Abstract
Silylenes are useful reactive intermediates for the stereoselective construction of compounds containing carbon–silicon bonds. Despite their synthetic utility, the development of either an enantioselective or diastereoselective metal-catalyzed silylene transfer reaction in which ligands on the metal catalyst control stereoselectivity has not been achieved. In this article, we report that the structure of the alkene is most important for controlling stereoselectivity in these reactions. The stereochemical course of kinetically controlled silacyclopropanation reactions was not affected by the nature or chirality of the ligands on the metal. When silylene transfer reactions were reversible, however, products can be formed with a high degree of diastereoselectivity (90:10 d.r.).
Introduction
The reactions of metal silylene complexes with various functionalized organic compounds provide routes to synthesize products with diverse structures.1 Methods to control the diastereoselectivity of silylene transfer reactions, however, are limited.2 By contrast to the well-established diastereoselective and enantioselective syntheses of cyclopropanes from metal carbenes,3 stereoselective silylene transfer to alkenes has been much more difficult to achieve.4 In the few reported examples, a sterically bulky substituent on the alkene prevented addition of the silylene to the more sterically encumbered face, resulting in diastereoselectivity.4 The only examples of obtaining single enantiomers from reactions using silylenes as reactive intermediates rely on the use of chiral enantiopure substrates5 or chiral auxiliaries.6 Although diastereoselective additions of nucleophiles to chiral silylenes have been reported,7 there is no known asymmetric or diastereoselective silylene transfer reaction using a chiral ligand as the source of stereodifferentiation.
To understand the role that ligands play in controlling stereoselectivity in silylene transfer reactions, we performed a systematic study of both silver- and copper-catalyzed silylene transfer reactions with chiral enantiopure alkenes to provide diastereomeric silylene transfer products (Figure 1). Silacyclopropanation reactions were examined in the presence of chiral ligands that differed in size, electron-donating ability, and absolute configuration. These studies provide evidence that the metal-catalyzed silacyclopropanation of alkenes involves a ligand-bound metal-silylene complex in which reactivity is determined by steric interactions between the ligand and the substituents on the alkene. Electronic factors are less significant in controlling both reactivity and stereoselectivity.
Figure 1.
Systematic study of diastereoselective silylene transfer reactions
Results and discussion
The effects of the structure of the catalyst on silylene transfer reactions were first evaluated using chiral non-racemic bis(homoallylic) ether 4 (eqn (1)) and diene 6 (eqn (2)). These substrates were designed to place the stereocenter at some distance from the reactive alkene functional group, but close enough to enable determination of facial selectivity on the alkene by measuring the ratio of diastereomers by nuclear magnetic resonance (NMR) spectroscopy. Initial experiments confirmed that the remote stereocenters of alkene 4 and diene 6 exerted no stereoselectivity on the silver salt-catalyzed silacyclopropanation reaction in the absence of chiral ligands. Control experiments established that the silylene transfer reaction was irreversible, so this selectivity is not the result of thermodynamic control. Consequently, these substrates could be used to evaluate the influence of chiral ligands on stereoselectivity in the absence of steric effects from the substrate.
 |
(1) |
 |
(2) |
The silacyclopropanation reaction was insensitive to the presence of the chiral ligands, however (Figure 2).8 Ligands had no effect on the ratio of isomers or the rate of silylene transfer. The presence of the enantiomeric ligands (R)- and (S)-BINAP9 and the quantity of them seemed to exert no influence on the extent of reactions as a function of time, as determined by 1H NMR spectroscopy. Only strongly donating ligands such as pyrrolidine 12 inhibited the reaction, indicating that the steric size of the ligands and the alkene did not affect the rate of silylene transfer. The fact that selectivity was independent of the presence of the ligand suggests that these ligands were likely not the optimal structures for achieving an enantioselective reaction.
Figure 2.
Effect of ligand on silver-catalyzed silylene transfer to 4
The reactivity of unsaturated substrates 4 and 6 were insensitive to reaction conditions.8 Changing the amount of silver catalyst from 1 mol % to 15 mol %, decreasing the temperature to –20 °C or –78 °C, and increasing the reaction time to 22 hours did not affect diastereoselectivity. Using polar and non-polar solvents such as toluene, trifluorotoluene or diethyl ether, chloroform, and 1,2-dichloroethene did not seem to affect the rate of the reaction, except for when tetrahydrofuran was used a solvent, in which case the reaction was completely inhibited.
