Abstract
There are inconsistencies in the literature regarding the association between gestational weight gain (GWG) and child adiposity. GWG is hypothesized to act on child adiposity directly through intrauterine programming and indirectly through birth weight. It is unclear if the relative importance of these pathways differs by prepregnancy BMI status. We analyzed data from 3600 participants of the nationally representative Early Childhood Longitudinal Study-Birth Cohort. Child BMI Z-score was calculated from height and weight measured at 5 y. Using linear regression, controlling for sociodemographics and family lifestyle, we examined prepregnancy BMI-specific associations between GWG and child BMI Z-score. There was a nonlinear association among normal (P < 0.001) and overweight mothers only (P = 0.013), such that GWG beyond the midpoint of the 2009 Institute of Medicine recommendations was associated with a significant increase in child BMI Z-score. After the addition of birth-weight-for-gestational-age and breastfeeding to the model, the association remained among normal-weight mothers (P = 0.005) and was slightly attenuated among overweight mothers (P = 0.09). No significant association was observed between GWG and child BMI Z-score among underweight or obese mothers. We used path analysis to decompose the total effect into direct and indirect effects. This indicated the presence of a stronger direct than indirect effect. In conclusion, low GWG is not associated with BMI Z-score among any prepregnancy BMI group. Excess GWG is associated with an increase in child BMI Z-score among normal and overweight mothers only. Prevention of excess GWG may be a strategy to prevent childhood obesity.
Introduction
The Institute of Medicine (IOM)8 and the NRC recommend that pregnant women gain weight within defined ranges specific to their prepregnancy BMI to achieve optimal birth outcomes such as birth weight and delivery mode (1). However, the IOM/NRC concluded that there was insufficient evidence on long-term outcomes of gestational weight gain (GWG), including child adiposity. The relationship between GWG and child adiposity is of particular interest given the obesity epidemic in the United States (2). GWG was previously hypothesized to act on child adiposity through both an indirect and direct mechanism. GWG is positively associated with birth weight and birth weight is positively associated with BMI later in life (3). In addition, GWG may be directly associated with an unfavorable intrauterine metabolic milieu (4, 5), which may program children for an increased risk for later obesity (6–8). This hypothesis is supported by animal studies (9), but in studies of humans it has yet to be determined if the direct effect is present and large enough to be meaningful due to study limitations. In addition, maternal lifestyle is related to both GWG (10–12) and child lifestyle (13, 14); consequently, human studies assessing the association between GWG and child adiposity may be confounded by lifestyle.
GWG has been associated with offspring anthropometric measures during infancy (15), childhood (16–22), and adulthood (23–25); however, due to inconsistencies and limitations of previous analyses, the presence and degree of mediation by birth weight remains unclear. Two studies used sibling analyses to control for shared lifestyle and genetics between mother and child (22, 25) and found either no association (22) or the presence of a direct intrauterine effect only in overweight and obese women (25). Two studies have controlled for select offspring lifestyle factors (16, 18), albeit no maternal factors, and found a positive association with minimal mediation by birth weight. Most other studies have not controlled for family lifestyle and therefore may be biased. Many of the previously published studies were based on historic cohorts (17, 21–25) and may have limited external generalizibility given the dramatic increase in both maternal and child obesity (26, 27) and changes in lifestyles (28, 29) since the data were collected.
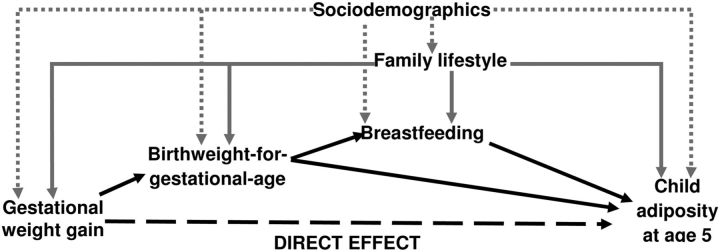
We extend previous studies by using measured variable path analysis (MVPA) to decompose the relationship between GWG and child adiposity at 5 y of age among a representative sample of children born in the United States in 2001. MVPA allows for the estimation of indirect effects. Driven by our conceptual framework (Fig. 1), we compared MVPA model fit statistics with and without the direct effect of GWG on child adiposity while controlling for sociodemographics and family lifestyle. We also assessed heterogeneity by maternal prepregnancy BMI, because it is unclear if associations between GWG and long-term outcomes differed by maternal prepregnancy BMI (1).
FIGURE 1.
Conceptual framework for the relationships between GWG and child adiposity measured at 5 y. Black solid lines represent the indirect effect of gestational weight gain on child adiposity.
Participants and Methods
Data source and study population.
The Early Childhood Longitudinal Study-Birth Cohort (ECLS-B) is a longitudinal study of a nationally representative cohort of children born in 2001. As required by the Department of Education, which sponsored the ECLS-B, we reported sample sizes rounded to the nearest 50 and presented all percentages as weighted population estimates. Study participants were selected from birth certificates collected by the National Center for Health Statistics' Vital Statistics System. Data from participants and their parents were collected in 5 waves at approximate child ages of 9 mo and 2, 4, 5, and 6 y. The 5-y interview was the last wave that included the entire cohort, because the 6-y interview was completed only among a subsample of children who had delayed entry into or repeated kindergarten. Therefore, we used the children's BMI measured when children were ∼5 y old as the outcome for the present analysis.
The ECLS-B enrolled 10,700 infants, oversampling select race-ethnic groups, twins, and infants born <2500 g. Only singleton, full-term (≥37 wk gestational age) infants (30) born to nondiabetic mothers (31, 32) were included in this analysis (n = 6700). Of the sample meeting our inclusion criteria, 850 children were not sampled at the 5-y wave due to budgetary restraints. An additional 1550 were lost to follow-up by the 5-y wave. To maintain a sample with demographics representative of all births in 2001, all data were weighted using ECLS-B sample weights to account for attrition and an unequal probability of selection. We performed a complete-subject analysis and excluded any records missing data for any of the study variables (n = 700). The final analytic sample consisted of 3600 children, weighted to be representative of ∼2.7 million children, ∼84% of the sample who met the inclusion criteria.
