Abstract
α-Tocopherol (α-Toc) enhances T cell function, whereas little is known in this regard for tocotrienols (T3), the less-known members of the vitamin E family. We pair-fed young (4 mo) and old (23 mo) C57BL/6 mice 0.1% Tocomin 50%, a mixture of T3 and α-Toc or a control diet containing an equal amount of α-Toc for 6 wk. As expected, lymphocyte proliferation was lower in the old mice compared with the young mice. Lymphocyte proliferation in the old T3 group was significantly higher than that in the old control group, whereas no significant difference was found in young mice. Splenocytes from old mice produced less interleukin (IL)-2, IL-4, IL-6, and IL-10 compared with young mice, whereas no significant age-related difference was found in IL-1β, tumor necrosis factor-α, and interferon-γ. T3 feeding was associated with a higher IL-1β production in old mice but not in young mice. Peritoneal macrophages from old mice produced significantly more IL-1β, IL-6, IL-10, and prostaglandin E2 (PGE2) compared with those from young mice. Mice of both ages fed T3 had higher production of IL-1β but not PGE2 or other cytokines. In the in vitro study, splenocytes isolated from young and old mice were supplemented with the purified form of each individual T3 (0.01–10 μmol/L) and mitogen-stimulated cell proliferation was determined. All T3 enhanced lymphocyte proliferation in old but not young mice with a potency order of α- > γ- > δ-T3. Together, these results suggest a beneficial effect of T3 in improving the age-related decline in T cell function.
Introduction
Vitamin E is the most effective chain-breaking, lipid-soluble antioxidant in biologic membranes of all cells. Its health benefits in various body systems have been demonstrated (1–3). Natural vitamin E is a mixture of 2 classes of compounds, tocopherols (Toc)7 and tocotrienols (T3), each having 4 homologs designated as α-, β-, γ-, and δ-. Among them, α-Toc is the major form of vitamin E found in diet and has the highest bioavailability and, thus, tissue concentrations. It is not surprising, therefore, that α-Toc is the form of vitamin E that has been used in a majority of studies and is best characterized. Recently, growing evidence has suggested that T3, the minor members of vitamin E, may have potential anticancer (4,5), antiangiogenic (6–9), blood cholesterol-lowering (10–12), antiatherosclerotic (13–15), and neuroprotective effects (16–18).
Studies have suggested that compared with Toc, T3 possess some distinct properties, such as their cholesterol-lowering effect. Further, T3 might be more effective in the biological effects shared with α-Toc. For example, T3 are shown to have a more profound effect than Toc in reducing the risk indices for developing atherosclerosis (14,15); α-T3 is more readily incorporated into the cell membrane and possesses higher antioxidant activity against lipid peroxidation than α-Toc (19,20). Aging is associated with a decline in immune response, which predisposes the elderly to higher incidence of infections and cancer. We and others have established through a number of animal and human studies that vitamin E (α-Toc) can enhance T cell-mediated immune function. Immune cells are particularly enriched in vitamin E, because their high PUFA content puts them at especially high risk for oxidative damage (21,22). Free radical damage to immune cell membrane lipids may ultimately impair their ability to respond normally to pathogenic challenge. Studies have shown that vitamin E deficiency impairs both humoral and cell-mediated immune functions (23,24), whereas supplementation with vitamin E above the recommended levels has been shown to enhance immune response, particularly in the aged (25–29). Furthermore, vitamin E-induced enhancement of immune functions in the aged is associated with significant improvement in resistance to influenza infection in aged mice (30,31) and a reduced risk of acquiring upper respiratory infections in nursing home residents (32). In all these studies, α-Toc is the form of vitamin E used exclusively and little is known about the immunological effects of other homologs. In an in vitro study, we found that β-, δ-, and γ-Toc are more potent in enhancing mitogen-induced lymphocyte proliferation and inhibiting production of T cell suppressive factor prostaglandin (PGE2) (33).
In contrast to the well-documented effect of α-Toc on improving the immune response and resistance to infectious diseases, information in this regard is essentially absent for T3. The only available information is from a recent human study showing that daily supplementation with 200 mg of T3-rich fraction for 56 d did not affect blood immune cell phenotype and production of cytokines interleukin (IL)-4 and interferon (IFN)-γ compared with placebo or the 200 mg of α-Toc group. However, several study limitations hampered the ability to reach a conclusion regarding immune-modulating effects of T3. Often, immunostimulatory effects of nutrients are exerted without a change in cell profile. Furthermore, nutrient intervention may affect production of some cytokines but leave others unchanged; thus, an overall conclusion about the effect of a nutrient on immune response cannot be reached by investigating a few indices. A selection of a broader spectrum of immune markers would be needed before a conclusion regarding T3 and immune response is reached. We therefore conducted the current study to determine the effect of dietary supplementation with a natural mixture of T3, the available form of T3 for dietary supplementation, on immune response of young and old mice using a wide range of immune indices. Furthermore, we utilized an in vitro model to further characterize the relative efficacy of each of the major constituents of T3.
Materials and Methods
Diets.
The basal diet was a modification of the AIN-93M diet (34) and purchased from Dyets (diet no. 102301). The modified AIN-93 diet was oil- and vitamin E-free. The amount of oil normally present in the AIN-93M diet is 4% soybean oil. T3 was premixed with soybean oil before being added to the basal diet. Tocomin 50% (kind gift from W.H. Leong, Carotech) is a mixture of natural T3 and α-Toc (12.2% α-T3, 2% β-T3, 6.2% δ-T3, 20.1% γ-T3, and 10.7% α-Toc). To prepare 1 kg of T3-supplemented diet, 1 g Tocomin 50% was added to 39 g vitamin E-stripped soybean oil, which was then mixed with 0.96 kg basal diet. This T3-supplemented diet contained 500 mg/kg of total vitamin E including 107 mg/kg α-Toc with the remainder as T3. One kilogram of control diet was prepared by adding 107 mg of d-α-Toc to 0.96 kg of the basal diet followed by mixing with 40 g of vitamin E-stripped soybean oil. Thus, both diets contained the same amount of α-Toc. In the in vitro study, a mouse nonpurified diet (Teklad 7012, Harlan Teklad) was used.
