Abstract
Background
Claudin 8 (CLDN8), an integral membrane protein that constitutes tight junctions in cell membranes, was recently implicated in tumor progression. However, its roles in colorectal cancer (CRC) progression and metastasis remain unknown.
Methods
In this study, we examined the effect of CLDN8 on the progression of CRC, including cell proliferation, migration, and invasion, and determines its underlying molecular mechanism using in vitro CRC cell lines and in vivo mouse xenograft models.
Results
We found that CLDN8 expression in human CRC tissues was significantly higher than that in adjacent normal tissues. The knockdown of CLDN8 markedly suppressed the proliferation, migration, and invasion of SW480 and HT-29 CRC cells, whereas the overexpression of CLDN8 notably promoted tumor progression in SW480 and HT-29 CRC cells. Mechanistic studies revealed that CLDN8 upregulated p-ERK (p-PKB/AKT) and MMP9 in CRC cells. Notably, the MAPK/ERK inhibitor PD98095 dramatically attenuated the effects of CLDN8 on p-ERK and MMP9. Moreover, PD98095 remarkably blocked the tumor-promoting activity of CLDN8. The knockdown of CLDN8 also inhibited the in vivo tumor growth in a nude mouse xenograft model. Collectively, CLDN8 promoted CRC cell proliferation, migration, and invasion, at least in part, by activating the MAPK/ERK signaling pathway.
Conclusion
These findings suggest that CLDN8 exhibits an oncogenic effect in human CRC progression.
Keywords: CLDN8, colorectal cancer, MAPK/ERK signaling
Introduction
Colorectal cancer (CRC) is one of the most common carcinomas worldwide and causes significant mortality.1–3 There are >1.2 million newly diagnosed cases of CRC and >600,000 deaths from this disease every year.4–6 Although recent advances in surgical resection techniques have increased the survival rate for patients with early-stage CRC, the long-term prognosis for most CRC patients remains poor, mainly due to recurrence and metastases.5,7,8 The molecular profiling (including DNA and proteins) of CRC has had increasing importance for the identification of prognostic biomarkers and the development of novel therapeutic strategies.3,4,6,8–12 However, the exact mechanisms underlying CRC development remain unknown. Therefore, identifying the key molecules involved in CRC progression may help provide novel therapeutic targets and improve the prognosis of CRC.
Claudin 8 (CLDN8), which is located in the cell membrane, is a member of the CLDN superfamily that constitutes tight junctions.13,14 CLDN8 is overexpressed in several human cancer cell lines and plays a vital role in the progression of several human cancers, including prostate cancer, renal cell carcinoma, and osteosarcoma.13–23 In prostate cancer, functional analyses of CLDN8 by overexpression and knockdown have indicated that CLDN8 promotes the migration, invasion, and metastasis of prostate cancer cells via intracellular signal transduction.13 These data indicate that CLDN8-mediated signaling pathways are involved in human tumor metastasis. However, the roles of CLDN8 in CRC cells have not been elucidated.
This study examines the effect of CLDN8 on the progression of CRC, including cell proliferation, migration, and invasion, and determines its underlying molecular mechanism using in vitro CRC cell lines and in vivo mouse xenograft models. Here, we demonstrate that CLDN8 promotes CRC cell proliferation, migration, and invasion via the MAPK/ERK signaling pathway. These findings suggest that CLDN8 plays an important role in regulating CRC progression and may serve as a prognostic biomarker for CRC.
Materials and methods
Tissue samples
Fresh CRC tumor tissue samples and corresponding adjacent normal tissues were obtained from 20 patients diagnosed with CRC following surgical resection through laparotomy. The demographic data are shown in Table 1. No distant metastasis was observed among these patients. All the patients did not receive neoadjuvant therapy, radiotherapy, or chemotherapy prior to surgery. Tissue samples were immediately frozen in liquid nitrogen for further analysis. The study protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University with written informed consent obtained from each patient. This study was conducted in accordance with the Declaration of Helsinki.
Table 1.
Demographic data of the patients
| Clinical variables | No. of patients (N=20) |
|---|---|
|
| |
| Gender | |
| Female | 12 |
| Male | 8 |
| Age | |
| Median | 55.2 |
| Range | 41–73 |
| Tumor location | |
| Rectum | 4 |
| Ascending | 3 |
| Cecum | 5 |
| Descending | 5 |
| Transverse | 1 |
| Sigmoid | 2 |
| Distant metastasis | |
| Yes | 0 |
| No | 20 |
Cell lines and cell culture
The human CRC cell lines SW480, HT-29, Caco-2, DLD-1, HCT116, and SW620, together with a normal colorectal epithelial cell line HCoEpiC were obtained from the Academia Sinica Cell Bank (Shanghai, China) and authenticated according to the American Type Culture Collection recommendations. The cell culture was conducted according to previous studies. In brief, SW480 and SW620 cells were cultured in Leibovitz’s L-15 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 µg/mL streptomycin (1% P/S). HT29 cells were cultured in McCoy’s medium (Thermo Fisher Scientific) supplemented with 10% FBS (Thermo Fisher Scientific) and 1% P/S. HCoEpiC, Caco-2, DLD-1, and HCT116 were maintained in Roswell Park Memorial Institute-1640 medium (HyClone) supplemented with 10% FBS (Thermo Fisher Scientific) and 1% P/S. All the cell cultures were conducted at 37°C under a humidified condition of 5% CO2.