Maternal prepregnancy BMI.
At the 9-mo interview, mothers were asked their current height and prepregnancy weight. We calculated prepregnancy BMI (kg/m2) and categorized mothers as underweight (BMI <18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), or obese (BMI ≥30.0) (1).
GWG.
The ECLS-B includes 2 sources of GWG: the child's birth certificate and maternal report at the 9-mo interview. We used GWG obtained from the birth certificate (81% of records); when unavailable, we used the maternal report. We previously compared the 2 GWG measures and found no meaningful differences in birth weight epidemiological measures of association between the 2 data sources (33). The National Center for Health Statistics codes weight loss during pregnancy as zero gain and codes any gains >44.5 kg (98 lb) as 44.5 kg on the birth certificate. We consistently coded GWG from the parent interview, such that records reporting weight loss during pregnancy (n = 50) were coded as zero gain. We excluded any records with GWG ≥44.5 kg (n < 50), because these values were rare and represented an unknown range of true values. Adequacy of GWG was defined according to the mother's prepregnancy BMI status on the basis of the 2009 IOM/NRC GWG recommendations (1): 12.5–18 kg (BMI <18.5), 11.5–16 kg (BMI 18.5–24.9), 7–11.5 kg (BMI 25.0–29.9), and 5–9 kg (BMI ≥30.0).
Child BMI.
At the 5-y interview, children were weighed and measured twice according to a standardized protocol (34). Age- and sex-specific BMI Z-scores and percentiles were calculated using the CDC's 2000 Growth Charts (35). We categorized children as underweight (BMI <5th percentile), normal weight (BMI 5th to <85th percentile), overweight (BMI 85th to <95th percentile), or obese (BMI ≥95th percentile).
Sociodemographics.
Maternal age at delivery, race/ethnicity, parity, marital status, and schooling were obtained from the child's birth certificate. Maternal participation in the Special Supplemental Nutrition Program for Women, Infants, and Children during pregnancy was ascertained during the 9-mo interview.
Family lifestyle.
We used multiple maternal and child variables to assess overall family lifestyle. Maternal use of tobacco during the last 3 mo of pregnancy was ascertained during the 9-mo interview. At the 4-y interview, mothers were asked how many days in a typical week they exercised ≥30 min. At each interview, mothers were weighed at least twice on a digital scale. We calculated the mother's change in weight (kg) from prepregnancy to the 5-y interview as an indicator of her lifestyle (36). If a mother's weight at the 5-y interview was unavailable, we used her weight measured at either the 4-y interview (9% of mothers) or the 6-y interview (<1% of mothers) to calculate weight change from prepregnancy.
Children's typical weekly intake of sugar-sweetened beverages and fast-food was assessed via the 4-y parent interview (37, 38). Children's weekday television viewing habits were assessed at the 4-y interview by asking parents about the child's typical weekday time (hours) spent watching television and videos/DVD (39). We summed the 2 measures to capture the total weekday television time for each child. Using principal component analysis, we created a composite score representing family lifestyle using all of the aforementioned lifestyle related variables. This new variable explained 25.1% of the shared and unique variance in the utilized maternal and child lifestyle-related variables. Higher scores correlated with less healthy lifestyle behaviors.
Mediating variables.
Using birth weight and gestational age obtained from the child's birth certificate, we calculated sex-specific birth-weight-for-gestational-age Z-scores according to a national reference (40). Breastfeeding status was assessed at the 9-mo and 2-y parent interviews. Duration was censored at the child's age at the 2-y interview if the mother indicated that she was still breastfeeding. We categorized breastfeeding duration for descriptive purposes and natural log-transformed it due to non-normality for models, where it was treated as a continuous variable.
Statistical analysis.
We computed weighted proportions or means ± SE for all variables and assessed differences across prepregnancy BMI category using chi-square statistics for proportions and ANOVA for continuous variables. We used ordinary least squares regression to estimate the independent association between GWG and child BMI Z-score across the entire range of GWG data, with the reference set at the midpoint of the 2009 IOM/NRC recommended range for each BMI category. Given the IOM and NRC's stated interest in examining nonlinearity in the associations between GWG and long-term outcomes (1) and that some prior studies have observed a nonlinear relationship (41), we included a quadratic term for GWG in all of our models. We tested and confirmed a statistical interaction among GWG, GWG2, and prepregnancy BMI category for the adjusted association with child BMI Z-score using a multiple partial F-test with 6 df (P = 0.10) and therefore stratified all analyses. Although the quadratic term for GWG was not significant within all prepregnancy BMI categories, we retained it in all models for consistency. A multiple partial F-test with 2 df was used to assess overall P value for GWG and GWG2.
We present regression coefficients per 5 kg GWG for 5 models. Model A presents the crude association, and model B is adjusted for sociodemographic covariates only. We additionally adjusted for family lifestyle in model C to demonstrate any independent effect lifestyle has on the estimates. The estimates from this model represent the total effect of GWG on child adiposity and do not differentiate the indirect and direct effects. Model D includes birth-weight-for-gestational-age, the natural log of breastfeeding duration, sociodemographic variables, and the lifestyle score. The estimates from this model represent the direct effect of GWG, independent of its effect on birth weight.