Animals.
Male young (4 mo) and old (23 mo) C57BL/6 mice were purchased from the National Institute on Aging colonies at Harlan Sprague Dawley, Inc. After a 2-wk acclimation period, mice were weight-matched for each age and placed into either the control or T3 diet group. Mice were housed individually and maintained in an environmentally controlled atmosphere (temperature 23°C, relative humidity 45%) with a 12-h-light/-dark cycle. Mice were provided free access to water and group pair-fed their respective diets for 6 wk. In the in vitro study, mice consumed the mouse nonpurified diet ad libitum. For the pair-feeding, mice in each age group were initially given a weighed portion of food daily. If any mouse did not eat the entire portion of food, the weighed portion for all mice was decreased to the amount that the mouse ate the previous day. If all mice consumed all of the diet, then the quantity of diet given was increased until an individual mouse did not consume all the food. By group feeding, we decreased the variability among the mice both within each diet group and among the diet groups. All mice were observed daily for clinical signs of disease; body weight was recorded weekly. At the end of the study, mice were killed by CO2 asphyxiation and exsanguination. All conditions and handling of the animals were approved by the Animal Care and Use Committee of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University and conducted according to the NIH Guidelines for the Care and Use of Laboratory Animals.
Splenocyte isolation.
Spleens were aseptically removed and placed in sterile RPMI 1640 (Biowhittaker) medium supplemented with 25 mmol/L HEPES (Invitrogen Gibco), 2 mmol/L glutamine (Gibco), 100 kU/L penicillin, and 100 mg/L streptomycin (Gibco). Medium supplemented as described will be referred to as complete RPMI. Single cell suspensions were prepared by gently disrupting spleens between 2 sterile frosted glass slides. Splenocytes were isolated via centrifugation (300 × g), and RBC were lysed using Gey's reagent. Splenocytes were washed twice with complete RPMI and viability was determined by trypan blue exclusion. Splenocytes were suspended in complete RPMI containing 5% heat-inactivated fetal bovine serum (Gibco) at appropriate density for different cultures. All the experiments were conducted at 37°C, an atmosphere of 5% CO2, and 95% humidity unless indicated otherwise.
Cellular composition of splenocytes.
The percentages of major cell types in spleen were determined using flow cytometry. Splenocytes (1 × 106 cells/sample) were stained in 4 sets of combinations: 1) CD3-FITC/CD4-APC/CD44-PE to identify total T cells and then determine CD4 naïve (CD44 low) and memory (CD44 high) T cells; 2) CD3-FITC/CD8-APC/CD44-PE to do the same as above but for CD8 T cells; 3) CD19-PE/NK1.1-FITC/F4/80-APC to identify B cells, natural killer cells, and macrophages (Mφ), respectively; and 4) CD4-FITC/CD25-APC to identify regulatory T cells (CD4+/CD25+ cells). Anti-CD16/32 (Fc block) and the isotype control corresponding to each specific antibody (Ab) were used. F4/80 Ab was from Caltag and all the other Ab and isotype controls were from BD PharMingen. Stained cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences); the results were analyzed using the software Summit 4.0 (DakoCytomation).
Mitogenic response of splenocytes.
Splenocytes (1 × 105 cells/well) in 96-well round-bottom plates (Becton Dickinson Labware) were cultured in the presence or absence of the T cell mitogen concanavalin A (Con A; Sigma) at 0.25, 0.5, 1.5, or 3 mg/L, phytohemagglutinin P (PHA; Difco, Laboratories) at 2.5, 5, or 20 mg/L, plate-coated anti-CD3 (BD Biosciences) at 1 or 5 mg/L, or plate-coated anti-CD3 (5 mg/L) plus soluble anti-CD28 (2 mg/L) (CD3/CD28) for 72 h.
For in vitro lymphocyte proliferation assay, splenocytes isolated from young and old C57BL/6 mice were incubated with α-, γ-, or δ-T3 at concentrations of 0.01–10 μmol/L for 4 h and then stimulated by Con A at optimal concentration (1.5 mg/L) for 72 h. T3 were kindly supplied by W.H. Leong (Carotech) with a purity of 87, 90, and 61% for α-, γ-, or δ-T3, respectively. Cultures were pulsed with 18.5 kBq [3H]-thymidine (Perkin Elmer) during the final 4 h of incubation. The cells were harvested onto glass fiber filter mats (Wallac) by a Tomtec harvester (Wallac) and cell proliferation was quantified as the amount of [3H]-thymidine incorporation into DNA as determined by liquid scintillation counting in a 1205 Betaplate counter (Wallac). Results are expressed as kBq.
Cytokine and PGE2 production by splenocytes.
Splenocytes (4 × 106 cells/well) in 24-well culture plates (Becton Dickinson Labware) were cultured in the presence of Con A (1.5 mg/L) or lipopolysaccharide (LPS) (1 mg/L) for 24 h for inflammatory cytokines IL-1β, IL-6, TNFα, and T cell suppressive lipid mediator PGE2 production, or in the presence of Con A (1.5 mg/L) or anti-CD3/CD28 (5, 2 mg/L) for 48 h for IFNγ, IL-2, IL-4, and IL-10 production. Cell-free supernatants were collected at the end of incubation and stored at −70°C for later analysis. All of the cytokines were measured using ELISA. The reagents for IL-1β were from R&D System; all other cytokines were from BD Biosciences. PGE2 was measured using RIA as previously described (33).
Cytokine and PGE2 production by Mφ.
Peritoneal exudate cells were obtained by peritoneal lavage and enriched for Mφ using the method of Kumagai et al. (35). Peritoneal Mφ prepared in this manner were at least 90% pure, as assessed by expression of F4/80. Mφ (∼5 × 105 cells/well) were incubated in 24-well culture plates in the presence of LPS (1 mg/L) for 24 h. Cell-free supernatants were analyzed for cytokines and PGE2 production. Cytokines and PGE2 were analyzed as described above and their concentrations were normalized with total cell protein.