The effects of the structure of the catalyst on silylene transfer reactions were then evaluated using chiral, non-racemic homoallylic ether 18.10 Both silver11 and copper12 catalysts were evaluated because of their propensity to form metal silylenes with silacyclopropane 2 and their ability to coordinate to various ligands.13
Silacyclopropanation of methyl ether 18 occurred with modest stereoselectivity with both copper and silver catalysts in the absence of a chiral ligand. Under either silver- (Table 1, entries 1–4) or copper- (Table 1, entries 5–7) catalyzed conditions, the reaction was irreversible, and the product ratio was consistently about 70:30. This selectivity was unaffected by the nature of the metal salt or its counterion. The high yields observed in the absence of a chiral ligand indicate that neither the steric hindrance of 18 nor the presence of an electron-withdrawing alkoxy group affected silylene transfer. The steric hindrance of this substrate also likely prevented rearrangement reactions from occurring.14
Table 1.
Silylene Transfer to Alkene 18
 | |||
|---|---|---|---|
| Entry | MX | Yielda | d.r.a |
| 1 | AgO2CCF3 | 77 | 71:29 |
| 2 | AgOTf | 73 | 71:29 |
| 3 | AgOTs | 96 | 71:29 |
| 4 | Ag3PO4 | 87 | 67:33 |
| 5 | [Cu(OTf)]2•PhH | 87 | 70:30 |
| 6 | CuCl | 79 | 71:29 |
| 7 | CuI | 69 | 57:43 |
Determined by 1H NMR Spectroscopy.
The effects of chiral ligands on the diastereoselectivity of the silacyclopropanation of alkene 18 were examined (Figure 3). In the presence of either a bidentate bis(phosphine)9 (8) or a tridentate bis(oxazoline)15 (20) ligand, no reaction was observed. A bidentate phosphine ligand could be too strongly electron-donating to allow the metal-catalyzed silylene transfer to occur; similar observations have been made in other systems.16 In the case of the tridentate ligand 7, there might not be an open coordination site for interaction with silacyclopropane 2 to initiate catalysis, considering the small number of ligands that can coordinate to copper or silver.13 In contrast, silylene transfer proceeded smoothly over 30 minutes when bidentate bis(oxazolines)17 (21) or mono(oxazoline) ligands18 (22) were used. The diastereoselectivity of the reaction, however, was not affected by the chiral ligand. Similar results were obtained with bis(homoallylic)ether 4 and a larger set of ligands.
Figure 3.
Effect of ligand on copper-catalyzed silylene transfer to 18
The lack of influence of the ligand on stereoselectivity could be explained in different ways. It could result because, in the transition state for silylene transfer, the substituents on the chiral ligand are too far from the developing stereocenter to control the facial selectivity. Alternatively, the homoallylic stereocenter could be large enough that it did not permit the development of an optimal geometry to observe ligand-controlled selectivity. Because the reactivity of 4 was also unaffected by most ligands used this possibility is less likely, however.
In contrast, when less sterically congested secondary homoallylic ethers were used, the reaction efficiency was sensitive to both the metal-ligand complex and the size of the substituent on the alkene. Racemic homoallylic ethers 23 and 24, which differ only in the size of the silyloxy group on the oxygen atom, were subjected to silylene transfer conditions (Table 2). Substrates protected as the larger tert-butyldiphenylsilyl ether (24) reacted more slowly than substrates protected as the smaller tert-butyldimethylsilyl ether 23.19 This effect was consistent for both silver and copper catalysts. This sensitivity to steric size of the substituent suggests some interaction of the alkene substituents with the approaching metal-silylene complex in the transition state for silacyclopropanation, considering that generation of the metal-silylene complex likely does not involve the alkenes 23 or 24.20
Table 2.
Steric Effects of Homoallylic Ethers 23 and 24
 | ||||
|---|---|---|---|---|
| Entry | MX | R | Conversion (%)a | d.r.a |
| 1 | AgOTs | Me | 55 | 62:48 |
| 2 | [Cu(OTf)]2•PhH | Me | 42 | 50:50 |
| 3 | AgOTs | Ph | 26 | 50:50 |
| 4 | [Cu(OTf)]2•PhH | Ph | 24 | 50:50 |
Determined by 1H NMR Spectroscopy.