Based on our a priori conceptual model (Fig. 1), we used MVPA to further examine the decomposition of the total effect of GWG on child BMI into direct and indirect effects. MVPA is an extension of linear regression that allows for the simultaneous estimation of direct and indirect effects. All endogenous variables were modeled continuously and we centered and rescaled GWG to stabilize the covariance. As with the ordinary least squares regression, we stratified by prepregnancy BMI, included the quadratic term for GWG, and adjusted for the set of covariates. We compared model fit with and without the direct effect of GWG. MVPA model fit was determined by considering multiple goodness-of-fit indices, namely, the model chi-square, the root mean square error of approximation, the comparative fit index, the Tucker Lewis index, and the Akaike Information Criteria (42). Due to the differing utility of each index (43), we simultaneously considered each index when examining fit of the various models. With the exception of obese mothers, where there was little difference between models, MVPA models with the direct effect of GWG on child BMI Z-score fit the data better. For consistency, we estimated all MVPA models with the direct effect of GWG on child BMI Z-score for all prepregnancy BMI groups. We plotted the total, direct, and indirect effects of GWG for each prepregnancy BMI group.
Data analysis was performed using SAS version 9.2 (SAS Institute) and Mplus version 6.1. We considered P values < 0.05 significant for main effects and P < 0.15 significant for multiplicative interactions due to the low power associated with tests of heterogeneity (44). All SE were estimated with Taylor Series approximations to account for the multistage, stratified, cluster design of the ECLS-B. This analysis was reviewed by a human participants coordinator at the CDC and determined not to involve human subjects.
Results
Among those not lost to attrition, we compared records retained in the analytic sample (n = 3600) with those excluded due to incomplete data (n = 700). Mothers in the analytic sample were older (27.3 ± 0.2 vs. 26.6 ± 0.3 y; P = 0.046) and were more likely to be non-Hispanic white (62.7 vs. 56.6%; P = 0.002), married (69.7 vs. 62.4%; P = 0.004), more educated (13.1 ± 0.1 vs. 12.0 ± 0.2 y; P < 0.001), to have not used Special Supplemental Nutrition Program for Women, Infants, and Children services during their pregnancy (60.9 vs. 47.0%; P < 0.0001), to have breastfed longer than 6 mo (34.8 vs. 25.3%; P = 0.004), and to be normal weight prior to pregnancy (57.5 vs. 45.6%; P < 0.001); and the children had a lower BMI Z-score at 5-y (0.67 ± 0.02 vs. 0.81 ± 0.06; P = 0.028) compared with those excluded from the sample due to incomplete data. No other significant differences between those excluded due to incomplete data and those retained in the analytic sample were detected across study variables (data not shown).
In the final sample, 4.7% of mothers were underweight, 57.5% were normal weight, 24.6% were overweight, and 13.2% were obese prior to pregnancy. At the 5-y interview, children were a mean age of 65 mo (range 57–74 mo) and 1.7% were underweight, 63.9% were normal weight, 18.6% were overweight, and 15.8% were obese. Several participant characteristics differed significantly across maternal prepregnancy BMI categories (Table 1).
TABLE 1.
Sample characteristics by maternal prepregnancy BMI status (ECLS-B, United States, 2001–2007)1
| Maternal prepregnancy BMI status | |||||
|---|---|---|---|---|---|
| Underweight | Normal Weight | Overweight | Obese | P value | |
| n 2 | 250 | 2150 | 750 | 450 | |
| Maternal sociodemographic variables | |||||
| Age, y | 25.2 ± 0.6 | 27.2 ± 0.2 | 27.7 ± 0.3 | 27.6± 0.4 | <0.001 |
| Race/ethnicity, % | <0.001 | ||||
| White, non-Hispanic | 62.1 | 65.0 | 62.6 | 52.9 | |
| Black, non-Hispanic | 11.8 | 12.0 | 12.8 | 25.3 | |
| Hispanic | 15.9 | 17.8 | 22.5 | 19.9 | |
| Asian/Pacific Islander/Native Hawaiian | 9.3 | 4.5 | 1.6 | 0.8 | |
| American Indian/Alaskan Native | 0.9 | 0.6 | 0.6 | 1.1 | |
| Married, % | 62.3 | 72.0 | 69.5 | 63.0 | 0.010 |
| Schooling, y | 12.6 ± 0.2 | 13.3 ± 0.1 | 12.9 ± 0.1 | 12.8 ± 0.2 | <0.001 |
| Primiparous, % | 52.6 | 44.8 | 39.5 | 30.6 | <0.001 |
| Utilized WIC services during pregnancy, % | 51.7 | 36.6 | 40.3 | 43.6 | 0.006 |
| Lifestyle variables | |||||
| Smoked during last 3 mo pregnancy, % | 23.4 | 10.3 | 8.0 | 13.2 | <0.001 |
| Maternal weekly exercise3, % | <0.001 | ||||
| 0 d | 46.9 | 40.5 | 41.3 | 47.4 | |
| 1–4 d | 30.7 | 43.9 | 42.5 | 41.0 | |
