanced in areas of the reservoir sediments experiencing seasonal water level fluctuations. Previous studies on reservoirs have suggested that sulfate re-cycling may be the mechanism responsible for this phenomenon (Evers et al., 2007, Selch et al., 2007, Sorensen et al., 2005). This hypothesis is supported by the findings from our study (e.g. Fig. 2C). However, our multivariate statistical analysis and correlations with sediment-porewater partition coefficients also identified that water level fluctuations may enhance inorganic Hg bioavailability through increased partitioning of Hg from the solid phase into the porewater and can increase the amount of DOC available for microbial populations. Both of these variables may be influenced by the enhanced breakdown of sediment organic matter in response to seasonal water level fluctuations and exposure of sediments to the air. Similar results have also been recently observed in Minnesota peatlands that have experienced variations in wetting and drying conditions in response to drought conditions (Wasik et al., 2015). The commonality of findings across disparate ecosystems and environmental drivers, which can be natural (drought) or anthropogenic (reservoir management), suggest that these processes can affect the Hg cycling across broad landscapes.
Fluctuation water levels is a common feature of many reservoir systems, particularly those managed for flood control. Similar wetting and drying cycles can also occur in natural water bodies, such as along floodplains or ombrotrophic bogs as a result of climatological variability (Singer et al., 2016). Combined with the global distribution of Hg pollution through atmospheric transport and deposition, the processes that resulted in increased MeHg production occurring at our study site may translate to other systems as well and may help explain why fish Hg levels tend to be particularly elevated in reservoirs compared to natural lakes (Kamman et al., 2005, Willacker et al., 2016). The degree to which water level fluctuations affect Hg methylation at a particular location is expected to vary depending on a host of site-specific conditions such as: the quantity and quality of organic carbon, the microbial community structure and abundance, whether sulfate or other electron acceptors become limited during the year, and the nature of inorganic Hg speciation and associations with solid phase sediment. Currently, management activities aimed at reducing the impacts of Hg contaminated sediments have mainly focused on actions like dredging and capping (Randall and Chattopadhyay, 2013), or more recently the addition of activated carbonor biochar (Gomez-Eyles et al., 2013). The results from our study suggest that reservoir water level management activities can effect Hg cycling, which may provide additional options and opportunities that can reduce MeHg production. Such activities may be particularly well suited to systems where the sediment Hg contamination is widely dispersed (e.g. atmospheric or river deposition) where traditional remediation options would be less feasible.
Supplementary Material
Acknowledgements
We wish to thank John DeWilde and Dave Krabbenhoft at the USGS for technical support of the isotopic methylation assays. We also thank the following EPA staff: Kira Lynch, Joe Goulet, Jennifer Crawford, Brent Richmond, Jed Januch, Arron Betts, Raymond Wu, Linda Myers, Joel Salter and Erik Peterson. We would also like to the thank the anonymous reviewers for their helpful comments on this manuscript. This work has been subjected to EPA administrative review and approved for publication. Any opinions expressed in this paper are those of the author(s) and do not, necessarily, reflect the official positions and policies of the USEPA or the funding agencies. Any mention of the products or trade names does not constitute recommendations for use by the USEPA.
References
- Ambers RKR, Hygelund BN, 2001. Contamination of two Oregon reservoirs by cinnabar mining and mercury amalgamation. Environ. Geol 40, 699e707. [Google Scholar]
- Anderson MR, Scruton DA, Williams UP, Payne JF, 1995. Mercury in fish in the smallwood reservoir, labrador, 21 years after impoundment. Water Air Soil Pollut 80, 927–930. [Google Scholar]
- Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL, 2003. Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. In: Cai Y, Braids OC (Eds.), Biogeochemistry of Environmentally Important Trace Elements, pp. 262–297.