Statistical analysis.
All results are expressed as means ± SE. Statistical analysis was conducted using SYSTAT 10 statistical software (Systat). Data were log-transformed to account for unequal variations. Data from the in vivo study were tested by 2-way ANOVA (age, diet, age × diet) except for body weight data, for which repeated-measures ANOVA was used. To assess the effects of different T3 at various concentrations, 3-way ANOVA was conducted. Fisher's least significance difference was conducted as the post hoc test when the interactions of 2-way or 3-way ANOVA were significant. Significance was set at P < 0.05.
Results
General condition of mice.
Mice in both age and diet groups remained healthy throughout the experiment as assessed by body weight and general physical activity. While not quantified, our daily observation of the mice indicated that in general, old mice were less active than young mice and diet did not seem to affect the activity of either age group. Daily food intake of group pair-fed mice varied from 3 to 3.5 g/d. Body weight did not differ between the diet groups in each age at the start and throughout the study (Supplemental Fig. 1). Old mice weighed ∼4.5 g more than young mice before dietary intervention. Weight increased during the feeding period in both age groups, but more so in young mice so that there was an age × time interaction (P < 0.0001) and at the end of the study the old mice were 3 g heavier than young mice (Supplemental Fig. 1).
Phenotypic profile of spleen cells.
Spleens of old mice compared with those of young mice had a lower percentage of total T cells, naïve CD4+, CD8+ T cells, and regulatory T cells and a higher percentage of memory CD4+ and CD8+ T cells and B cells, whereas no age-related difference was observed in other cell types, including total CD4+ T cells, total CD8+ T cells, Mφ, and NK cells. T3 supplementation did not affect the percentage of any cell type in spleen (Supplemental Table 1).
Ex vivo lymphocyte proliferation.
As expected and consistent with the previous studies, old mice had significantly lower levels of lymphocyte proliferation compared with young mice under most stimulation conditions (Table 1). Proliferation of splenocytes of young and old mice differed significantly except those stimulated with PHA at 2.5 and 5 mg/L. Although there was a significant overall diet effect for all stimulation conditions, young and old mice responded to the dietary intervention differently. Age and diet interacted significantly such that not all effects in cells from old mice occurred in those from young mice (Table 1).
TABLE 1.
Effect of dietary supplementation with T3 on lymphocyte proliferation of young and old mice1
| Young | Old | P-value | |||||
|---|---|---|---|---|---|---|---|
| Stimulation | Control | T3 | Control | T3 | Age | T3 | Age × T3 |
| Con A, mg/L | kBq | ||||||
| 0.25 | 0.17 ± 0.03 | 0.21 ± 0.04 | 0.06 ± 0.01 | 0.17 ± 0.05 | 0.005 | 0.013 | 0.116 |
| 0.5 | 0.81 ± 0.08 | 0.99 ± 0.11 | 0.31 ± 0.09 | 0.47 ± 0.09 | <0.001 | 0.026 | 0.116 |
| 1.5 | 3.44 ± 0.26 | 3.99 ± 0.15 | 0.70 ± 0.15 | 1.53 ± 0.29* | <0.001 | 0.002 | 0.041 |
| 3 | 1.85 ± 0.26 | 2.85 ± 0.37 | 0.17 ± 0.04 | 0.34 ± 0.06 | <0.001 | 0.001 | 0.545 |
| PHA, mg/L | |||||||
| 2.5 | 0.13 ± 0.03 | 0.13 ± 0.01 | 0.12 ± 0.03 | 0.28 ± 0.06* | 0.067 | 0.034 | 0.027 |
| 5 | 0.18 ± 0.03 | 0.24 ± 0.02 | 0.12 ± 0.02 | 0.48 ± 0.14** | 0.194 | 0.005 | 0.044 |
| 20 | 0.12 ± 0.01 | 0.16 ± 0.01 | 0.05 ± 0.01 | 0.09 ± 0.02 | <0.001 | 0.003 | 0.385 |
| CD3, mg/L | |||||||
| 1 | 1.94 ± 0.16 | 2.23 ± 0.12 | 0.33 ± 0.04 | 0.58 ± 0.07 | <0.001 | 0.002 | 0.070 |
| 5 | 2.55 ± 0.15 | 2.61 ± 0.08 | 0.38 ± 0.07 | 0.73 ± 0.09** | <0.001 | 0.002 | 0.005 |
| CD3/CD28 | 2.36 ± 0.15 | 2.57 ± 1.04 | 0.45 ± 0.07 | 0.77 ± 0.09* | <0.001 | 0.003 | 0.030 |
Values are means ± SE, n = 11–13. Asterisks indicate different from the corresponding control: * P < 0.05 and ** P < 0.01.
Ex vivo production of inflammatory cytokines and PGE2 by Mφ and splenocytes.
Peritoneal Mφ from old mice produced significantly more IL-1β, IL-6, IL-10, and PGE2 compared with those from young mice (Table 2). Splenocytes from old mice produced less IL-6 compared with those from young mice. No significant age-related difference was found in IL-1β, TNFα, and PGE2 production. The mice fed T3 had higher splenocyte IL-1β production compared with the mice fed the control diet (Table 3).
TABLE 2.