| 5–7 d | 22.3 | 15.6 | 16.2 | 11.6 | |
| Maternal weight change from prepregnancy,4kg | 9.7 ± 1.1 | 8.6 ± 0.3 | 10.5 ± 0.7 | 8.4 ± 0.9 | <0.001 |
| Child weekly sugar sweetened beverage consumption,3% | 0.21 | ||||
| Never | 26.8 | 29.6 | 27.8 | 24.3 | |
| 1–3 times/wk | 38.3 | 36.0 | 33.6 | 34.3 | |
| 4–6 times /wk | 3.8 | 6.6 | 7.5 | 6.2 | |
| 1 time/d | 18.7 | 14.8 | 19.1 | 19.1 | |
| ≥2 times/d | 12.4 | 12.9 | 12.0 | 16.2 | |
| Child weekly fast-food consumption,3% | 0.26 | ||||
| Never | 23.5 | 24.8 | 23.6 | 22.8 | |
| 1–3 times/wk | 69.8 | 66.3 | 64.7 | 62.6 | |
| 4–6 times/wk | 1.1 | 2.1 | 3.0 | 4.0 | |
| 1 time/d | 3.7 | 4.5 | 5.7 | 8.5 | |
| ≥2 times/d | 1.9 | 2.3 | 2.9 | 2.2 | |
| Child typical weekday screen time,3h | 3.5 ± 0.3 | 3.6 ± 0.1 | 3.6 ± 0.1 | 4.0 ± 0.2 | 0.001 |
| Family lifestyle score5 | 0.003 ± 0.003 | −0.003 ± 0.001 | 0.002 ± 0.002 | 0.007 ± 0.002 | <0.001 |
| Mediating variables | |||||
| Birth-weight-for-gestational age Z-score | −0.42 ± 0.09 | −0.08 ± 0.03 | 0.12 ± 0.04 | 0.13 ± 0.06 | <0.001 |
| Breastfeeding duration, % | 0.012 | ||||
| Never or <1 mo | 26.9 | 26.0 | 30.1 | 37.7 | |
| 1–5 mo | 42.9 | 36.1 | 36.8 | 35.8 | |
| 6–11 mo | 18.6 | 19.7 | 18.4 | 13.9 | |
| ≥12 mo | 11.6 | 18.1 | 14.7 | 12.6 | |
| Maternal exposures | |||||
| GWG, kg | 16.0 ± 0.7 | 15.2 ± 0.2 | 13.9 ± 0.3 | 11.1 ± 0.4 | <0.001 |
| Adequacy by 2009 IOM/NRC recommendations, % | <0.001 | ||||
| Inadequate | 29.8 | 21.8 | 8.7 | 19.0 | |
| Adequate | 42.3 | 40.4 | 30.7 | 28.1 | |
| Excessive | 27.9 | 37.8 | 60.7 | 52.9 | |
| Child outcomes at 5 y | |||||
| BMI Z-score | 0.32 ± 0.09 | 0.54 ± 0.03 | 0.82 ± 0.04 | 1.04 ± 0.05 | <0.001 |
| Weight status, % | <0.001 | ||||
| Underweight | 2.4 | 1.8 | 1.5 | 1.0 | |
| Normal weight | 77.7 | 68.7 | 56.7 | 51.7 | |
| Overweight | 14.1 | 16.9 | 22.5 | 20.4 | |
| Obese | 5.8 | 12.5 | 19.3 | 26.9 | |
Values are mean ± SE or percentages. ECLS-B, Early Childhood Longitudinal Study-Birth Cohort; GWG, gestational weight gain; IOM, Institute of Medicine; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
Sample size rounded to the nearest 50 per data agreement with the Department of Education.
Assessed when children were ∼4 y.
Weight change from prepregnancy assessed when children were ∼5 y.
Based on principal component analysis with maternal smoking during pregnancy, exercise, and weight change from prepregnancy, child sugar-sweetened beverage and fast-food consumption, and screen time.
The associations between GWG and child BMI Z-score at 5 y stratified by prepregnancy BMI status are presented in Table 2. Regression estimates from model C were plotted in Figure 2 and represent the overall adjusted association between GWG and child BMI Z-score.
TABLE 2.
Association between GWG and child BMI Z-score at 5 y, by maternal prepregnancy BMI status (ECLS-B, United States, 2001–2007)1
| Maternal prepregnancy BMI status | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Underweight n = 2502 | Normal weight n = 21502 | Overweight n = 7502 | Obese n = 4502 | |||||||||
| Model3 | GWG | GWG2 | P 4 | GWG | GWG2 | P 4 | GWG | GWG2 | P 4 | GWG | GWG2 | P 4 |
| A | 0.35 (−0.37, 0.87) | −0.03 (−0.13, 0.06) | 0.29 | −0.12 (−0.27, 0.02) | 0.03 (0.01, 0.05) | <0.001 | −0.11 (−0.34, 0.12) | 0.04 (0.00, 0.08) | 0.004 | 0.00 (−0.17, 0.18) | 0.00 (−0.03, 0.03) | 0.99 |
| B | 0.42 (−0.34, 1.19) | −0.05 (−0.15, 0.05) | 0.49 | −0.08 (−0.22, 0.07) | 0.02 (0.01, 0.05) | <0.001 | −0.07 (−0.29, 0.14) | 0.03 (−0.01, 0.07) | 0.010 | −0.03 (−0.21, 0.15) | 0.00 (−0.03, 0.03) | 0.72 |
| C | 0.42 (−0.34, 1.19) | −0.05 (−0.15, 0.05) | 0.49 | −0.08 (−0.22, 0.07) | 0.02 (0.01, 0.05) | <0.001 | −0.04 (−0.25, 0.17) | 0.03 (−0.01, 0.06) | 0.013 | −0.03 (−0.21, 0.15) | 0.00 (−0.03, 0.03) | 0.71 |
| D | 0.51 (−0.24, 1.27) | −0.06 (−0.16, 0.03) | 0.40 | −0.08 (−0.22, 0.06) | 0.02 (0.00, 0.04) | 0.005 | −0.06 (−0.27, 0.15) | 0.02 (−0.01, 0.06) | 0.09 | −0.04 (−0.23, 0.16) | 0.00 (−0.04, 0.03) | 0.31 |
All estimates are β (95% CI) per 5 kg GWG. ECLS-B, Early Childhood Longitudinal Study-Birth Cohort; GWG, gestational weight gain.
Sample size rounded to the nearest 50 per data agreement with the Department of Education.
Model A, unadjusted; model B, adjusted for sociodemographic characteristics; model C, adjusted for sociodemographic characteristics and family lifestyle score; model D, adjusted for sociodemographic characteristics, family lifestyle score, birth-weight-for-gestational-age Z-score, and breastfeeding duration.
P for the overall association of GWG and GWG2.
FIGURE 2.