To determine the feasibility of the development of an asymmetric silylene transfer reaction, silacyclopropanation of enantiomerically enriched alkene (S)-23, prepared using an asymmetric allylation reaction,21 was evaluated. Silacyclopropanation with both silver and copper catalysts occurred with modest diastereoselectivity (eqn (3)). In the case of the copper-catalyzed reaction, the selectivity with the chiral non-racemic alkene (60:40 d.r.) was higher than for the racemic alkene (50:50 d.r.). Although this difference is small, it is beyond experimental error, and it was reproducible. This observation might result if complexes containing two alkenes were present in the transition state for silacyclopropanation.22
 |
(3) |
The use of chiral ligands for copper in the silacyclopropanation of homoallylic ether 23 did not alter the diastereoselectivity to any appreciable degree (Figure 4). The presence of these ligands also had little effect on the rates of conversion of the reaction. This situation differs from that observed during silylene transfer to homoallylic ether 18, where the presence of ligands 8 and 20 (Figure 3, above) and bis(homoallylic) ether 4, where ligand 12 (Figure 2, above), blocked silacyclopropanation. These results suggest that the lack of reactivity with homoallylic ether 18 was not caused by ineffective catalysis by a complex between ligand 20 and the copper salt. Instead, it suggests that the resulting metal-ligand-silylene complex was too sterically hindered to transfer the silylene unit to the hindered alkene, therefore terminating the catalytic cycle for silylene transfer.20
Figure 4.
Ligand screen for alkene 23.
Silylene transfer to the enantiomeric homoallylic ether (R)-23 and enantiomeric ligands (R)- and (S)-BINAP were evaluated in this series to discern how diastereomeric pairs of components might affect the efficiency of the silylene transfer reaction (eqn (4)). These experiments did not result in any variation in diastereoselectivity or conversion, again indicating that bis(phosphines) are the not optimal ligands for developing enantioselective processes involving metal silylenes. The results do indicate, however, that mismatched stereochemistry was not the source of diminished yields and poor selectivities when phosphines were used. They also indicate that the ligand is located far from the reactive alkene during the silylene transfer step. Similar results were obtained with ether 4 and the enantiomeric BINAP ligands.
 |
(4) |
Experiments with allylic silyl ethers, such as the chiral, non-racemic alkene 28, provided additional evidence that the silylene transfer reaction is sensitive to the size of the metal-ligand complex (eqn (5)). When alkene 28 was subjected to the silylene transfer conditions in either the absence or presence of chiral ligands, diastereoselectivity was not affected.8 Trends in reactivity, however, were evident. When ligands were employed, reaction times were extended and conversion was diminished. In particular, the use of bulky phosphine ligands such as (S,S)-NORPHOS (30) and (S,S)-DIOP (31) completely inhibited the reaction (eqn (6)), in contrast to their minimal effects with homoallylic silyl ethers (eqn (4)).23
 |
(5) |
 |
(6) |
Despite the difficulty associated with coaxing sterically encumbered alkenes to react, stereoselectivity could be influenced if the silylene transfer reaction were reversible. Although the silver-catalyzed reaction of the hindered triisopropylsilyl ether 28 was irreversible, the copper-catalyzed reaction was reversible (eqn (7)). When the copper-catalyzed reaction was allowed to equilibrate overnight, a 90:10 ratio of isomers was obtained. Even after this long reaction time, however, rearrangement to allylic silanes was not observed.24
 |
(7) |
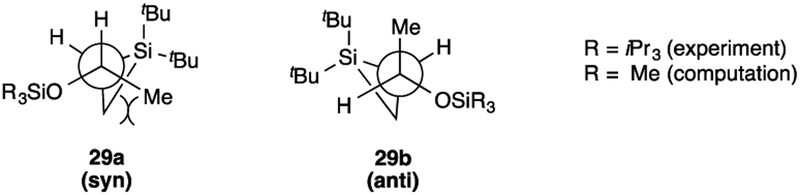
Although the stereochemical configuration of the major diastereomer of silacyclopropane 29 could not be assigned unambiguously, the thermodynamic product is likely to be the anti isomer 29b (Figure 5). This assignment was made by considering the conformational preferences of these sterically congested products. Conformational analysis of each isomer of silacyclopropane was aided by density-functional theory calculations (B3LYP/6–31G*) on a model system with a SiMe3 group in place of the more computationally demanding iPr3Si group.25 Low-energy conformers of both isomers would likely place the large silyloxy group anti to the di-tert-butylsilyl group. The syn isomer 29a places the methyl group gauche to the methylene unit of the silacyclopropane (and therefore also near a tert-butyl group), an interaction that is absent in the lowest energy conformers of the anti isomer 29b. Computationally, this interaction leads to an approximately 1 kcal/mol difference in energy between the isomers. Considering that the experiments involved the larger iPr3Si ether,19 these interactions should be even more destabilizing, leading to the selectivity observed (eq (7)).