Immunohistochemistry (IHC) staining
IHC staining was carried out on paraformaldehyde-fixed paraffin tissue sections strictly according to the manufacturer’s instructions.13 In brief, to block endogenous peroxidase, 0.3% H2O2 was added to 5 µm thick slides. After that, the slides were incubated in 10% BSA for 30 minutes. The slides were then incubated overnight with the anti-CLDN8 antibody (1:75; Abcam, Shanghai, China) or Ki-67 (1:400; Cell Signaling Technology, Shanghai, China) and visualized with 3,3′-diami-nobenzidine solution. The degree of IHC staining was scored as previously described.13 The final weighed expression score (0–8) was obtained by calculating the intensity values of IHC staining and the percentage of positive cells.
Overexpression and knockdown of CLDN8
To overexpress CLDN8, the full-length open reading frame of CLDN8 was cloned into pcDNA3.1, pCMV4-FLAG, and pEGFP-N1 vectors. The transfection of SW480 and HT-29 cells was performed with Lipofectamine 2000 (Thermo Fisher Scientific). Transfected SW480 and HT-29 cells stably expressing CLDN8 were obtained after selection with G418 (500 µg/mL; Thermo Fisher Scientific) for at least 2 weeks. For knockdown of CLDN8, SW480 and HT-29 cells were transfected with CLDN8 shRNA plasmid with a sequence as follows: 5′-GCCAUCCUUGGCAUGAAAUGCACCA-3′. The overexpression and knockdown of CLDN8 were determined by Western blot (WB) analyses.
Cell proliferation
To evaluate the effects of CLDN8 on CRC, SW480 and HT-29 cells (4×103 cells/well) were seeded into 96-well plates. Cell proliferation ability was analyzed 24, 48, 72, and 96 hours post-infection using a Cell Counting Kit-8 (CCK-8) Assay Kit (Dojindo Molecular Technologies, Rockville, MD, USA). The OD value of each well was measured at 490 nm under an automatic microplate reader.
Wound healing assay
Six-well plates were seeded with 1×106 SW480 and HT-29 cells until they formed a cell monolayer. The confluent cultures were then carefully scratched with a sterile 1 mL pipette tip to generate a wound, which was then washed and cultured in complete medium without FBS. At 0 hour and after 24 hours, the wounds were photographed under a light microscope, and the wound closure percentage (%) was evaluated using TScratch software.
Invasion assay
Invasion assay was performed using a Transwell system (Corning Incorporated, Corning, NY, USA). The 8 µm pore-sized polycarbonate membrane was coated with Matrigel (BD Biosciences, San Jose, CA, USA). Also, 5×104 cells/well were suspended in 200 µL serum-free medium and were added to the upper chamber, while 600 µL medium containing 15% FBS was added to the lower well of each chamber. After 24 hours of incubation at 37°C, the migrated cells in the lower chambers were stained with 0.1% crystal violet, which were then counted using light microscopy.
RNA isolation and quantitative reverse transcription PCR (qRT-PCR)
Total RNA was obtained using Trizol (Thermo Fisher Scientific) according to the manufacturer’s instructions, which was then reverse transcribed into cDNA by using an RT reagent kit (Takara, Kusatsu, Japan). qRT-PCR was performed as previously described using an SYBR Green PCR Kit (Takara) on the ABI StepOne Plus System. The sequences of primers used were as follows: CLDN8, forward: 5′-TGAATGTTGCCCAAAAACGTG-3′ and reverse: 5′-GCGATGGGAAGGTATCGAGTATC′; GAPDH, the internal control, forward: 5′-CTGGGCTACACTGAGCACC-3′ and reverse: 5′-AAGTGGTCGTTGAGGGCAATG-3′. The value of 2−DDCt was used to evaluate relative gene expression.
WB analysis
For the extraction of proteins from tissue samples, RIPA lysis buffer (Beyotime Biotechnology, Beijing, China) was used on ice for 60 minutes. After the protein concentrations were measured by using a bicinchoninic acid protein assay kit (Beyotime Biotechnology), equivalent amounts of lysates were loaded and separated in 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were then blocked with 5% nonfat milk containing 0.1% Tween-20 for 1 hour and incubated overnight at 4°C with primary antibodies including anti-CLDN8 (1:1,000; Abcam), MMP9 (1:1,000; CST), ERK (1:1,000; CST), p-ERK (1:1,000; CST), E-cadherin (1:1,000; CST), N-cadherin (1:1,000; CST), and GAPDH (1:1,000; CST), respectively. Then a corresponding HRP-labeled secondary antibody (1:5,000; Beyotime Biotechnology) was applied to the membranes and incubated for 2 hours at room temperature. An enhanced chemiluminescence detection reagent (EMD Millipore) was used to detect protein signaling, and a Bio-Rad XRS chemiluminescence detection system (Bio-Rad) was used to evaluate the protein expression.
Subcutaneous xenografts model
Female Balb/c nude mice aged 4–6 weeks (18–20 g, n=8/group) were subcutaneously injected with SW480 and HT-29 with CLDN8 knockdown using CLDN8 shRNA plasmid (CLDN8-knock) or the negative control plasmid (Knock-NC) (2×106 cells/mouse; eight mice/group). At the end point (4 weeks after tumor cell inoculation), the tumor-bearing mice were sacrificed and tumors were harvested and weighted. All experimental procedures were approved by the Animal Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Statistical analyses
All experiments in this study were repeated independently at least three times. Statistical differences between the results of each group were evaluated using Student’s t-test or one-way ANOVA with a Bonferroni post hoc test when appropriate. Statistical procedures were undertaken using SPSS 23.0 software (IBM Corporation, Armonk, NY, USA). Data were presented as mean ± SD. A P-value <0.05 was considered statistically significant.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University. All subjects provided written informed consent. Animal studies were approved and conducted strictly in accordance with the institutional ethical guidelines of the Committee on the Use of Live Animals in Teaching and Research of Zhengzhou University.