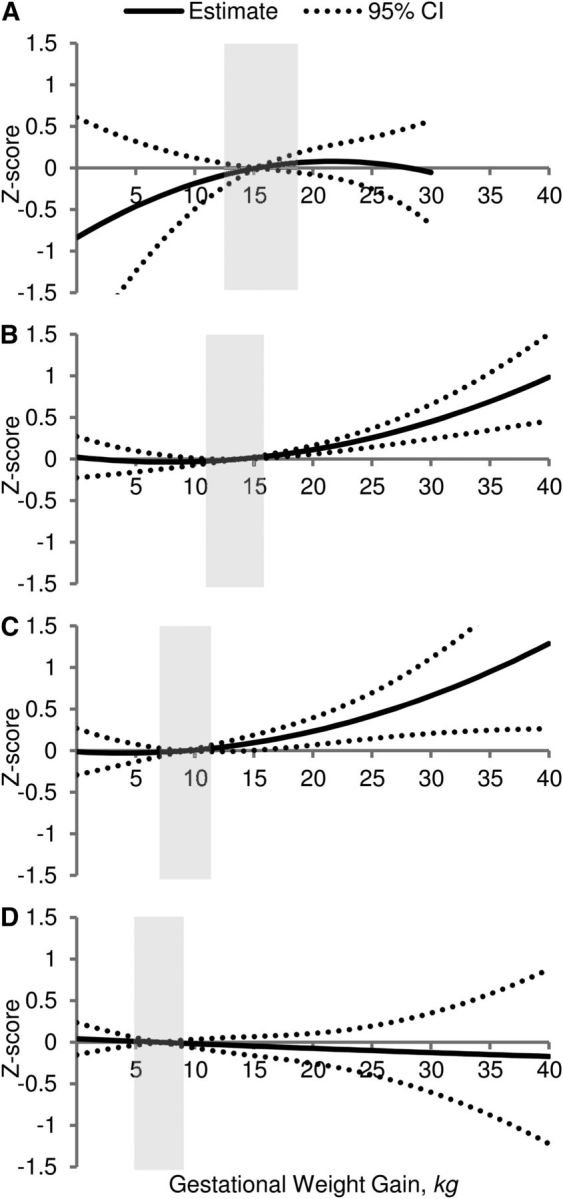
Adjusted association between GWG and child BMI Z-score at 5 y among underweight (n = 250) (A), normal-weight (n = 2150) (B), overweight (n = 750) (C), and obese (n = 450) (D) mothers. Values are β ± 95% CI. Shaded boxes represent IOM/NRC 2009 recommended ranges. ECLS-B, United States, 2001–2007. All models adjusted for sociodemographic characteristics and unhealthy family lifestyle score. ECLS-B, Early Childhood Longitudinal Study-Birth Cohort; GWG, gestational weight gain; IOM, Institute of Medicine.
MVPA model estimates indicated that across all prepregnancy BMI categories, there was a positive association between GWG and infant birth-weight-for-gestational-age (data not shown). With the exception of children of underweight mothers, birth-weight-for-gestational-age was positively associated with child BMI Z-score. No significant associations were observed between birth-weight-for-gestational-age and breastfeeding duration or between breastfeeding duration and child BMI Z-score. Figure 3 shows that for normal and overweight mothers, the direct effect is stronger than the indirect effect.
FIGURE 3.
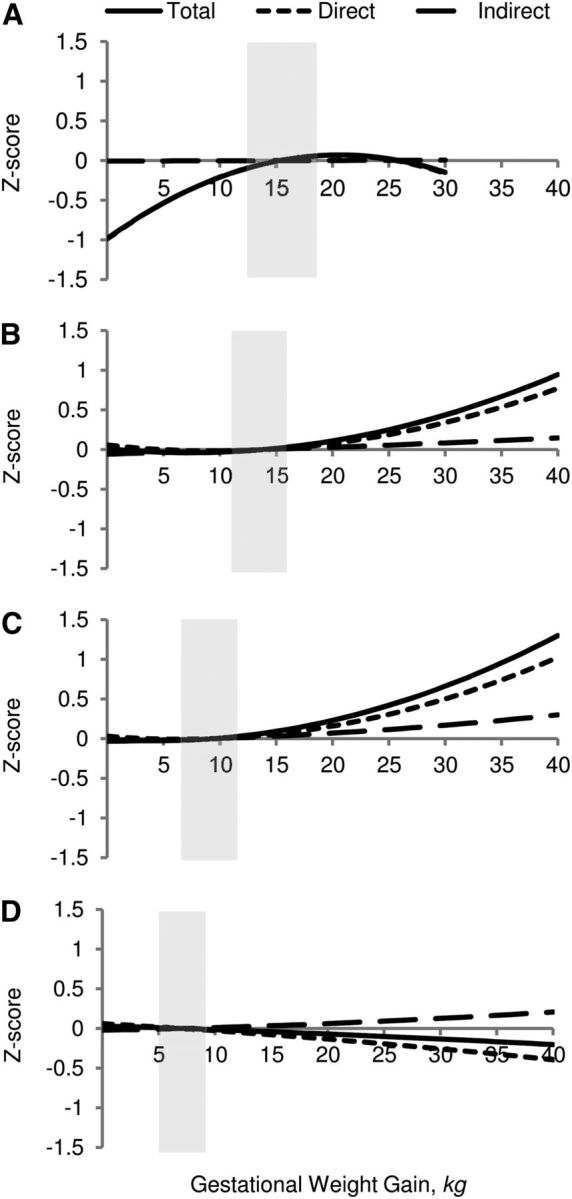
Total (solid line), direct (short dashed line), and indirect (long dashed line) effect of the relationship between GWG and child BMI Z-score at 5 y among underweight (n = 250) (A), normal-weight (n = 2150) (B), overweight (n = 750) (C), and obese (n = 450) (D) mothers. Shaded boxes represent IOM/NRC 2009 recommended ranges. ECLS-B, United States, 2001–2007. All models adjusted for sociodemographic characteristics and unhealthy family lifestyle score. ECLS-B, Early Childhood Longitudinal Study-Birth Cohort; GWG, gestational weight gain; IOM, Institute of Medicine.