Figure 5.
Low energy conformers of syn and anti 29
A variety of mechanisms for metal-catalyzed silylene transfer can be envisioned (Figure 6). The simplest mechanism (a) is one in which the metal facilitates the liberation of a free silylene, which then undergoes concerted silacyclopropanation with an alkene. This mechanism is unlikely, however. If this mechanism were operating, then the influence of the ligand on reaction rate would have been the same for all substrates, which was not the case.
Figure 6.
Possible mechanisms for silylene transfer to an alkene
A second mechanism, which involves dissociation of the ligand from the metal silylene prior to transfer to the alkene (b), can also be discounted. In such a mechanism, the ligand could not completely inhibit the reactivity of the silylene unless the silver catalyst were completely sequestered by the ligand. That response would be consistent for all alkenes examined, which is not the case: some ligands slowed reactions with some alkenes but not with others. This second mechanism can also be discounted.
The most reasonable explanation for these results involves a mechanism in which both the ligand and alkene are involved in the stereochemistry-determining step (c). The rate of any particular reaction would depend upon the combination of the two factors, the substitution of the alkene and the nature of the ligand on the metal-silylene complex. This trend was observed: the lowest levels of reactivity were observed when a strongly donating ligand was combined with a sterically encumbered alkene. The fact that the ligand could not control the facial selectivity only indicates that that interaction between the ligand and the substituents on the alkene did not differentiate between diastereomeric transition states.
Conclusion
In conclusion, the formation of single diastereomers of silacyclopropanes is possible if the reaction were reversible and the alkene sufficiently bulky to lead to a stereoselective silylene transfer step. Through a systematic study of different alkenes, we determined that steric factors outweigh electronic ones in the metal-catalyzed transfer of silylenes to alkenes. These observations are consistent with a mechanism involving the silylene, metal atom, ligand, and alkene in the stereochemistry-determining step.
Experimental details
General Procedures.
General Procedures are provided as Supplementary Information
Representative Procedure for Silylene Transfer without Ligand (Silacyclopropanes 5a and 5b).
To a solution of alkene 4 (0.100 mL, 0.496 M in C6D6, 0.0496 mmol), silacyclopropane 2 (0.110 mL, 0.750 M in C6D6, 0.0830 mmol), mesitylene (0.0020 mL, 0.014 mmol, internal standard), and AgO2CCF3 (0.110 mL, 0.014 M in C6D6, 0.0015 mmol) in C6D6 (0.185 mL). Silacyclopropanes 5a and 5b were formed in 88% combined yield based on comparison of the area of the standard peak (δ 6.72) and the silacyclopropane protons (54:46 d.r.): 1H NMR (600 MHz, C6D6, diagnostic peaks) δ 4.08–3.99 (m, 2H), 1.69–1.59 (m, 2H), 1.24 (d, J = 6.1, 3H), 1.23 (d, J = 6.1, 3H), 1.17–1.15 (m, 42H), 1.15 (s, 9H), 1.039 (s, 9H), 1.037 (s, 9H), 0.94–0.87 (m, 4H), 0.32–0.24 (m, 2H); 13C NMR (150 MHz, C6D6, diagnostic peaks) δ 69.37, 69.36, 43.63, 43.58, 24.40, 24.36, 19.43, 19.38, 18.89, 18.86, 18.81, 18.78, 14.9, 14.8, 13.33, 13.26, 4.2, 4.1; 29Si NMR (99 MHz, C6D6, diagnostic peaks) δ –49.0, –49.1.
Vinylsilacyclopropanes 7a and 7b.