Results
CLDN8 is upregulated in CRC cell lines and clinical human CRC samples
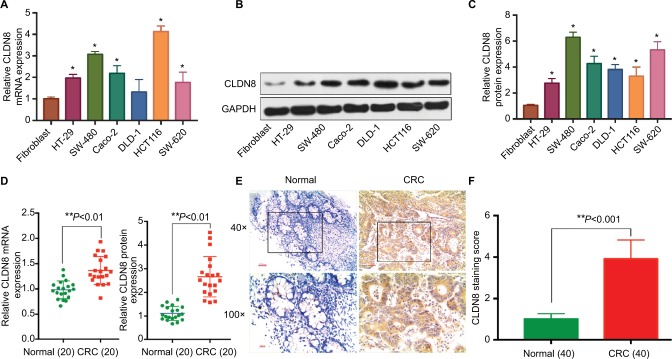
qRT-PCR and WB analysis revealed that the CLDN8 mRNA and protein levels were significantly higher in CRC cell lines (SW480, HT-29, Caco-2, HCT116, and SW620) and CRC tissues compared to the normal colorectal epithelial cell line HCoEpiC and adjacent normal tissues (P<0.05; Figure 1A–D). IHC staining again confirmed that CLDN8 protein was upregulated in CRC samples compared to corresponding adjacent normal tissues (P<0.05; Figure 1E, F).
Figure 1.
CLDN8 is overexpressed in CRC cell lines and clinical human CRC samples.
Notes: The results of qRT-PCR (A) and Western blotting analysis (B, C) showed that CLDN8 mRNA and protein levels were significantly higher in CRC cell lines (SW480, HT-29, Caco-2, HCT116, and SW620 cells) compared to normal colorectal epithelial cell line HCoEpiC. Western blotting and PCR analysis (D) and IHC staining (E, F) showed that CLDN8 protein and mRNA were overexpressed in CRC samples when compared with the corresponding adjacent normal tissues. *P<0.05 and **P<0.01 when compared with HCoEpiC or the normal tissue.
Abbreviations: CRC, colorectal cancer; IHC, immunohistochemistry; qRT-PCR, quantitative reverse transcription PCR.
CLDN8 promotes CRC cell proliferation in vitro
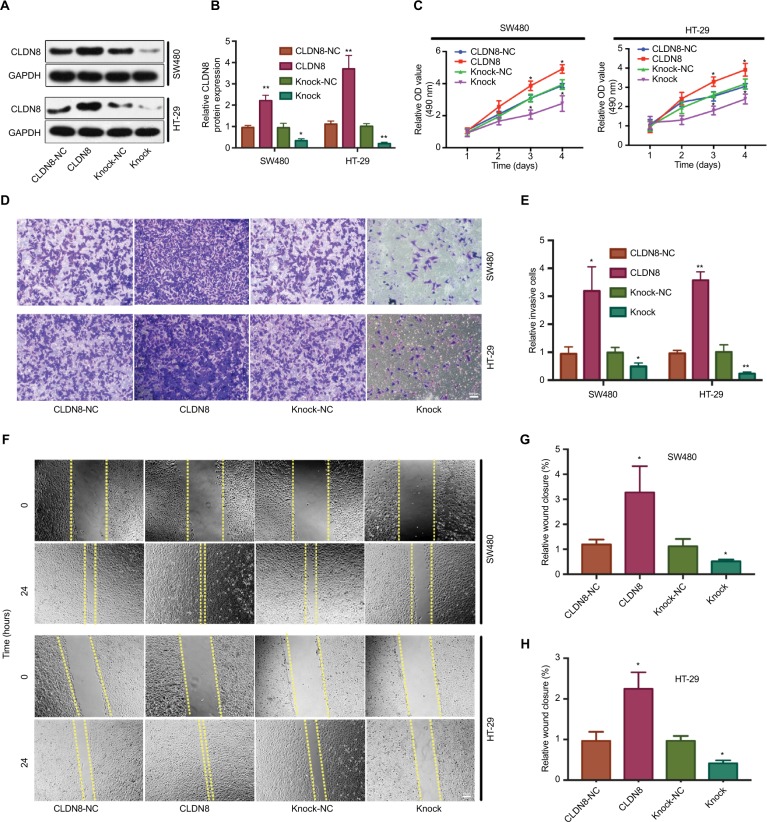
To explore the function of CLDN8 in CRC, we first transfected SW480 and HT-29 cells with a plasmid to overexpress and knock down CLDN8 expression. WB confirmed successful transfection and stable establishment in both SW480 and HT-29 cells (Figure 2A, B). The CCK-8 assay revealed that CLDN8 overexpression promoted the proliferation of SW480 and HT-29 cells, while CLDN8 knockdown significantly decreased proliferation in a time-dependent manner (Figure 2C).
Figure 2.
Effects of CLDN8 on CRC cell proliferation migration and invasion.
Notes: (A, B) Western blot analysis confirmed the successful transfection and stable establishment in both SW480 and HT-29 cells. (C) The results of CCK-8 assay revealed that CLDN8 overexpression promoted the proliferation of SW480 and HT-29 cells, while CLDN8 knockdown significantly decreased the proliferation rate in a time-dependent manner. (D, E) The results of Transwell invasion assay showed that upregulation of CLDN8 expression in SW480 and HT-29 cells significantly enhanced cell invasion, which was inhibited by CLDN8 knockdown. (F–H) Wound healing assay showed that CLDN8 overexpression promoted SW480 and HT-29 cells migration, whereas downregulation of CLDN8 markedly reduced cell migration. The meaning of each group name: CLDN8-NC, the negative control group of CLDN8 overexpression; CLDN8, overexpression of CLDN8; Knock-NC, the negative control group of TROP2 knockdown; Knock, knockdown of CLDN8. *P<0.05 when compared with CLDN8-NC. ; **P<0.01 when compared with CLDN8-NC. Bar = 100μm.