We performed a sensitivity analysis excluding mothers with hypertension, as reported on the child's birth certificate. No meaningful differences were observed in the estimates (data not shown).
Discussion
We used a contemporary, nationally representative cohort to examine the relationship between GWG and child adiposity measured at 5 y. We observed heterogeneity by maternal prepregnancy BMI status. There was a significant overall association between GWG and child adiposity among normal and overweight mothers only, such that child BMI Z-score increased significantly with GWG above the midpoint of the current IOM/NRC recommendations. Heterogeneity by prepregnancy BMI is consistent with the well-documented interaction between GWG and prepregnancy BMI with respect to short-term obstetric outcomes (1) and is supported by most of the recent studies examining child anthropometric outcomes and GWG (15, 17, 18, 24, 25). Notably, we found that among all prepregnancy BMI groups, there was no association between low GWG and child adiposity. This suggests that lowering GWG recommendations may not lower child BMI; however, prevention of excess GWG among normal weight and overweight mothers may be an important strategy for the prevention of childhood obesity.
We examined how the overall association between GWG and child BMI Z-score was decomposed into direct and indirect effects. The direct effect not mediated through birth weight was significant among normal-weight mothers only; however, there was a suggestion that there may be a direct effect among overweight mothers as well. Thus, excess GWG may have a lasting effect on child adiposity independent of its effect through birth weight, which was a much smaller component of the overall association with child BMI Z-score. Previous studies have found that excess GWG is associated with poor dietary quality (11) and an unfavorable inflammatory and hormonal intrauterine metabolic environment (4), which may be associated with developmental programming of obesity. Specific evidence from animal studies suggest that overnutrition during pregnancy is associated with altered fetal adipogenesis, appetite control, and possibly even a preference for high-fat, high-sugar foods (6–8). We hypothesize that the direct effect was not observed among obese mothers given the intrauterine metabolic environment associated with maternal prepregnancy obesity (45) and therefore the direct effect may be masked by the competing risk associated with high prepregnancy BMI.
Our findings were independent of maternal sociodemographics and family lifestyle. Accounting for lifestyle as a confounder of the association between GWG and child adiposity was important given that maternal lifestyle is strongly associated with GWG (10, 12) as well as child lifestyle and obesity (13, 14). We controlled for lifestyle through the use of a composite score developed using various indicators of an unhealthy maternal and child lifestyle. A higher composite score correlated with unhealthier lifestyle behaviors. Although this variable was developed independently of maternal prepregnancy BMI, we found that it was significantly different across maternal BMI groups, with the lowest scores among normal-weight mothers and the highest scores among obese mothers. The inclusion of the family lifestyle score slightly attenuated the association between GWG and child BMI Z-score among overweight mothers. In addition, by stratifying by prepregnancy BMI status, we partially controlled for shared lifestyle and genetics associated with adiposity (46). We could not, however, control for confounding due to genetic factors related to GWG, but the evidence supporting common genetic factors between excess GWG and child adiposity is less strong (47, 48).
We did not observe a significant association between GWG and child's BMI Z-score among underweight mothers. Only a small proportion of mothers were underweight prior to pregnancy and when measured at 5 y, most of their children had a healthy, normal weight. In contrast, a large proportion of children born to obese mothers were either overweight or obese at 5 y. The lack of an overall association between GWG and child's BMI Z-score may reflect the many factors contributing to childhood obesity among children born to obese mothers (7, 46, 49) or that the total effect can be detected only when assessed at more proximal time points (41). Our principal finding of no overall association among obese mothers should not be interpreted as suggesting that obese mothers can gain across the entire spectrum of GWG, because excess GWG in obese mothers has many other adverse consequences such as macrosomia and cesarean delivery (1).
Our study used a contemporary, diverse, population-based cohort to examine the relationship between GWG and child adiposity at 5 y. Our results are strengthened by the fact that in this cohort, child BMI was obtained from height and weight measured at least twice by trained fieldworkers and have been shown to have high reliability (34). Also, GWG in this study was obtained from the birth certificate for >80% of the cohort. The birth certificate has previously been shown to be a reliable source of GWG (50, 51). We used a quadratic model to allow for the flexibility to detect a J-shaped or U-shaped relationship between GWG and child BMI Z-score. Some previous studies have suggested an increased risk of child obesity with low GWG (19, 24), although our results suggest that there is no adverse or beneficial effect of low GWG. In the absence of this quadratic term, we would have concluded that, among normal-weight and overweight mothers, low GWG was associated with a decrease in child BMI Z-score. Last, we used MVPA, which provided a more detailed decomposition of the direct and indirect effects.
Our study is not without limitations. First, although it is based on a nationally representative cohort, as with most longitudinal studies, there was attrition in the sample. Although we do not have data on the reasons that families may have chosen to discontinue participation, all data were weighted to account for attrition and maintain a study sample with demographics similar to the target population. Also, our analytic sample included a greater proportion of records of mothers who tend to have better obstetric outcomes than those who were excluded due to missing data; however, it is unclear if, and to what degree, this may have biased our findings. We calculated prepregnancy BMI from maternal weight that was self-reported by mothers at the 9-mo interview. Mothers may have differentially underreported their prepregnancy weight and, even though it is possible that they were misclassified, prior studies have found that although some mothers are misclassified, the association with prepregnancy BMI and obstetric outcomes was not meaningfully different (52). It is possible that we lacked adequate power to detect an association between GWG and child adiposity among underweight women (n = 250). Although we used MVPA to examine direct and indirect effects, as with any observational study, the presence of direct and indirect effects does not necessarily imply causality. Also, MVPA assumes that all variables are free of measurement error (42). Whereas we know that the ECLS-B measurements of child height and weight were highly reliable (r = 1.000) (34), it is unclear if measurement error among other variables may have biased our results. Last, we have considered lifestyle to be a confounder of these associations; however, it is possible that GWG may affect postpartum lifestyle and therefore lifestyle may alternatively be a mediator. We have presented all associations with and without lifestyle in the model. Furthermore, although we included many maternal and child variables in our composite score for family lifestyle, there may be other important variables. In our study, we did not have any data on maternal diet or exercise either before or during pregnancy.