Effect of dietary supplementation with T3 on basal and LPS-stimulated cytokine and PGE2 production by Mφ of young and old mice1
| Young | Old | P-value | |||||
|---|---|---|---|---|---|---|---|
| Control | T3 | Control | T3 | Age | T3 | Age × T3 | |
| IL-1β | pg/μg protein | ||||||
| LPS | 0.7 ± 0.2 | 1.1 ± 0.2 | 1.5 ± 0.3 | 1.6 ± 0.1 | <0.001 | 0.183 | 0.343 |
| IL-6 | |||||||
| Medium | 0.9 ± 0.6 | 2.4 ± 1.7 | 3.3 ± 1.1 | 5.5 ± 1.7 | 0.007 | 0.331 | 0.809 |
| LPS | 60 ± 24 | 68 ± 16 | 82 ± 22 | 193 ± 49 | 0.002 | 0.013 | 0.856 |
| TNFα | |||||||
| LPS | 4.5 ± 0.7 | 5.3 ± 1.0 | 5.0 ± 0.8 | 7.8 ± 1.0 | 0.070 | 0.077 | 0.489 |
| IL-10 | |||||||
| LPS | 1.8 ± 0.4 | 3.0 ± 0.5 | 20.0 ± 6.7 | 14.6 ± 2.8 | <0.001 | 0.374 | 0.192 |
| PGE2 | |||||||
| Medium | 1.7 ± 0.5 | 2.2 ± 1.0 | 7.8 ± 2.4 | 10.0 ± 3.5 | <0.001 | 0.920 | 0.674 |
| LPS | 85 ± 17 | 112 ± 10 | 242 ± 50 | 321 ± 129 | 0.001 | 0.385 | 0.199 |
Values are means ± SE, n = 11–13.
TABLE 3.
Effect of dietary supplementation with T3 on basal and LPS- or ConA-stimulated cytokine and PGE2 production by splenocytes of young and old mice1
| Young | Old | P-value | |||||
|---|---|---|---|---|---|---|---|
| Control | T3 | Control | T3 | Age | T3 | Age × T3 | |
| IL-1β | ng/L | ||||||
| LPS | 76 ± 8 | 87 ± 6 | 77 ± 13 | 134 ± 20 | 0.271 | 0.019 | 0.266 |
| IL-6 | |||||||
| Con A | 497 ± 53 | 621 ± 68 | 231 ± 61 | 287 ± 103 | <0.001 | 0.335 | 0.918 |
| LPS | 626 ± 87 | 762 ± 76 | 863 ± 219 | 854 ± 106 | 0.413 | 0.217 | 0.922 |
| TNFα | |||||||
| Con A | 133 ± 25 | 130 ± 23 | 222 ± 10 | 162 ± 49 | 0.796 | 0.744 | 0.999 |
| LPS | 215 ± 21 | 213 ± 16 | 319 ± 75 | 366 ± 74 | 0.062 | 0.503 | 0.550 |
| PGE2 | |||||||
| Con A | 247 ± 26 | 324 ± 46 | 276 ± 41 | 293 ± 83 | 0.636 | 0.453 | 0.313 |
| LPS | 726 ± 136 | 937 ± 153 | 1225 ± 548 | 1175 ± 282 | 0.510 | 0.327 | 0.993 |
| Medium | 79 ± 8 | 124 ± 19 | 127 ± 19 | 127 ± 21 | 0.298 | 0.302 | 0.204 |
Values are means ± SE, n = 10–12.
Ex vivo production of T cell cytokines by splenocytes.
Splenocytes from old mice produced less IL-2, IL-4, and CD3/CD28-stimulated IL-10 compared with those from young mice, whereas no significant age-related difference was found in IFNγ and Con A-stimulated IL-10. T3 feeding did not affect the production of any of these cytokines in either young or old mice (Table 4).
TABLE 4.
Effect of dietary supplementation with T3 on Con A- or anti-CD3-induced Th1 and Th2 cytokine production by splenocytes of young and old mice1
| Young | Old | P-value | |||||
|---|---|---|---|---|---|---|---|
| Control | T3 | Control | T3 | Age | T3 | Age × T3 | |
| IL-2, ng/L | |||||||
| Con A | 561 ± 98 | 412 ± 50 | 240 ± 46 | 398 ± 97 | <0.005 | 0.799 | 0.174 |
| CD3/CD28 | 2181 ± 489 | 1527 ± 267 | 1276 ± 695 | 1049 ± 350 | 0.003 | 0.680 | 0.684 |
| IFNγ, μg/L | |||||||
| Con A | 45 ± 4 | 72 ± 16 | 73 ± 24 | 85 ± 20 | 0.243 | 0.693 | 0.627 |
| CD3/CD28 | 517 ± 48 | 616 ± 64 | 754 ± 233 | 573 ± 109 | 0.988 | 0.869 | 0.296 |
| IL-4, ng/L | |||||||
| Con A | 23.1 ± 5.8 | 16.2 ± 4.1 | 0.6 ± 0.1 | 2.7 ± 1.4 | 0.031 | 0.466 | 0.156 |
| CD3/CD28 | 488 ± 71 | 479 ± 36 | 213 ± 72 | 164 ± 31 | <0.001 | 0.777 | 0.930 |
| IL-10 | |||||||
| Con A, ng/L | 709 ± 91 | 863 ± 163 | 1056 ± 240 | 641 ± 207 | 0.617 | 0.308 | 0.380 |
| CD3/CD28, μg/L | 10.4 ± 1.6 | 14.0 ± 2.7 | 7.7 ± 1.3 | 6.9 ± 1.4 | 0.004 | 0.796 | 0.298 |
Values are means ± SE, n = 10–12.
In vitro lymphocyte proliferation.