The representative procedure for the synthesis of silacyclopropanes was followed using diene 6 (0.100 mL, 0.510 M in C6D6, 0.0510 mmol), silacyclopropane 2 (0.110 mL, 0.750 M in C6D6, 0.0830 mmol), mesitylene (0.0020 mL, 0.014 mmol, internal standard), and AgO2CCF3 (0.085 mL, 0.018 M in C6D6, 0.0015 mmol) in C6D6 (0.205 mL). Vinylsilacyclopropanes 7a and 7b were formed in 93% combined yield based on comparison of the area of the standard peak (δ 6.72) and the alkene protons (51:49 d.r.): 1H NMR (500 MHz, C6D6, diagnostic peaks) δ 5.95 (dd, J = 15.1, 7.6, 1H), 5.89 (dd, J = 15.2, 7.5, 1H), 5.64–5.55 (m, 2H), 1.36–1.34 (m, 6H), 1.13 (s, 9H), 1.12 (s, 9H), 0.98 (s, 9H), 0.97 (s, 9H), 0.77–0.71 (s, 2H); 13C NMR (125 MHz, C6D6, diagnostic peaks) δ 132.5, 132.4, 130.5, 130.2, 71.0, 70.7, 30.3, 30.2, 19.9, 19.8, 18.88, 18.86, 17.7, 17.4, 3.3, 2.9; 29Si NMR (99 MHz, C6D6, diagnostic peaks) δ –48.1, –48.2.
Silacyclopropanes 19a and 19b.
The representative procedure for the synthesis of silacyclopropanes was followed using alkene 18 (0.100 mL, 0.505 M in C6D6, 0.0505 mmol), silacyclopropane 2 (0.100 mL, 0.548 M in C6D6, 0.0548 mmol), mesitylene (0.0040 mL, 0.028 mmol, internal standard), and AgOTs (0.100 mL, 0.025 M in C6D6, 0.0025 mmol) in C6D6 (0.200 mL). Silacyclopropanes 19a and 19bb were formed in 96% combined yield based on comparison of the area of the standard peak (δ 6.72) and the methoxy protons (71:29 d.r.): 1H NMR (600 MHz, C6D6, diagnostic peaks) δ 3.12 (s, 1.2H), 3.05 (s, 3H), 2.51 (dd, J = 15.7, 2.2, 1H), 1.99 (dd, J = 15.5, 6.2, 0.4H), 1.18 (s, 3.6H), 1.14 (s, 9H), 1.07 (s, 3.6H), 1.05 (s, 9H), 0.40–0.36 (m, 1H), 0.30 (dd, J = 10.6, 9.5, 0.4H); 13C NMR (125 MHz, C6D6, diagnostic peaks) δ 87.3, 87.2, 53.5, 53.1, 51.0, 50.9, 48.4, 47.9, 45.8, 45.5, 42.4, 42.3, 38.8, 38.7, 31.2, 31.0, 30.4, 30.3, 22.2, 22.0, 21.8, 21.7, 13.5, 12.6, 7.8, 7.2; 29Si NMR (99 MHz, C6D6) δ –49.2, –49.8.
Silacyclopropanes 25a and 25b.
The representative procedure for the synthesis of silacyclopropanes was followed using alkene (S)-23 (0.100 mL, 0.503 M in C6D6, 0.0503 mmol), silacyclopropane 2 (0.100 mL, 0.550 M in C6D6, 0.0550 mmol), mesitylene (0.0020 mL, 0.014 mmol, internal standard), and [CuOTf]2•PhH (0.060 mL, 0.013 mM in C6D6, 0.0016 mmol Cu) in C6D6 (0.240 mL). Silacyclopropanes 25a and 25b were formed in 82% yield based on comparison of the area of the standard peak (δ 6.72) and the methine protons (52:48 d.r.): 1H NMR (500 MHz, C6D6, diagnostic peaks) δ 7.44 (d, J = 7.5, 2H), 7.41 (d, J = 7.5, 2H), 7.19 (t, J = 7.6, 4H), 7.10–7.07 (m, 2H), 4.86–4.80 (m, 2H), 2.43–2.28 (m, 2H), 2.09–1.98 (m, 2H), 1.18–1.13 (m, 1H), 1.08 (s, 9H), 1.06 (s, 9H), 1.04 (s, 9H and m, 1H), 1.04 (s, 9H), 1.03 (s, 9H), 1.02 (s, 18H), 0.91–0.85 (m, 2H), 0.31–0.23 (m, 2H), 0.17 (s, 3H), 0.16 (s, 3H), –0.02 (s, 3H), –0.04 (s, 3H); 13C NMR (125 MHz, C6D6, diagnostic peaks) δ 147.1, 146.8, 127.73, 127.69, 126.83, 126.76, 78.5, 77.1, 44.8, 44.7, 31.2, 31.1, 30.18, 30.15, 26.6, 26.5, 11.5, 10.5, 3.9, 3.7, –3.87, –3.91, –4.1, –4.3; 29Si NMR (99 MHz, C6D6, diagnostic peaks) δ –48.7, –49.6.