Abbreviations: CCK-8, Cell Counting Kit-8; CRC, colorectal cancer.
CLDN8 promotes CRC cell migration and invasion
To determine the effect of CLDN8 on CRC cell migration and invasion, a wound healing assay and a Transwell invasion assay were performed. The Transwell invasion assay showed that upregulation of CLDN8 expression in SW480 and HT-29 cells significantly enhanced cell invasion, which was inhibited by CLDN8 knockdown (Figure 2D, E). Similar results were obtained from the wound healing assay, which showed that CLDN8 overexpression promoted SW480 and HT-29 cell migration, whereas downregulation of CLDN8 markedly reduced cell migration (Figure 2F–H). These data indicate that CLDN8 promotes the migration and invasion of CRC cells in culture.
CLDN8 enhances the MAPK/ERK signaling pathway in CRC cells
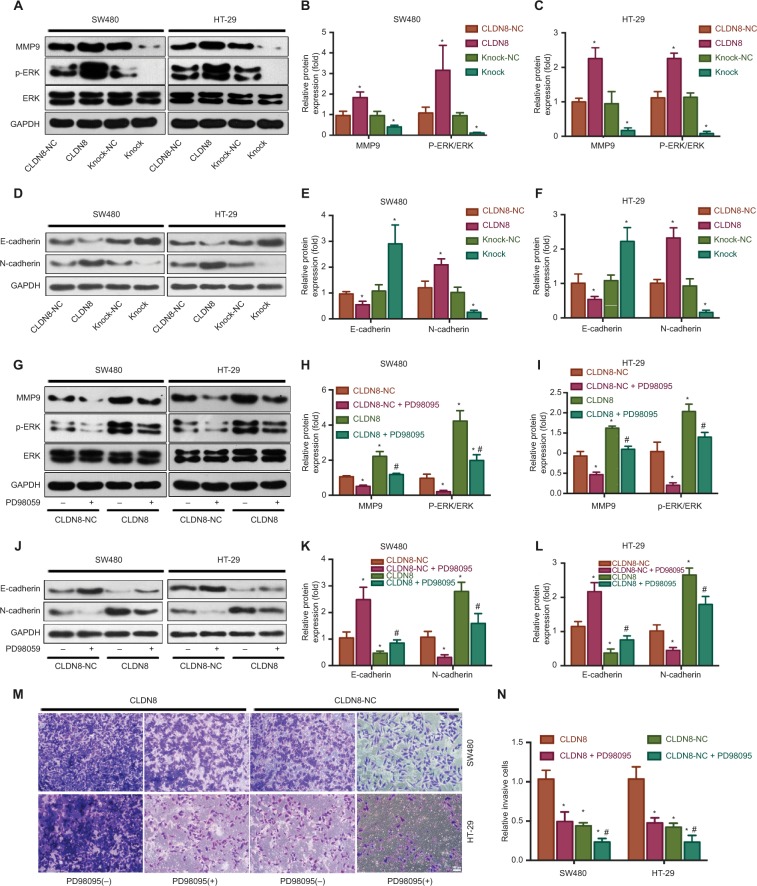
To examine the underlying mechanism by which CLDN8 participates in the proliferation, migration, and invasion of CRC cells, MAPK/ERK,24,25 one of the most common key regula tors of CRC development, was evaluated by WB. As shown in Figure 3A–C, the expression of MMP9 was significantly upregulated in the CLDN8 overexpression group. In addition, CLDN8 overexpression significantly enhanced the level of p-ERK, whereas CLDN8 knockdown reduced its expression (Figure 3A–C). Subsequently, we applied PD98059, a synthetic inhibitor of the MAPK/ERK pathway, to confirm the involvement of ERK in the CLDN8-mediated regulation of CRC cells. As expected, treatment of CLDN8-overexpressing SW480 and HT-29 cells with PD98059 significantly impaired CLDN8-induced ERK phosphorylation (Figure 3G–I). Moreover, enhanced invasion of CRC cells in response to CLDN8 overexpression was reduced after treatment with PD98059 (Figure 3M, N). These results confirm that CLDN8 activates the MAPK/ERK signaling pathway in CRC cells.
Figure 3.
CLDN8 enhances the MAPK/ERK signaling pathway in CRC cells.
Notes: (A–C) The expression of MMP9 was significantly upregulated in CLDN8 overexpression group. In addition, CLDN8 overexpression significantly enhanced the levels of p-ERK, whereas CLDN8 knockdown reduced their expression level. (D–F) CLDN8 overexpression remarkably inhibited the protein expression level of E-cadherin, whereas CLDN8 knockdown effectively upregulated its expression. In contrast, the expression level of N-cadherin was upregulated under CLDN8 overexpression, but downregulated under CLDN8 knockdown. (G–I) Treatment of CLDN8-overexpressed SW480 and HT-29 cells with PD98059 significantly impaired CLDN8-induced ERK phosphorylation. *P<0.05 when compared with CLDN8-NC. (J–L) Application of PD98059 significantly impaired CLDN8-induced E-cadherin inhibition and N-cadherin enhancement, respectively. (M, N) Enhanced invasion of CRC cells in response to CLDN8 overexpression was reduced after the application of PD98059. *P<0.05 when compared with CLDN8; #P<0.05 when compared with CLDN8-NC. CLDN8-NC, the negative control group of CLDN8 overexpression. Bar = 100μm.
Abbreviation: CRC, colorectal cancer.