Like most other T3 preparations currently available for dietary supplementation studies, Tocomin 50% used in the present study is a concentrated mixture of all forms of T3 and α-Toc. Therefore, after observing that feeding Tocomin 50% enhanced lymphocyte proliferation, we sought to determine to what extent the individual T3 contributed to this effect. We found that all the T3 tested (excluding β-T3, a minor T3 in Tocomin 50%, for which a purified form is not available) enhanced Con A-stimulated proliferation of lymphocytes differently depending on age and type of T3. Three-way ANOVA revealed a significant age effect, diet effect, and interaction of age × concentration × T3 type; i.e. in the cells from old mice but not young mice, all T3 significantly affected cell proliferation in a dose-dependant manner and this effect of T3 had a varied potency with different types in the order of α- > γ- > δ-T3, which was based on the following tests (Fig. 1). The lowest effective concentration resulting in significant enhancement was 0.02, 0.04, and 0.15 μmol/L for α-, γ-, and δ-T3, respectively, but the enhancement was similar for all T3. The difference in T cell proliferation between 0.02 μmol/L α-T3 and its control (0) was significantly larger than that between 0.02 μmol/L γ-T3 or δ-T3 and their own control (0). When comparing the difference between 0.04 μmol/L and control for all T3, we found that the difference for γ-T3 was less than that in α-T3 (P < 0.001) but greater than that in δ-T3 (P = 0.024). Although all the T3 had a comparable maximum effect on lymphocyte proliferation, the lowest concentrations needed for reaching the maximum effect were 0.15, 0.3, and 0.625 μmol/L for α-, γ-, and δ-T3, respectively. The difference in lymphocyte proliferation between 0.15 μmol/L α-T3 and its control (0) was larger than that between 0.15 μmol/L g-T3 and its control (0) (P = 0.002) and, in turn, the difference between 0.15 μmol/L and control in γ-T3 was larger than that in δ-T3 (P = 0.009). Although the enhancement was still at its plateau with α-T3 up to 10 μmol/L, it sharply dropped for γ-T3 and δ-T3, which was due to decreased cell viability (data not shown), probably a result of cytotoxicity. Similar to the findings in the in vivo supplementation, lymphocyte proliferation was not enhanced by any type of T3 in young mice (Fig. 1).
FIGURE 1 .
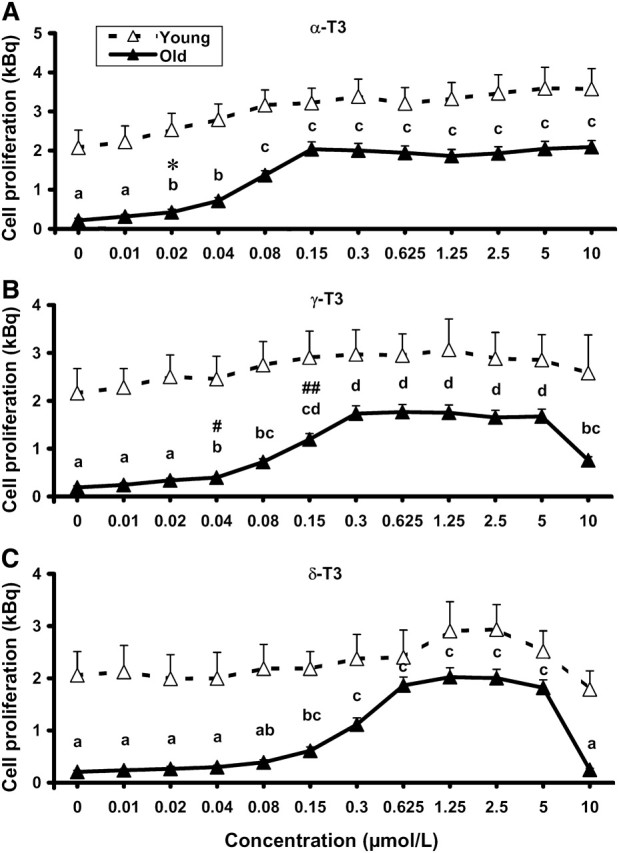
Effect of in vitro supplementation with α-T3 (A), γ-T3 (B), and δ-T3 (C) on lymphocyte proliferation of young and old mice. Splenocytes from C57BL/6 mice fed a nonpurified mouse diet were incubated with various concentrations of α-, γ-, or δ-T3 for 4 h and stimulated by with 1.5 mg/L Con A for 72 h. Values are mean ± SEM, n = 6. Three-way ANOVA revealed a significant age effect and an interaction of age × concentration × T3 type. For each variable, labeled means without a common letter differ, P < 0.05. *The difference from its control (0) is greater than that for γ-T3 or δ-T3 at the same concentration (P < 0.01). #The difference from its control (0) is less than that for α-T3 (P< 0.001) and greater than that for δ-T3 (P < 0.05) at the same concentration. The difference from its control (0) is less than that for α-T3 (P < 0.01) and greater than that in δ-T3 (P < 0.01) at the same concentration.
Discussion
T3 have been viewed as “minor” forms of vitamin E because: 1) their natural sources are very limited and it is impractical to obtain a biologically meaningful amount by consuming whole foods; 2) their metabolic fate is poorly understood; 3) their negligible tissue concentrations disqualify them as a major dietary antioxidant; and 4) their biological effects have been much less demonstrated relative to their Toc cousins. However, increasing evidence has suggested that T3 have notably distinct biological properties not shared by the Toc family, as summarized by Sen et al. (36) in an excellent review. While the effect of Toc (mainly α-Toc) on immune cell functions is relatively well documented, the information on T3 is lacking in this regard. In the current study, we found that T3 significantly enhanced the ability of lymphocytes to proliferate upon stimulation with T cell mitogens or T cell receptor Ab and this effect was mainly observed in old mice. The T3 preparations (including Tocomin 50%) currently used in most of the reported studies are the natural forms of T3 that contain varied amounts of all T3 homologs and a substantial amount of α-Toc. α-Toc has been shown to affect a wide spectrum of cell functions; in particular, we and others have repeatedly demonstrated a T cell-enhancing effect of α-Toc in mice and humans. Both control and T3 diets in the present study contained 107 mg/kg α-Toc, which is slightly more than that present in the standard AIN93 diet (75 mg/kg). In our previous studies, we used 500 mg/kg α-Toc, a dose roughly equivalent to 200 mg/d for humans. Because the control and T3 diets contained the same amount of α-Toc, the observed differences between mice fed the T3 and control diets in the present study should largely represent the effect of T3. Although a synergistic effect between T3 and α-Toc cannot be ruled out, our in vitro studies indicate that T3 have immune-stimulating effects that are independent of α-Toc.