Silacyclopropanes 26a and 26b.
The representative procedure for the synthesis of silacyclopropanes was followed using alkene 24 (0.019 g, 0.051 mmol), silacyclopropane 2 (0.100 mL, 0.550 M in C6D6, 0.0550 mmol), mesitylene (0.0020 mL, 0.014 mmol, internal standard), and AgO2CCF3 (0.0004 g, 0.0022 mmol) in C6D6 (0.400 mL). Silacyclopropanes 26a and 26b were formed in 26% combined yield based on comparison of the area of the standard peak (δ 6.72) and the methine protons (50:50 d.r.): 1H NMR (500 MHz, C6D6, diagnostic peaks) δ 7.91–7.88 (m, 4H), 7.67–7.65 (m, 2H), 7.63–7.62 (m, 1H), 7.46–7.44 (m, 2H), 7.37–7.36 (m, 1H), 4.99 (dd, J = 8.5, 4.9, 1H), 4.90–4.80 (m, 1H), 2.32 (dd, J = 8.3, 6.7, 2H), 2.29–2.24 (m, 2H), 1.20 (s, 9H), 1.18 (s, 9H), 0.97 (s, 9H), 0.91 (s, 18H), 0.85 (s, 9H), 0.56 (dd, J = 12.2, 10.8, 1H), 0.08 (dd, J = 10.6, 9.2, 1H), –0.02 to –0.06 (m, 1H).
Silacyclopropanes 29a and 29b:
Note: Enantiomeric substrate ent-28 was used. The representative procedure for synthetises of silacyclopropanes was followed using alkene ent-28 (0.100 mL, 0.485 M in C6D6, 0.0485 mmol), silacyclopropane 2 (0.100 mL, 0.550 M in C6D6, 0.0550 mmol), mesitylene (0.0020 mL, 0.014 mmol, internal standard) and AgO2CCF3 (0.100 mL, 0.0145 M in C6D6, 0.00145 mmol) in C6D6 (0.200 mL). Silacyclopropanes ent-29a and ent-29b were formed in 77% yield based on comparison of the area of the standard peak (δ 6.72) and the methine proton (54:46 d.r.): 1H NMR (600 MHz, C6D6, diagnostic peaks) δ 4.22–4.12 (m, 2H), 1.48 (d, J = 6.0, 3H), 1.46 (d, J = 6.0, 3H), 1.18 (s, 9H), 1.08 (s, 9H), 1.06 (s, 9H), 0.97 (s, 9H), 0.77 (dd, J = 12.7, 10.9, 1H), 0.58 (dd, J = 11.1, 9.3, 1H), 0.19 (t, J = 10.4, 1H); 13C NMR (125 MHz, C6D6, diagnostic peaks) δ 72.8, 72.1, 31.00, 30.96, 30.22, 30.18, 29.6, 29.5, 19.2, 19.1, 19.0, 13.9, 13.7, 4.1, 1.0; 29Si NMR (99 MHz, C6D6, diagnostic peaks) δ –47.4, –48.9.
Supplementary Material
Acknowledgements
This research was supported by the National Science Foundation (CHE-1362709). CZR thanks the Margaret Strauss Kramer Graduate Fellowship (NYU) for fellowship support. K. A. W. thanks the Global Research Institute at NYU Florence for a fellowship. The NMR spectra collected were supported by an S10 grant from the National Institutes of Health (OD016343). We thank Dr. Chin Lin (NYU) for his assistance with NMR spectroscopy, mass spectrometry, and optical rotation.