To further determine whether the influence of CLDN8 on ERK signaling and MMP-mediated invasion was based on the loss of E-cadherin, additional WB analyses were conducted. As is shown in Figure 3D–F, CLDN8 overexpression remarkably inhibited the protein expression level of E-cadherin, whereas CLDN8 knockdown effectively upregulated its expression. In contrast, the expression level of N-cadherin was upregulated under CLDN8 overexpression, but downregulated under CLDN8 knockdown. Subsequently, application of PD98059 significantly impaired the CLDN8-induced E-cadherin inhibition and N-cadherin enhancement, respectively (Figure 3J–L). Thus, CLDN8-induced loss of E-cadherin and N-cadherin enrichment may at least partly contribute to the downstream ERK signaling activation and MMP-mediated invasion.
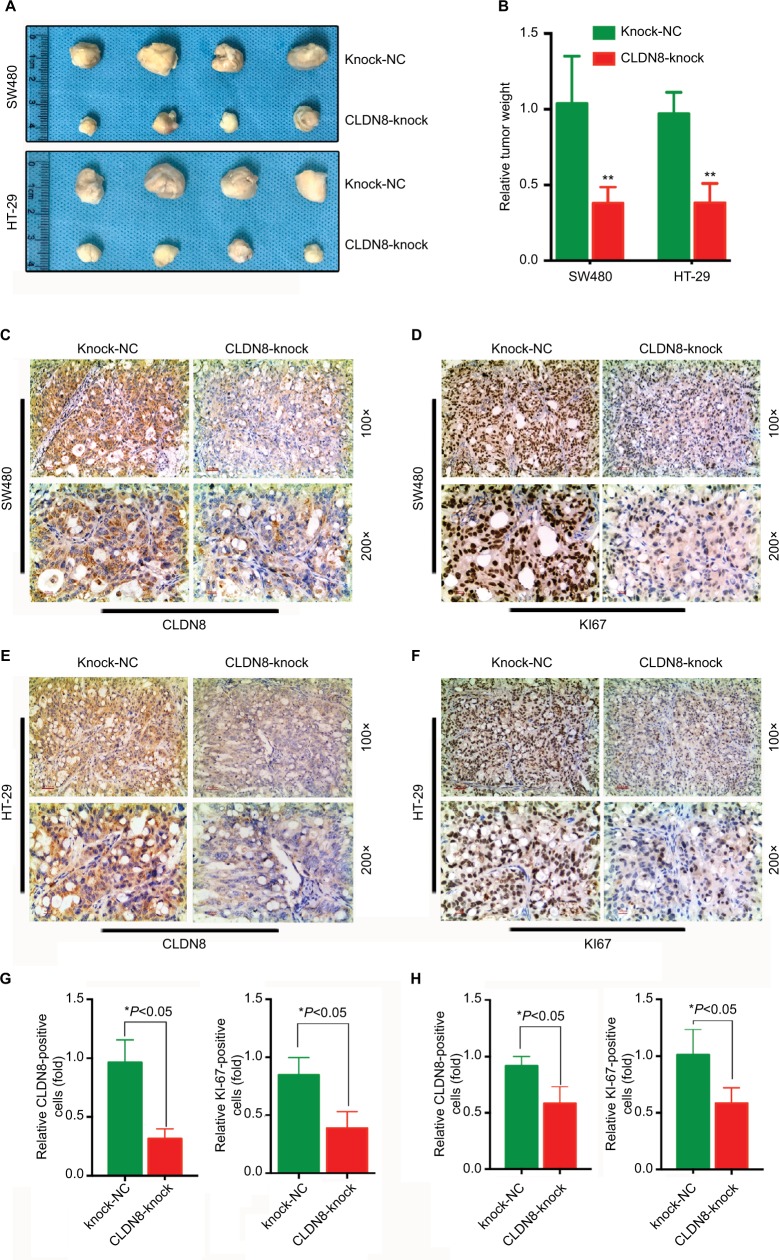
Knockdown of CLDN8 inhibits CRC growth in nude mice
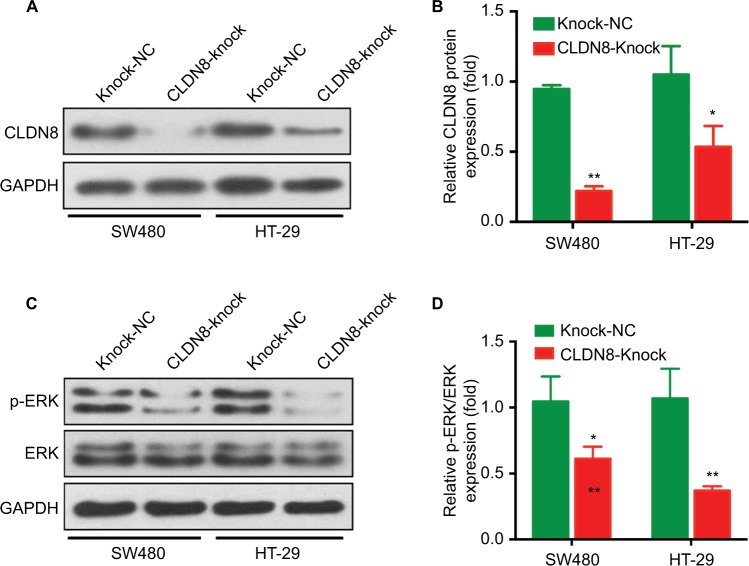
To investigate whether CLDN8 affects the growth of CRC cells in vivo, CLDN8-knockdown SW480 and HT-29 cells, which were created using the CLDN8 shRNA plasmid (CLDN8-knock) or negative control plasmid (Knock-NC), were subcutaneously injected into nude mice. CLDN8 knockdown markedly reduced the tumor weight in vivo (Figure 4A, B). The knockdown of CLDN8 was further confirmed by IHC staining (Figure 4C, E, G, H) and WB analysis (Figure 5A, B), and tumors formed by CLDN8-knockdown cells exhibited a lower percentage of Ki-67 than control tumors (Figure 4D, F, G, H). In addition, by conducting WB analysis using tumor xenograft lysates, we found significantly reduced p-ERK expression in the CLDN8-knockdown group when compared with the negative controls (Figure 5C, D). Collectively, these results suggest that the knockdown of CLDN8 inhibits CRC growth in vivo.