This is the first study, to our knowledge, reporting that T3 enhances T cell function. First, we demonstrated that the T3-induced enhancement in T cell response is not due to a change in cellular composition of spleen, particularly the percentage of T cells, because no difference was found in any cell type between control and T3-fed mice of each age group. Because T3 and α-Toc are members of the vitamin E family and both enhance T cell proliferation with a similar age-related pattern, we attempted to determine whether their effect is mediated through common mechanisms. Our previous studies on α-Toc have shown that vitamin E enhances T cell proliferation by directly acting on T cells and by indirectly inhibiting production of T cell suppressive molecule PGE2, mainly from Mφ (37–40). One cytokine that has been indicated to mediate the effect of vitamin E on T cells is IL-2, a T cell growth factor thought to play a key role in T cell proliferation. Consistent with previous reports, we observed in this study a lower IL-2 production by old mice compared with the young. However, T3 supplementation did not significantly affect PGE2 or IL-2 production in either age group. Because the α-Toc–induced increases in IL-2 and decreases in PGE2 production have been shown repeatedly and were found to be responsible for the α-Toc–induced increase in T cell proliferation in old mice, these data suggest that T3 may enhance T cell proliferation through mechanisms not entirely shared with α-Toc.
Proinflammatory cytokines have been shown to facilitate T cell activation and proliferation. IL-1β is one such cytokine. As a costimulator, IL-1β works with antigens or mitogens to induce T cell activation (41,42). Of note, IL-1β production in splenocytes increased 14% in young and 74% in old mice, which corresponds to the age-related difference in T3-induced enhancement of T cell proliferation. However, whether and to what extent this T3-induced increase in IL-1β production contributes to its enhancement of T cell proliferation remains to be further investigated. Additionally, how T3 increases IL-1β production is yet to be answered. Furthermore, IL-1β is known to induce PGE2 synthesis, but in this study we did not observe a significant effect of T3 on PGE2 production. It is possible that the IL-1–induced increase in PGE2 production was counteracted by a direct effect of T3 on PGE2 production. Our previous studies with α-Toc showed a significant reduction in cyclooxygenase activity and PGE2 production by α-Toc (43). Further studies are needed to differentiate these possibilities. Because we have not found an effect of α-Toc on IL-1β production in our previous studies (26,44), these results further suggest that T3 might have a distinct effect on immune cell functions compared with α-Toc. Given that IL-1β is a proinflammatory cytokine and it is involved in pathogenesis of a number of age-associated diseases, recommending increased intake of T3 to improve T cell function in the aged should be considered with caution. Further studies to determine the clinical benefits of T3 for the aged are needed.
Because individual T3 of high purity are not available in usable amounts for an in vivo feeding study, we utilized the available purified α-, γ-, and δ-T3 to conduct an in vitro supplementation study to determine the relative potency of T3. We found that all 3 T3 enhanced T cell proliferation but with varied potency in the order of α- > γ- > δ-T3. It is interesting to note that, similar to our previous observation with different Toc isomers (33), α-T3 was the safest based on its effect on cell viability. In addition, several studies have shown that among T3, α-T3 is the most bioavailable. Oral administration of the Tocomin 50% to rats showed that bioavailability of α-T3 was 3-fold that of γ- and δ-T3 (45), probably because α-T3 is preferentially absorbed compared with γ- and δ-T3 (46). Taken together, these data suggest that α-T3 is the main contributor to the observed in vivo effect of Tocomin 50% on T cell proliferation. Tissue concentrations of T3 are very low or undetectable if the diet is not supplemented with T3 or T3-rich foods such as palm oil. Oral T3 can be delivered to different tissues with varied efficacy (47–49). Human and animal studies have shown that plasma T3 can reach high nanomolar to low micromolar concentrations after oral administration. For example, after a single 300-mg oral dose of mixed T3 (containing α-, γ-, and δ-T3 at a 2:4:1 ratio) was given to healthy participants, the peak plasma concentrations were 1.36, 1.41, and 0.35 μmol/L for α-, γ-, and δ-T3, respectively (50). Consistent with this, the participants consuming 250 mg/d of individual T3 (α-, γ-, or δ-T3, isolated from Tocomin 50%) for 8 wk had mean plasma concentrations of T3 at 0.98, 0.54, and 0.07 μmol/L for α-, γ-, and δ-T3, respectively (51). The T3 diet used in the present study contained ∼400 mg/kg of total T3. On average, a mouse of 30 g consumes 3.5 g diet/d, which can be translated to a daily dose of 1.4 mg or 46.67 mg/kg body weight. Based on the isocaloric calculation (52,53), this dose is equivalent to 3.33 mg/kg body weight or 233 mg/d for a person of 70 kg. This dose is close to those utilized in human studies, i.e. 250 mg/d by O'Byrne et al. (51) and 300 mg/d by Yap et al. (50) as mentioned above. Thus, the effective ranges of T3 found in our in vitro study would be achievable in tissues after oral intake; indeed, the results from our feeding study strongly support this notion.
In summary, we demonstrated in this study that dietary supplementation with T3 resulted in enhanced T cell proliferation, which indicates that bioavailability of T3 allows them to reach tissues at levels adequate to impact immune cell functions. We further found that all of the T3 tested can promote T cell proliferation, albeit with different efficacy. Similar to α-Toc, T3 enhance T cell proliferation more dramatically in old mice. Unlike α-Toc, however, T3 do not affect IL-2 and PGE2 but increase IL-1β production, suggesting that the 2 families of vitamin E do not have a similar effect on all aspects of immune cell functions. While further studies are needed to determine the mechanism of T3 and its relative efficacy compared with Toc as well as its clinical implications, the data described here present novel findings showing for the first time that T3 enhances T cell function, particularly in old mice.
Supplementary Material
Body weights of young and old mice fed control or T3 diet for 6 wk. Values are mean ± SE, n = 13. There was a significant age effect and significant age × time interaction by repeated measures ANOVA (P < 0.0001) while no diet effect was detected. Y/C: young control; Y/T3: young T3; O/C: old control; O/T3: old T3.