Footnotes
Electronic Supplementary Information (ESI) available: Additional experimental details, ligands used, and 1H and 13C NMR spectra of new compounds. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Franz AK and Woerpel KA, Acc. Chem. Res, 2000, 33, 813–820. [DOI] [PubMed] [Google Scholar]
- 2.Ćiraković J, Driver TG and Woerpel KA, J. Org. Chem, 2004, 69, 4007–4012. [DOI] [PubMed] [Google Scholar]
- 3.a) Lebel H, Marcoux J-F, Molinaro C and Charette AB, Chem. Rev, 2003, 103, 977–1050; [DOI] [PubMed] [Google Scholar]; b) Bartoli G, Bencivenni G and Dalpozzo R, Synthesis, 2014, 46, 979–1029. [Google Scholar]
- 4.Driver TG, Franz AK and Woerpel KA, J. Am. Chem. Soc, 2002, 124, 6524–6525. [DOI] [PubMed] [Google Scholar]
- 5.Calad SA and Woerpel KA, Org. Lett, 2007, 9, 1037–1040. [DOI] [PubMed] [Google Scholar]
- 6.Ventocilla CC and Woerpel KA, J. Am. Chem. Soc, 2011, 133, 406–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Sanji T, Fujiyama H, Yoshida K and Sakurai H, J. Am. Chem. Soc, 2003, 125, 3216–3217; [DOI] [PubMed] [Google Scholar]; b) Sanji T, Mitsugi H, Tanaka M, Fujiyama H and Sakurai H, Organometallics, 2006,25, 6159–6161. [Google Scholar]
- 8.See the Supplementary Information for more details.
- 9.For a review discussing this family of phosphine ligands, see: Shimizu H, Nagasaki I and Saito T, Tetrahedron, 2005, 61, 5405–5432.
- 10.Homoallylic ether 18 was prepared from (R)-camphor and literature precedent suggests that the alkoxy substituent occupies the exo position. See: Dimitrov V, Simova S and Kostova K, Tetrahedron, 1996, 52, 1699–1706.
- 11.Ćiraković J, Driver TG and Woerpel KA, J. Am. Chem. Soc, 2002, 124, 9370–9371. [DOI] [PubMed] [Google Scholar]
- 12.Franz AK and Woerpel KA, J. Am. Chem. Soc, 1999, 121, 949–957. [Google Scholar]
- 13.For a discussion of copper and silver coordination chemistry, see: Baker L-J, Bowmaker GA, Hart RD, Harvey PJ, Healy PC and White AJ, Inorg. Chem, 1994, 33, 3925–3931.
- 14.Cleary PA and Woerpel KA, Org. Lett, 2005, 7, 5531–5533. [DOI] [PubMed] [Google Scholar]
- 15.For structural studies of tridentate bis(oxazoline) ligands to silver and copper salts, see: Kuai H-W, Cheng X-C, Li D-H, Hu T and Zhu X-H, J. Solid State Chem, 2015, 65–75.
- 16.For a review covering silver-catalyzed atom-transfer reactions, including enantioselective transformations, see: Li Z and He C, Eur. J. Org. Chem, 2006, 4313–4322.
- 17.For structural studies of bidentate bis(oxazoline) ligands to silver and copper salts, see: Čaplar V, Raza Z, Roje M, Tomišić V, Horvat G, Požar J, Piantanida I and Žinić M, Tetrahedron, 2004, 60, 8079–8087.
- 18.For applications of mono(oxazoline) ligands in enantioselective catalysis, see, for example: Brunner H, Störiko R and Rominger F, Eur. J. Inorg. Chem, 1998, 771–781; Shibatomi K, Narayama A, Soga Y and Muto T, Org. Lett, 2011, 13, 2944–2947.
- 19.Replacing a methyl group on the silicon atom with a phenyl group increases the volume of a silyl group, see: Panek JS, Prock A, Eriks K and Giering WP, Organometallics, 1990, 9, 2175–2176.
- 20.Driver TG and Woerpel KA, J. Am. Chem. Soc, 2004, 126, 9993–10002. [DOI] [PubMed] [Google Scholar]
- 21.Keck GE, Tarbet KH and Geraci LS, J. Am. Chem. Soc, 1993, 115, 8467–8468. [Google Scholar]
- 22.For a discussion of the origin of non-linear effects in asymmetric synthesis, see: Blackmond DG, Acc. Chem. Res, 2000, 33, 402–411.
- 23.For asymmetric transformations using DIOP and NORPHOS, see: Alexakis A, Burton J, Vastra J and Mangeney P, Tetrahedron: Asymmetry, 1997, 8, 3987–3990.
- 24.a) Bourque LE, Cleary PA and Woerpel KA, J. Am. Chem. Soc, 2007, 129, 12602–12603; [DOI] [PubMed] [Google Scholar]; b) Bourque LE, Haile PA, Loy JMN and Woerpel KA, Tetrahedron, 2009, 65, 5608–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Details of the computational studies are provided as Supplementary Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.