The current GWG recommendations were established primarily for the purpose of optimizing birth weight and other short-term obstetric outcomes. At that time, data were insufficient to support child adiposity as an influential outcome (1). Recently, there has been concern that recommendations may be too high given the current obesity epidemic (53). On the other hand, with respect to child adiposity, our data, in accord with other studies (15, 17, 18, 23), suggest that there is no benefit of low GWG; however, low weight gain should not be considered benign in the context of other outcomes, particularly small-for-gestational-age (1). In addition, excess GWG was associated with an increase in child adiposity among normal and overweight mothers. We also found that a large proportion of women gained more than the IOM/NRC-recommended GWG ranges, with the greatest proportion (61%) among mothers who were already overweight. Our study provides the first nationally representative estimates of the proportion of women who gain excessively during pregnancy. Determining how to prevent excess GWG is a critical next step given the increased risk for adverse short-term obstetric outcomes (1). As indicated by our study, among children born to normal-weight and overweight mothers, excess GWG was associated with a modestly increased BMI Z-score at 5 y. It is important to note that obesity in young children has been shown to be highly predictive of adult BMI (54) and thus early prevention is critical. Some diet and physical activity trials have been successful in the prevention of excess GWG (55); however, most have not been powered to examine the effect on outcomes.
Our study adds to the literature by supporting the association between GWG and child adiposity, specifically among normal-weight and overweight mothers. Although current recommendations were primarily established for short-term outcomes, our data suggest that these recommendations may also have a beneficial reduction in later child adiposity and do not provide support for reducing the amount of pregnancy weight gain that is recommended. Regardless of maternal prepregnancy BMI, prevention of excess GWG is important for short-term birth outcomes (1) and may be a strategy in the prevention of childhood obesity.
Acknowledgments
S.N.H. and A.J.S. designed the project conception, developed the overall research plan, and analyzed data and performed statistical analyses; A.J.S. provided study oversight; D.W.S. provided statistical support; S.N.H., A.J.S., D.W.S., L.A.S., U.R., and A.D.S. interpreted the data; S.N.H. wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Abbreviations
- ECLS-B
Early Childhood Longitudinal Study-Birth Cohort
- GWG
gestational weight gain
- IOM
Institute of Medicine
- MVPA
measured variable path analysis
Footnotes
Supported in part under an appointment to the CDC, administered by the Oak Ridge Institute for Science and Education under contract no. DE-AC05-06OR23100 between the U.S. Department of Energy and Oak Ridge Associated Universities. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Literature Cited
- 1. Institute of Medicine and NRC Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll M, Curtin L, Lamb M, Flegal K. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–9. [DOI] [PubMed] [Google Scholar]
- 3. Oken E, Gillman M. Fetal origins of obesity. Obes Res. 2003;11:496–506. [DOI] [PubMed] [Google Scholar]
- 4. Do Carmo S, Forest J-C, Gigure Y, Masse A, Lafond J, Rassart E. Modulation of apolipoprotein D levels in human pregnancy and association with gestational weight gain. Reprod Biol Endocrinol. 2009;7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaarawy M, el Mallah SY. Leptin and gestational weight gain: relation of maternal and cord blood leptin to birth weight. J Soc Gynecol Investig. 1999;6:70–3. [DOI] [PubMed] [Google Scholar]
- 6. Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–98. [DOI] [PubMed] [Google Scholar]
- 7. Oken E. Maternal and child obesity: the causal link. Obstet Gynecol Clin North Am. 2009;36:361–77. [DOI] [PubMed] [Google Scholar]
- 8. Mühlhausler BS, Adam C, McMillen IC. Maternal nutrition and the programming of obesity: the brain. Organogenesis. 2008;4:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stuebe A, Oken E, Gillman M. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201:58e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Streuling I, Beyerlein A, Rosenfeld E, Schukat B, von Kries R. Weight gain and dietary intake during pregnancy in industrialized countries: a systematic review of observational studies. J Perinat Med. 2011;39:123–9. [DOI] [PubMed] [Google Scholar]
- 12. Althuizen E, van Poppel MNM, Seidell JC, van Mechelen W. Correlates of absolute and excessive weight gain during pregnancy. J Womens Health (Larchmt). 2009;18:1559–66. [DOI] [PubMed] [Google Scholar]
- 13. Cameron AJ, Crawford D, Salmon J, Campbell K, McNaughton SA, Mishra GD, Ball K. Clustering of obesity-related risk behaviors in children and their mothers. Ann Epidemiol. 2011;21:95–102. [DOI] [PubMed] [Google Scholar]
- 14. Hart CN, Raynor HA, Jelalian E, Drotar D. The association of maternal food intake and infants' and toddlers' food intake. Child Care Health Dev. 2010;36:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deierlein AL, Siega-Riz AM, Adair LS, Herring AH. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr. 2011;158:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oken E, Taveras E, Kleinman K, Rich-Edwards J, Gillman M. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr. 2008;87:1818–24. [DOI] [PubMed] [Google Scholar]
- 18. Oken E, Rifas-Shiman S, Field A, Frazier AL, Gillman M. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, Robinson SM. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am J Clin Nutr. 2010;91:1745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fraser A, Tilling K, Macdonald Wallis C, Sattar N, Brion MJ, Benfield L, Ness A, Deanfield J, Hingorani A, Nelson SM, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF. Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J. 2012;16:1215–23. [DOI] [PubMed] [Google Scholar]