Effect of dietary supplementation with T3 on spleen cell phenotype of young and old mice1,2
Acknowledgments
We thank Stephanie Marco for help in the preparation of the manuscript. D.W. and S.N.M. designed the research; Z.R., M.P., M.C.D., D.S., and D.W. conducted research; Z.R. and D.W. analyzed data; Z.R., D.W., and S.N.M. wrote the paper; and D.W. had primary responsibility for final content. All authors read and approved the final manuscript.
Abbreviations
- Toc
tocopherol
- Con A
concanavalin A
- FBS
fetal bovine serum
- IL
interleukin
- IFN
interferon
- LPS
lipopolysaccharide
- Mφ
macrophage
- PGE2
prostaglandin E2
- PHA
phytohemagglutinin
- T3
tocotrienol
- Th
helper T cells
- Toc
tocopherol
Footnotes
Supported by the USDA, Agriculture Research Service under contract number 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Literature Cited
- 1. Clarke MW, Burnett JR, Croft KD. Vitamin E in human health and disease. Crit Rev Clin Lab Sci. 2008;45:417–50. [DOI] [PubMed] [Google Scholar]
- 2. Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev. 2005;205:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mustacich DJ, Bruno RS, Traber MG. Vitamin E. Vitam Horm. 2007;76:1–21. [DOI] [PubMed] [Google Scholar]
- 4. Wada S, Satomi Y, Murakoshi M, Noguchi N, Yoshikawa T, Nishino H. Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett. 2005;229:181–91. [DOI] [PubMed] [Google Scholar]
- 5. Miyazawa T, Shibata A, Sookwong P, Kawakami Y, Eitsuka T, Asai A, Oikawa S, Nakagawa K. Antiangiogenic and anticancer potential of unsaturated vitamin E (tocotrienol). J Nutr Biochem. 2009;20:79–86. [DOI] [PubMed] [Google Scholar]
- 6. Inokuchi H, Hirokane H, Tsuzuki T, Nakagawa K, Igarashi M, Miyazawa T. Anti-angiogenic activity of tocotrienol. Biosci Biotechnol Biochem. 2003;67:1623–7. [DOI] [PubMed] [Google Scholar]
- 7. Miyazawa T, Shibata A, Nakagawa K, Tsuzuki T. Anti-angiogenic function of tocotrienol. Asia Pac J Clin Nutr. 2008;17Suppl 1:253–6. [PubMed] [Google Scholar]
- 8. Miyazawa T, Tsuzuki T, Nakagawa K, Igarashi M. Antiangiogenic potency of vitamin E. Ann N Y Acad Sci. 2004;1031:401–4. [DOI] [PubMed] [Google Scholar]
- 9. Nakagawa K, Shibata A, Yamashita S, Tsuzuki T, Kariya J, Oikawa S, Miyazawa T. In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J Nutr. 2007;137:1938–43. [DOI] [PubMed] [Google Scholar]
- 10. Black TM, Wang P, Maeda N, Coleman RA. Palm tocotrienols protect ApoE +/− mice from diet-induced atheroma formation. J Nutr. 2000;130:2420–6. [DOI] [PubMed] [Google Scholar]
- 11. Raederstorff D, Elste V, Aebischer C, Weber P. Effect of either gamma-tocotrienol or a tocotrienol mixture on the plasma lipid profile in hamsters. Ann Nutr Metab. 2002;46:17–23. [DOI] [PubMed] [Google Scholar]
- 12. Baliarsingh S, Beg ZH, Ahmad J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis. 2005;182:367–74. [DOI] [PubMed] [Google Scholar]
- 13. Qureshi AA, Salser WA, Parmar R, Emeson EE. Novel tocotrienols of rice bran inhibit atherosclerotic lesions in C57BL/6 ApoE-deficient mice. J Nutr. 2001;131:2606–18. [DOI] [PubMed] [Google Scholar]
- 14. Naito Y, Shimozawa M, Kuroda M, Nakabe N, Manabe H, Katada K, Kokura S, Ichikawa H, Yoshida N, et al. Tocotrienols reduce 25-hydroxycholesterol-induced monocyte-endothelial cell interaction by inhibiting the surface expression of adhesion molecules. Atherosclerosis. 2005;180:19–25. [DOI] [PubMed] [Google Scholar]
- 15. Theriault A, Chao JT, Gapor A. Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis. 2002;160:21–30. [DOI] [PubMed] [Google Scholar]
- 16. Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, Akaike A. Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology. 2004;47:904–15. [DOI] [PubMed] [Google Scholar]
- 17. Khanna S, Roy S, Slivka A, Craft TK, Chaki S, Rink C, Notestine MA, DeVries AC, Parinandi NL, et al. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36:2258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127–42. [DOI] [PubMed] [Google Scholar]
- 19. Serbinova E, Kagan V, Han D, Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med. 1991;10:263–75. [DOI] [PubMed] [Google Scholar]
- 20. Kamat JP, Devasagayam TP. Tocotrienols from palm oil as potent inhibitors of lipid peroxidation and protein oxidation in rat brain mitochondria. Neurosci Lett. 1995;195:179–82. [DOI] [PubMed] [Google Scholar]
- 21. Hatam LJ, Kayden HJ. A high-performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J Lipid Res. 1979;20:639–45. [PubMed] [Google Scholar]
- 22. Coquette A, Vray B, Vanderpas J. Role of vitamin E in the protection of the resident macrophage membrane against oxidative damage. Arch Int Physiol Biochim. 1986;94:S29–34. [PubMed] [Google Scholar]
- 23. Gebremichael A, Levy EM, Corwin LM. Adherent cell requirement for the effect of vitamin E on in vitro antibody synthesis. J Nutr. 1984;114:1297–305. [DOI] [PubMed] [Google Scholar]
- 24. Kowdley KV, Mason JB, Meydani SN, Cornwall S, Grand RJ. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology. 1992;102:2139–42. [DOI] [PubMed] [Google Scholar]
- 25. Lee CY, Man-Fan Wan J. Vitamin E supplementation improves cell-mediated immunity and oxidative stress of Asian men and women. J Nutr. 2000;130:2932–7. [DOI] [PubMed] [Google Scholar]