- Benoit JM, Gilmour CC, Mason RP, Heyes A, 1999. Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ. Sci. Technol 33, 951–957. [Google Scholar]
- Bloom NS, Gill GA, Cappellino S, Dobbs C, McShea L, Driscoll C, Mason R, Rudd J, 1999. Speciation and cycling of mercury in Lavaca Bay, Texas, sediments. Environ. Sci. Technol 33, 7–13. [Google Scholar]
- Bodaly RA, Jansen WA, Majewski AR, Fudge RJP, Strange NE, Derksen AJ, Green DJ, 2007. Postimpoundment time course of increased mercury concentrations in fish in hydroelectric reservoirs of northern Manitoba, Canada. Archives Environ. Contam. Toxicol 53, 379–389. [DOI] [PubMed] [Google Scholar]
- Branfireun BA, Heyes A, Roulet NT, 1996. The hydrology and methylmercury dynamics of a Precambrian Shield headwater peatland. Water Resour. Res 32, 1785–1794. [Google Scholar]
- Branfireun BA, Roulet NT, Kelly CA, Rudd JWM, 1999. In situ sulphate stimulation of mercury methylation in a boreal peatland: toward a link between acid rain and methylmercury contamination in remote environments. Glob. Biogeochem. Cycles 13, 743–750. [Google Scholar]
- Brigham ME, Krabbenhoft DP, Olson ML, DeWild JF, 2002. Methylmercury in flood-control impoundments and natural waters of northwestern Minnesota, 1997–99. Water Air Soil Pollut 138, 61–78. [Google Scholar]
- Buckman KL, Marvin-DiPasquale M, Taylor VF, Chalmers A, Broadley HJ, Agee J, Jackson BP, Chen CY, 2015. Influence of a chlor-alkali superfund site on mercury bioaccumulation in periphyton and low-trophic level fauna. Environ. Toxicol. Chem 34, 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compeau GC, Bartha R, 1985. Sulfate-reducing bacteria - principal methylators of mercury in anoxic estuarine sediment. Appl. Environ. Microbiol 50, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LR, Morgans DL, Thoms B, Villenueve D, 2013. Extreme precipitation appears a key driver of mercury transport from the watershed to Cottage Grove Reservoir, Oregon. Environ. Pollut 176, 178–184. [DOI] [PubMed] [Google Scholar]
- DeWild JF, Olson Mark L., Olund Shane D., 2002. Determination of Methyl Mer- cury by Aqueous Phase Ethylation, Followed by Gas Chromatographic Separation with Cold Vapor Atomic Fluorescence Detection. Report 01–445 Geological Survey, Middleton, WI: U.S. [Google Scholar]
- DeWild JF, Olund Shane D., Olson Mark L., Tate Michael T., 2004. Method for the Preparation and Analysis of Solids and Suspended Solids for Methylmercury, Techniques and Methods, Book 5, Chapter A-7 Geological Survey, U.S. [Google Scholar]
- Domagalski J, 2001. Mercury and methylmercury in water and sediment of the sacramento river basin, California. Appl. Geochem 16, 1677–1691. [Google Scholar]
- Eckley CS, Hintelmann H, 2006. Determination of mercury methylation potentials in the water column of lakes across Canada. Sci. Total Environ 368, 111–125. [DOI] [PubMed] [Google Scholar]
- Eckley CS, Luxton TP, McKernan JL, Goetz J, Goulet J, 2015. Influence of reservoir water level fluctuations on sediment methylmercury concentrations downstream of the historical Black Butte mercury mine, OR. Appl. Geochem 61, 284–293. [Google Scholar]
- Ecology & Environment, 1998. Black Butte Mine Site Inspection Report US Environmental Protection Agency, p. 317. [Google Scholar]
- Evers DC, Han Y-J, Driscoll CT, Kamman NC, Goodale MW, Lambert KF, Holsen TM, Chen CY, Clair TA, Butler T, 2007. Biological mercury hotspots in the northeastern United States and southeastern Canada. Bioscience 57, 29e43. [Google Scholar]
- Golding GR, Kelly CA, Sparling R, Loewen PC, Rudd JWM, Barkay T, 2002. Evidence for facilitated uptake of Hg(II) by Vibrio anguillarum and Escherichia coli under anaerobic and aerobic conditions. Limnol. Oceanogr 47, 967–975. [Google Scholar]
- Gomez-Eyles JL, Yupanqui C, Beckingham B, Riedel G, Gilmour C, Ghosh U, 2013. Evaluation of biochars and activated carbons for in situ remediation of sediments impacted with organics, mercury, and methylmercury. Environ. Sci. Technol 47, 13721–13729. [DOI] [PubMed] [Google Scholar]