Figure 4.
Knockdown of CLDN8 expression inhibits CRC growth in nude mice.
Notes: (A, B) CLDN8 knockdown markedly reduced tumor weight in vivo. (C, E) IHC staining confirmed the knockdown expression of CLDN8 in SW480 and HT-29–induced tumor. (D, F) IHC staining showed that tumors formed by CLDN8-knockdown cells exhibited lower positive percentage of Ki-67 than control tumors. (G) Quantitative analysis of IHC staining in SW480-induced tumor. (H) Quantitative analysis of IHC staining in HT-29–induced tumor. The meaning of each group name: Knock-NC, the negative control group of TROP2 knockdown; CLDN8-knock, knockdown of CLDN8. *P<0.05 when compared with Knock-NC; **P<0.01 when compared with Knock-NC.
Abbreviations: CRC, colorectal cancer; IHC, immunohistochemistry; WB, Western blot.
Figure 5.
Knockdown of CLDN8 expression inhibits MAPK/ERK signaling pathway in vivo.
Notes: (A, B) WB analysis confirmed the knockdown expression of CLDN8 in SW480 and HT-29–induced tumor. *P<0.05 when compared with Knock-NC; **P<0.01 when compared with Knock-NC. Knock-NC, the negative control group of TROP2 knockdown.
Abbreviations: CRC, colorectal cancer; WB, Western blot.
Discussion
We provided evidence that CLDN8 acts as a tumor-promoting gene in human CRC, partially by activating the MAPK/ERK signaling pathway. CLDN8 mRNA and protein levels were upregulated in human CRC cell lines compared with the normal colorectal epithelial cell line HCoEpiC. WB analysis and IHC staining also revealed stronger protein expression of CLDN8 in CRC tissues compared with matched adjacent normal tissues. CLDN8 overexpression promoted the growth of CRC cells. In contrast, the knockdown of CLDN8 in CRC cells repressed the development of CRC both in vitro and in vivo. Mechanistically, CLDN8 activated the MAPK/ERK signaling pathway and PD980059 treatment blocked the effects of CLDN8 in CRC. Moreover, by researching the influence of CLDN8 on epithelial–mesenchymal transition–related biomarkers, we found that the CLDN8-induced loss of E-cadherin and N-cadherin enrichment may also at least partly contribute to the downstream ERK signaling activation and MMP-mediated invasion.
CLDN8, a member of the claudin protein family, which includes CLDN1–27, is an important transmembrane protein component of tight junction strands. CLDN8 and tight junctions are responsible for creating barriers to cytokines, molecules, mediators, solutes, and ions. Moreover, several studies have demonstrated that claudins, along with occludins, and junction adhesion molecules are essential structural elements in carcinogenesis. Indeed, claudins are overexpressed in several human cancer cell lines and tissues and act as tumor-promoting genes. The results of the present study are consistent with those of previous studies regarding the upregulation of CLDN1 and CLDN226–28 and CLDN813–15 in tumor tissues relative to controls. In this study, we found that not only the mRNA, but also the protein level of CLDN8 were overexpressed in CRC tissues and cell lines when compared with the normal controls. The functional role of CLDN8 found in this study was also consistent with previous studies. In prostate cancer, CLDN8 promoted the migration, invasion, and metastasis of prostate cancer cells via intracellular signal transduction.13 In osteosarcoma, high levels of CLDN8 contribute to its malignant proliferation.15 To some extent, these two previous studies support our finding: CLDN8 promotes tumor proliferation, migration, and invasion. On the other hand, based on a study published in 2007,18 CLDN8 was reported to be downregulated in CRC. Another study analyzed the mRNA level of CLDN8 in CRC tissues from 26 patients and revealed the downregulation of CLDN8 in CRC samples.17 However, Zhao et al22 reported that although CLDN8 was downregulated in clinical tissues from patients with oral squamous cell carcinoma (OSCC) compared to normal controls, patients with higher expression of CLDN8 had poorer overall survival, suggesting that CLDN8 promotes OSCC progression. Thus far, there has been no other report on CLDN8 protein expression in CRC tissues or on the functional effect of CLDN8 on CRC cells. Future studies are required to uncover whether this contradictory finding is due to the different roles of CLDN8 at different stages during cancer progression.
The MAPK/ERK pathway is a pivotal signaling pathway involved in the development of several cancers, including CRC.24,25,29 Uncontrolled tumor cell survival and proliferation are usually regulated by the MAPK/ERK pathway.25,30,31 In prostate cancer cells, the MAPK/ERK signaling pathway supports cellular metabolism, proliferation, and survival.32 Alteration of the MAPK/ERK pathway is also strongly implicated in CRC pathogenesis.24,25,33,34 Previous constitutive genomic studies also demonstrated that MAPK signaling pathway is one of the most frequently deregulated pathways in CRC.35,36 MMP9, as a key member of the zinc-dependent endopeptidases family, has been widely accepted to be involved in tumor cell proliferation and invasion.37,38 Kim et al39 reported that CD147 induces the expression of MMP9 via activation of MAPK/ERK in macrophages. Further research by Xu et al34 demonstrated that the ERK inhibitor decreased MMP expression, cell invasion, and migration in CRC. Therefore, further study about the role of CLDN8 on MAPK/ERK/MMP-related pathway may help uncover the underlying molecular mechanism of CLDN8’s regulating effect on CRC. In the current study, CLDN8 promoted the expression of p-ERK via the MAPK/ERK pathway, which was abrogated by treatment with an MAPK inhibitor. Consistent with previous studies, the inhibition of MAPK/ERK was associated with decreased CRC cell proliferation and migration. Thus, we conclude that the promoting effect of CLDN8 on CRC cell lines may be, at least in part, due to the activation of MAPK/ERK signaling.