- 22. Branum AM, Parker JD, Keim SA, Schempf AH. Prepregnancy body mass index and gestational weight gain in relation to child body mass index among siblings. Am J Epidemiol. 2011;174:1159–65. [DOI] [PubMed] [Google Scholar]
- 23. Schack Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sørensen TI. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes (Lond). 2010;34:67–74. [DOI] [PubMed] [Google Scholar]
- 24. Stuebe AM, Forman MR, Michels KB. Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. Int J Obes. 2009;33:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawlor DA, Lichtenstein P, Fraser A, Langstrom N. Does maternal weight gain in pregnancy have long-term effects on offspring adiposity? A sibling study in a prospective cohort of 146,894 men from 136,050 families. Am J Clin Nutr. 2011;94:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. [DOI] [PubMed] [Google Scholar]
- 27. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–32. [DOI] [PubMed] [Google Scholar]
- 28. Nielsen SJ, Siega Riz A, Popkin B. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res. 2002;10:370–8. [DOI] [PubMed] [Google Scholar]
- 29. Poti JM, Popkin BM. Trends in energy intake among US children by eating location and food source, 1977–2006. J Am Diet Assoc. 2011;111:1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lampl M, Kusanovic J, Erez O, Gotsch F, Espinoza J, Gonclaves L, Lee W, Gomez R, Nien JK, Frongillo EA, et al. Growth perturbations in a phenotype with rapid fetal growth preceding preterm labor and term birth. Am J Hum Biol. 2009;21:782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morisset A-S, Tchernof A, Dub M-C, Veillette J, Weisnagel SJ, Robitaille J. Weight gain measures in women with gestational diabetes mellitus. J Womens Health (Larchmt). 2011;20:375–80. [DOI] [PubMed] [Google Scholar]
- 32. Lampl M, Jeanty P. Exposure to maternal diabetes is associated with altered fetal growth patterns: A hypothesis regarding metabolic allocation to growth under hyperglycemic-hypoxemic conditions. Am J Hum Biol. 2004;16:237–63. [DOI] [PubMed] [Google Scholar]
- 33. Hinkle SN, Sharma AJ, Schieve LA, Ramakrishnan U, Swan DW, Stein AD. Reliability of gestational weight gain reported postpartum: a comparison to the birth certificate. Matern Child Health J. Epub 2012 Jun 16. [DOI] [PubMed]
- 34. Najarian M, Snow K, Lennon J, Kinsey S. Early Childhood Longitudinal Study, Birth Cohort (ECLS-B), preschool–kindergarten 2007 psychometric report (NCES 2010–009). Washington, DC: National Center for Education Statistics, Institute of Education Sciences, U.S. Department of Education; 2010. [Google Scholar]
- 35. Grummer Strawn L, Reinold C, Krebs N. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Morb Mortal Wkly Rep. 2010;59: RR-9:1–15. [PubMed] [Google Scholar]
- 36. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dubois L, Farmer A, Girard M, Peterson K. Regular sugar-sweetened beverage consumption between meals increases risk of overweight among preschool-aged children. J Am Diet Assoc. 2007;107:924–34. [DOI] [PubMed] [Google Scholar]
- 38. Bowman SA, Gortmaker S, Ebbeling C, Pereira M, Ludwig D. Effects of fast-food consumption on energy intake and diet quality among children in a national household survey. Pediatrics. 2004;113:112–8. [DOI] [PubMed] [Google Scholar]
- 39. Dennison BA, Erb T, Jenkins P. Television viewing and television in bedroom associated with overweight risk among low-income preschool children. Pediatrics. 2002;109:1028–35. [DOI] [PubMed] [Google Scholar]
- 40. Oken E, Kleinman K, Rich Edwards J, Gillman M. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shrout PE. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422. [PubMed] [Google Scholar]
- 42. Kline RB. Principles and practice of structural equation modeling. 3rd ed New York: Guilford Press; 2011. [Google Scholar]
- 43. Lt H, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 44. Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia: Wolters Kluwer Health/Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 45. Armitage JA, Poston L, Taylor P. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. [DOI] [PubMed] [Google Scholar]
- 46. Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008;87:398–404. [DOI] [PubMed] [Google Scholar]
- 47. Stuebe A, Lyon H, Herring A, Ghosh J, Wise A, North KE, Siega-Riz AM. Obesity and diabetes genetic variants associated with gestational weight gain. Am J Obstet Gynecol. 2010;203:283e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lawlor DA, Fraser A, Macdonald-Wallis C, Nelson SM, Palmer TM, Davey Smith G, Tilling K. Maternal and offspring adiposity-related genetic variants and gestational weight gain. Am J Clin Nutr. 2011;94:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wardle J, Guthrie C, Sanderson S, Birch L, Plomin R. Food and activity preferences in children of lean and obese parents. Int J Obes Relat Metab Disord. 2001;25:971–7. [DOI] [PubMed] [Google Scholar]
- 50. Buescher PA, Taylor KP, Davis MH, Bowling JM. The quality of the new birth certificate data: a validation study in North Carolina. Am J Public Health. 1993;83:1163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zollinger TW, Przybylski M, Gamache R. Reliability of Indiana birth certificate data compared to medical records. Ann Epidemiol. 2006;16:1–10. [DOI] [PubMed] [Google Scholar]
- 52. Bodnar LM, Siega-Riz AM, Simhan HN, Diesel JC, Abrams B. The Impact of exposure misclassification on associations between prepregnancy BMI and adverse pregnancy outcomes. Obesity (Silver Spring). 2010;18:2184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Artal R, Lockwood C, Brown H. Weight gain recommendations in pregnancy and the obesity epidemic. Obstet Gynecol. 2010;115:152–5. [DOI] [PubMed] [Google Scholar]
- 54. Brisbois TD, Farmer AP, McCargar LJ. Early markers of adult obesity: a review. Obes Rev. 2012;13:347–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Skouteris H, Hartley Clark L, McCabe M, Milgrom J, Kent B, Herring SJ, Gale J, et al. Preventing excessive gestational weight gain: a systematic review of interventions. Obes Rev. 2010;11:757–68. [DOI] [PubMed] [Google Scholar]