- Graham AM, Aiken GR, Gilmour CC, 2012. Dissolved organic matter enhances microbial mercury methylation under sulfidic conditions. Environ. Sci. Technol 46, 2715–2723. [DOI] [PubMed] [Google Scholar]
- Hall BD, St Louis VL, Rolfhus KR, Bodaly RA, Beaty KG, Paterson MJ, Cherewyk KA, 2005. Impacts of reservoir creation on the biogeochemical cycling of methyl mercury and total mercury in Boreal Upland Forests. Eco- systems 8, 248–266. [Google Scholar]
- Hamelin S, Amyot M, Barkay T, Wang Y, Planas D, 2011. Methanogens: principal methylators of mercury in lake periphyton. Environ. Sci. Technol 45, 7693–7700. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF, 2004. Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ. Sci. Technol 38, 1487–1495. [DOI] [PubMed] [Google Scholar]
- He T, Lu J, Yang F, Feng X, 2007. Horizontal and vertical variability of mercury species in pore water and sediments in small lakes in Ontario. Sci. Total Environ 386, 53–64. [DOI] [PubMed] [Google Scholar]
- Hollweg TA, Gilmour CC, Mason RP, 2009. Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental margin. Mar. Chem 114, 86–101. [Google Scholar]
- Hsu-Kim H, Kucharzyk KH, Zhang T, Deshusses MA, 2013. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review. Environ. Sci. Technol 47, 2441–2456. [DOI] [PubMed] [Google Scholar]
- Hurley JP, Benoit JM, Babiarz CL, Shafer MM, Andren AW, Sullivan JR, Hammond R, Webb DA, 1995. Influences of watershed characteristics on mercury levels in Wisconsin rivers. Environ. Sci. Technol 29, 1867–1875. [DOI] [PubMed] [Google Scholar]
- Johnson WP, Swanson N, Black B, Rudd A, Carling G, Fernandez DP, Luft J, Van Leeuwen J, Marvin-DiPasquale M, 2015. Total- and methyl-mercury concentrations and methylation rates across the freshwater to hypersaline continuum of the Great Salt Lake, Utah, USA. Sci. Total Environ 511, 489–500. [DOI] [PubMed] [Google Scholar]
- Kamman NC, Burgess NM, Driscoll CT, Simonin HA, Goodale W, Linehan J, Estabrook R, Hutcheson M, Major A, Scheuhammer AM, Scruton DA, 2005. Mercury in freshwater fish of northeast North America - a geographic perspective based on fish tissue monitoring databases. Ecotoxicology 14, 163–180. [DOI] [PubMed] [Google Scholar]
- Kehrig HA, Costa M, Moreira I, Malm O, 2002. Total and methylmercury in a Brazilian estuary, Rio de Janeiro. Mar. Pollut. Bull 44, 1018–1023. [DOI] [PubMed] [Google Scholar]
- Kerin EJ, Gilmour CC, Roden E, Suzuki MT, Coates JD, Mason RP, 2006. Mercury methylation by dissimilatory iron-reducing bacteria. Appl. Environ. Microbiol 72, 7919–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JK, Saunders FM, Lee RF, Jahnke RA, 1999. Coupling mercury methylation rates to sulfate reduction rates in marine sediments. Environ. Toxicol. Chem 18, 1362e1369. [Google Scholar]
- Korthals ET, Winfrey MR, 1987. Seasonal and spatial variations in mercury methylation and demethylation in an oligotrophic lake. Appl. Environ. Micro- biol 53, 2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson JH, Maki RP, Knights BC, Gray BR, 2014. Can mercury in fish be reduced by water level management? Evaluating the effects of water level fluctuation on mercury accumulation in yellow perch (Perca flavescens). Ecotoxicology 23, 1555–1563. [DOI] [PubMed] [Google Scholar]
- Lehnherr I, St Louis VL, Kirk JL, 2012. Methylmercury cycling in high Arctic wetland ponds: controls on sedimentary production. Environ. Sci. Technol 46, 10523–10531. [DOI] [PubMed] [Google Scholar]
- Lewis ME, Brigham ME, 2004. Processing of water samples: Low Level Mercury 5.6.4.B. In: Wilde FD, Radtke DB, Gibs, Jacob, Iwatsubo RT (Eds.), U.S. Geological Survey Techniques of Water-Resources Investigations USGS, p. 26. [Google Scholar]
- Marvin-DiPasquale M, Lutz MA, Brigham ME, Krabbenhoft DP, Aiken GR, Orem WH, Hall BD, 2009. Mercury cycling in stream ecosystems. 2. Benthic methylmercury production and bed sediment-pore water partitioning. Environ. Sci. Technol 43, 2726–2732. [DOI] [PubMed] [Google Scholar]
- Mitchell CPJ, Branfireun BA, Kolka RK, 2008. Spatial characteristics of net methylmercury production hot spots in peatlands. Environ. Sci. Technol 42, 1010–1016. [DOI] [PubMed] [Google Scholar]