To our knowledge, this is the first study that investigated the functional effect of CLDN8 on CRC. However, we acknowledge several important limitations of this study. First, we did not evaluate the correlation between CLDN8 expression and patient prognosis. Second, due to the small number of included patients, we were unable to evaluate the roles of CLDN8 at different stages of CRC and the role of CLDN8 in CRC metastasis. In addition, the number of mice is relatively small for a xenograft study, which may reduce the robustness of the conclusions of this study. Considering the complex mechanisms underlying CRC development and endogenous biological differences in CRC cell lines, additional studies are needed.
Conclusion
CLDN8 promoted CRC cell proliferation, migration, and invasion, at least in part, by activating the MAPK/ERK signaling pathway. These findings suggest that CLDN8 exhibits an oncogenic effect in human CRC progression.
Data sharing statement
Please contact the author for data requests.
Footnotes
Author contributions
WL: conception and design of the research; BC: acquisition of data; AR and BC: analysis and interpretation of data; QZ: statistical analysis; BC: drafting the manuscript; WL: revision of manuscript for important intellectual content. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ding C, Luo J, Li L, et al. Gab2 facilitates epithelial-to-mesenchymal transition via the MEK/ERK/MMP signaling in colorectal cancer. J Exp Clin Cancer Res. 2016;35(1) doi: 10.1186/s13046-015-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo YH, Wang LQ, Li B, et al. Wnt/β-catenin pathway transactivates microRNA-150 that promotes EMT of colorectal cancer cells by suppressing CREB signaling. Oncotarget. 2016;7(27):42513–42526. doi: 10.18632/oncotarget.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawaguchi N, Tashiro K, Taniguchi K, et al. Nogo-B (Reticulon-4B) functions as a negative regulator of the apoptotic pathway through the interaction with c-FLIP in colorectal cancer cells. Biochim Biophys Acta. 2018;1864:2600–2609. doi: 10.1016/j.bbadis.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Wei Y, Feng Q, et al. Ribosomal protein S15a promotes malignant transformation and predicts poor outcome in colorectal cancer through misregulation of p53 signaling pathway. Int J Oncol. 2016;48(4):1628–1638. doi: 10.3892/ijo.2016.3366. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Zhang H, Chen Y, et al. miR-598 inhibits metastasis in colorectal cancer by suppressing JAG1/Notch2 pathway stimulating EMT. Exp Cell Res. 2017;352(1):104–112. doi: 10.1016/j.yexcr.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Li G, Liu L, Wang Y, Li X, Gong J. AF1q mediates tumor progression in colorectal cancer by regulating AKT signaling. Int J Mol Sci. 2017;18(5):987. doi: 10.3390/ijms18050987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong-Xu W, Jia L, Su-Juan Z. MicroRNA-185 is a novel tumor suppressor by negatively modulating the Wnt/β-catenin pathway in human colorectal cancer. Indian J Cancer. 2015;52(7):182–185. doi: 10.4103/0019-509X.186576. [DOI] [PubMed] [Google Scholar]
- 8.Lee TY, Liu CL, Chang YC, et al. Increased chemoresistance via Snail-Raf kinase inhibitor protein signaling in colorectal cancer in response to a nicotine derivative. Oncotarget. 2016;7(17):23512–23520. doi: 10.18632/oncotarget.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng WW, Hu Q, Zr L, et al. KDM4B promotes DNA damage response via STAT3 signaling and is a target of CREB in colorectal cancer cells. Mol Cell Biochem. 2018;449(1–2):81–90. doi: 10.1007/s11010-018-3345-5. [DOI] [PubMed] [Google Scholar]
- 10.He XS, Guo LC, Du MZ, et al. The long non-coding RNA NON-HSAT062994 inhibits colorectal cancer by inactivating Akt signaling. Oncotarget. 2017;8(40):68696–68706. doi: 10.18632/oncotarget.19827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi M, Funayama R, Ohnuma S, Unno M, Nakayama K. Wnt-β-catenin signaling regulates abcc3 (MRP3) transporter expression in colorectal cancer. Cancer Sci. 2016;107(12):1776–1784. doi: 10.1111/cas.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mu Y, Zou H, Chen B, Fan Y, Luo S. FAM83D knockdown regulates proliferation, migration and invasion of colorectal cancer through inhibiting FBXW7/Notch-1 signalling pathway. Biomed Pharmacother. 2017;90:548–554. doi: 10.1016/j.biopha.2017.03.073. [DOI] [PubMed] [Google Scholar]
- 13.Ashikari D, Takayama K, Obinata D, Takahashi S, Inoue S. CLDN8, an androgen-regulated gene, promotes prostate cancer cell proliferation and migration. Cancer Sci. 2017;108(7):1386–1393. doi: 10.1111/cas.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu TH, Chung RH, Wang SC, et al. Missense mutation at CLDN8 associated with a high plasma interferon gamma-inducible protein 10 level in methadone-maintained patients with urine test positive for morphine. PLoS One. 2017;12(11):e0187639. doi: 10.1371/journal.pone.0187639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Yang Y, Hao P, Ding X. Claudin 8 contributes to malignant proliferation in human osteosarcoma U2OS cells. Cancer Biother Radiopharm. 2015;30(9):400–404. doi: 10.1089/cbr.2015.1815. [DOI] [PubMed] [Google Scholar]