- Mitchell CPJ, Gilmour CC, 2008. Methylmercury production in a Chesapeake Bay salt marsh. J. Geophys. Research-Biogeosciences 113. [Google Scholar]
- Montgomery S, Lucotte M, Rheault I, 2000. Temporal and spatial influences of flooding on dissolved mercury in boreal reservoirs. Sci. Total Environ 260, 147–157. [DOI] [PubMed] [Google Scholar]
- Olund SD, DeWild, John F, Olson Mark L., Tate, Michael T, 2004. Method for the Preparation and Analysis of Solids and Suspended Solids for Total Mercury, Techniques and Methods, Book 5, Chapter A-8 U.S. Geological Survey. [Google Scholar]
- Randall PM, Chattopadhyay S, 2013. Mercury contaminated sediment sites-An evaluation of remedial options. Environ. Res 125, 131–149. [DOI] [PubMed] [Google Scholar]
- Schaefer JK, Rocks SS, Zheng W, Liang L, Gu B, Morel FMM, 2011. Active transport, substrate specificity, and methylation of Hg(II) in anaerobic bacteria. Proc. Natl. Acad. Sci. U. S. A 108, 8714–8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J, Castelle S, Blanc G, Dabrin A, Masson M, Lanceleur L, Bossy C, 2010. Mercury methylation in the sediments of a macrotidal estuary (Gironde Estuary, south-west France). Estuar. Coast. Shelf Sci 90, 80–92. [Google Scholar]
- Schartup AT, Balcom PH, Mason RP, 2014. Sediment-porewater partitioning, total sulfur, and methylmercury production in estuaries. Environ. Sci. Technol 48, 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selch TM, Hoagstrom CW, Weimer EJ, Duehr JP, Chipps SR, 2007. Influence of fluctuating water levels on mercury concentrations in adult walleye. Bull. Environ. Contam. Toxicol 79, 36–40. [DOI] [PubMed] [Google Scholar]
- Singer MB, Harrison LR, Donovan PM, Blum JD, Marvin-DiPasquale M, 2016. Hydrologic indicators of hot spots and hot moments of mercury methylation potential along river corridors. Sci. Total Environ 568, 697–711. [DOI] [PubMed] [Google Scholar]
- Sorensen JA, Kallemeyn LW, Sydor M, 2005. Relationship between mercury accumulation in young-of-the-year yellow perch and water-level fluctuations. Environ. Sci. Technol 39, 9237–9243. [DOI] [PubMed] [Google Scholar]
- St Louis VL, Rudd JWM, Kelly CA, Bodaly RA, Paterson MJ, Beaty KG, Hesslein RH, Heyes A, Majewski AR, 2004. The rise and fall of mercury methylation in an experimental reservoir. Environ. Sci. Technol 38, 1348–1358. [DOI] [PubMed] [Google Scholar]
- Louis VL St., Rudd JWM, Kelly CA, Beaty KG, Bloom NS, Flett RJ, 1994. Importance of wetlands as sources of methyl mercury to Boreal forest ecosystems. Can. J. Fish. Aquatic Sci 51, 1065–1076. [Google Scholar]
- Ullrich SM, Tanton TW, Abdrashitova SA, 2001. Mercury in the aquatic environment: a review of factors affecting methylation. Crit. Rev. Environ. Sci. Technol 31, 241–293. [Google Scholar]
- United States Environmental Protection Agency, 2009. Guidance for Labeling Externally Validated Laboratory Analytical Data for Superfund Use U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response, Washington, DC. [Google Scholar]
- United States Environmental Protection Agency, 2011. National Listing of FIsh Advisories, 2011 EPA 820/F-13/058, Washington, DC. [Google Scholar]
- United States Geological Survey, 2006. Collection of water samples (ver. 2.0): U.S. Geological Survey Techniques of Water-Resources Investigations
- Wasik JKC, Engstrom DR, Mitchell CPJ, Swain EB, Monson BA, Balogh SJ, Jeremiason JD, Branfireun BA, Kolka RK, Almendinger JE, 2015. The effects of hydrologic fluctuation and sulfate regeneration on mercury cycling in an experimental peatland. J. Geophys. Research-Biogeosciences 120, 1697–1715. [Google Scholar]
- Watras CJ, Morrison KA, Kent A, Price N, Regnell O, Eckley C, Hintelmann H, Hubacher T, 2005. Sources of methylmercury to a wetland-dominated lake in northern Wisconsin. Environ. Sci. Technol 39, 4747–4758. [DOI] [PubMed] [Google Scholar]
- Willacker JJ, Eagles-Smith CA, Lutz MA, Tate MT, Lepak JM, Ackerman JT, 2016. Reservoirs and water management influence fish mercury concentrations in the western United States and Canada. Sci. Total Environ 568, 739–748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