- 16.Bray JD, Zhang Z, Winneker RC, Lyttle CR. Regulation of gene expression by PRA-910, a novel progesterone receptor modulator, in T47D cells. Steroids. 2003;68(10-13):995–1003. doi: 10.1016/s0039-128x(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 17.Bujko M, Kober P, Mikula M, Ligaj M, Ostrowski J, Siedlecki JA. Expression changes of cell–cell adhesion-related genes in colorectal tumors. Oncol Lett. 2015;9(6):2463–2470. doi: 10.3892/ol.2015.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gröne J, Weber B, Staub E, et al. Differential expression of genes encoding tight junction proteins in colorectal cancer: frequent dys-regulation of claudin-1, -8 and -12. Int J Colorectal Dis. 2007;22(6):651–659. doi: 10.1007/s00384-006-0197-3. [DOI] [PubMed] [Google Scholar]
- 19.Katoh M, Katoh M. CLDN23 gene, frequently down-regulated in intestinal-type gastric cancer, is a novel member of claudin gene family. Int J Mol Med. 2003;11(6):683–689. [PubMed] [Google Scholar]
- 20.Qi J, Yang Y, Hao P, Xu J. Transcription factor SOX9 promotes osteosarcoma cell growth by repressing claudin-8 expression. Tohoku J Exp Med. 2017;241(1):55–63. doi: 10.1620/tjem.241.55. [DOI] [PubMed] [Google Scholar]
- 21.Shangkuan WC, Lin HC, Chang YT, et al. Risk analysis of colorectal cancer incidence by gene expression analysis. PeerJ. 2017;5(8):e3003. doi: 10.7717/peerj.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X, Sun S, Zeng X, Cui L. Expression profiles analysis identifies a novel three-mRNA signature to predict overall survival in oral squamous cell carcinoma. Am J Cancer Res. 2018;8(3):450–461. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Ma X, Zhang Y, et al. Establishment of a miRNA–mRNA regulatory network in metastatic renal cell carcinoma and screening of potential therapeutic targets. Tumour Biol. 2016;37(12):15649–15663. doi: 10.1007/s13277-016-5135-6. [DOI] [PubMed] [Google Scholar]
- 24.Dahlmann M, Okhrimenko A, Marcinkowski P, et al. RAGE mediates S100A4-induced cell motility via MAPK/ERK and hypoxia signaling and is a prognostic biomarker for human colorectal cancer metastasis. Oncotarget. 2014;5(10):3220–3233. doi: 10.18632/oncotarget.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han HB, Gu J, Ji DB, et al. PBX3 promotes migration and invasion of colorectal cancer cells via activation of MAPK/ERK signaling pathway. World J Gastroenterol. 2014;20(48):18260–18270. doi: 10.3748/wjg.v20.i48.18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huo Q, Kinugasa T, Wang L, et al. Claudin-1 protein is a major factor involved in the tumorigenesis of colorectal cancer. Anticancer Res. 2009;29(3):851–857. [PubMed] [Google Scholar]
- 27.Kinugasa T, Huo Q, Higashi D, et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007;27(6A):3729–3734. [PubMed] [Google Scholar]
- 28.Singh AB, Sharma A, Smith JJ, et al. Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells. Gastroenterology. 2011;141(6):2140–2153. doi: 10.1053/j.gastro.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragusa M, Statello L, Maugeri M, et al. Specific alterations of the microRNA transcriptome and global network structure in colorectal cancer after treatment with MAPK/ERK inhibitors. J Mol Med. 2012;90(12):1421–1438. doi: 10.1007/s00109-012-0918-8. [DOI] [PubMed] [Google Scholar]
- 30.Xavier CP, Lima CF, Fernandes-Ferreira M, Pereira-Wilson C. Salvia fruticosa, Salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: the role in MAPK/ERK pathway. Nutr Cancer. 2009;61(4):564–571. doi: 10.1080/01635580802710733. [DOI] [PubMed] [Google Scholar]
- 31.Xiang S, Xiang T, Xiao Q, Li Y, Shao B, Luo T. Zinc-finger protein 545 is inactivated due to promoter methylation and functions as a tumor suppressor through the Wnt/β-catenin, PI3K/Akt and MAPK/ERK signaling pathways in colorectal cancer. Int J Oncol. 2017;51(3):801–811. doi: 10.3892/ijo.2017.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang C, Wang S, Qin C, et al. TRIM36, a novel androgen-responsive gene, enhances anti-androgen efficacy against prostate cancer by inhibiting MAPK/ERK signaling pathways. Cell Death Dis. 2018;9(2):155. doi: 10.1038/s41419-017-0197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian XQ, Guo FF, Sun DF, et al. Downregulation of ZNF278 arrests the cell cycle and decreases the proliferation of colorectal cancer cells via inhibition of the ERK/MAPK pathway. Oncol Rep. 2017;38(6):3685–3692. doi: 10.3892/or.2017.6031. [DOI] [PubMed] [Google Scholar]
- 34.Xu T, Zhou M, Peng L, et al. Upregulation of CD147 promotes cell invasion, epithelial-to-mesenchymal transition and activates MAPK/ERK signaling pathway in colorectal cancer. Int J Clin Exp Pathol. 2014;7(11):7432–7441. [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Artacho-Cordón F, Ríos-Arrabal S, Lara PC, Artacho-Cordón A, Calvente I, Núñez MI. Matrix metalloproteinases: potential therapy to prevent the development of second malignancies after breast radiotherapy. Surg Oncol. 2012;21(3):e143–e151. doi: 10.1016/j.suronc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Overall CM, Kleifeld O. Tumour microenvironment – opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6(3):227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 39.Kim J-Y, Kim W-J, Kim H, Suk K, Lee W-H. The stimulation of CD147 induces MMP-9 expression through ERK and NF-κB in macrophages: implication for atherosclerosis. Immune Netw. 2009;9(3):90–97. doi: 10.4110/in.2009.9.3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]