- 26. Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JG, Morrow FD, Rocklin R, Blumberg JB. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr. 1990;52:557–63. [DOI] [PubMed] [Google Scholar]
- 27. Meydani SN, Meydani M, Verdon CP, Shapiro AA, Blumberg JB, Hayes KC. Vitamin E supplementation suppresses prostaglandin E1 (2) synthesis and enhances the immune response of aged mice. Mech Ageing Dev. 1986;34:191–201. [DOI] [PubMed] [Google Scholar]
- 28. Sakai S, Moriguchi S. Long-term feeding of high vitamin E diet improves the decreased mitogen response of rat splenic lymphocytes with aging. J Nutr Sci Vitaminol (Tokyo). 1997;43:113–22. [DOI] [PubMed] [Google Scholar]
- 29. Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380–6. [DOI] [PubMed] [Google Scholar]
- 30. Han SN, Wu D, Ha WK, Beharka A, Smith DE, Bender BS, Meydani SN. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. 2000;100:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayek MG, Taylor SF, Bender BS, Han SN, Meydani M, Smith DE, Eghtesada S, Meydani SN. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J Infect Dis. 1997;176:273–6. [DOI] [PubMed] [Google Scholar]
- 32. Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu D, Meydani M, Beharka AA, Serafini M, Martin KR, Meydani SN. In vitro supplementation with different tocopherol homologues can affect the function of immune cells in old mice. Free Radic Biol Med. 2000;28:643–51. [DOI] [PubMed] [Google Scholar]
- 34. Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 35. Kumagai K, Itoh K, Hinuma S, Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29:17–25. [DOI] [PubMed] [Google Scholar]
- 36. Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med. 2007;28:692–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adolfsson O, Huber BT, Meydani SN. Vitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J Immunol. 2001;167:3809–17. [DOI] [PubMed] [Google Scholar]
- 38. Beharka AA, Wu D, Han SN, Meydani SN. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mech Ageing Dev. 1997;93:59–77. [DOI] [PubMed] [Google Scholar]
- 39. Marko MG, Ahmed T, Bunnell SC, Wu D, Chung H, Huber BT, Meydani SN. Age-associated decline in effective immune synapse formation of CD4 (+) T cells is reversed by vitamin E supplementation. J Immunol. 2007;178:1443–9. [DOI] [PubMed] [Google Scholar]
- 40. Wu D, Meydani SN. Age-associated changes in immune and inflammatory responses: impact of vitamin E intervention. J Leukoc Biol. 2008;84:900–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joseph SB, Miner KT, Croft M. Augmentation of naive, Th1 and Th2 effector CD4 responses by IL-6, IL-1 and TNF. Eur J Immunol. 1998;28:277–89. [DOI] [PubMed] [Google Scholar]
- 42. Kawakami K, Yamamoto Y, Kakimoto K, Onoue K. Requirement for delivery of signals by physical interaction and soluble factors from accessory cells in the induction of receptor-mediated T cell proliferation. Effectiveness of IFN-gamma modulation of accessory cells for physical interaction with T cells. J Immunol. 1989;142:1818–25. [PubMed] [Google Scholar]
- 43. Wu D, Mura C, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Physiol. 1998;275:C661–8. [DOI] [PubMed] [Google Scholar]
- 44. Han SN, Meydani M, Wu D, Bender BS, Smith DE, Vina J, Cao G, Prior RL, Meydani SN. Effect of long-term dietary antioxidant supplementation on influenza virus infection. J Gerontol A Biol Sci Med Sci. 2000;55:B496–503. [DOI] [PubMed] [Google Scholar]
- 45. Yap SP, Yuen KH, Lim AB. Influence of route of administration on the absorption and disposition of alpha-, gamma- and delta-tocotrienols in rats. J Pharm Pharmacol. 2003;55:53–8. [DOI] [PubMed] [Google Scholar]
- 46. Ikeda I, Imasato Y, Sasaki E, Sugano M. Lymphatic transport of alpha-, gamma- and delta-tocotrienols and alpha-tocopherol in rats. Int J Vitam Nutr Res. 1996;66:217–21. [PubMed] [Google Scholar]
- 47. Khanna S, Patel V, Rink C, Roy S, Sen CK. Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic Biol Med. 2005;39:1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ikeda S, Tohyama T, Yoshimura H, Hamamura K, Abe K, Yamashita K. Dietary alpha-tocopherol decreases alpha-tocotrienol but not gamma-tocotrienol concentration in rats. J Nutr. 2003;133:428–34. [DOI] [PubMed] [Google Scholar]
- 49. Okabe M, Oji M, Ikeda I, Tachibana H, Yamada K. Tocotrienol levels in various tissues of Sprague-Dawley rats after intragastric administration of tocotrienols. Biosci Biotechnol Biochem. 2002;66:1768–71. [DOI] [PubMed] [Google Scholar]
- 50. Yap SP, Yuen KH, Wong JW. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J Pharm Pharmacol. 2001;53:67–71. [DOI] [PubMed] [Google Scholar]
- 51. O'Byrne D, Grundy S, Packer L, Devaraj S, Baldenius K, Hoppe PP, Kraemer K, Jialal I, Traber MG. Studies of LDL oxidation following alpha-, gamma-, or delta-tocotrienyl acetate supplementation of hypercholesterolemic humans. Free Radic Biol Med. 2000;29:834–45. [DOI] [PubMed] [Google Scholar]
- 52. Lambert JD, Lee MJ, Diamond L, Ju J, Hong J, Bose M, Newmark HL, Yang CS. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab Dispos. 2006;34:8–11. [DOI] [PubMed] [Google Scholar]
- 53. Schneider K, Oltmanns J, Hassauer M. Allometric principles for interspecies extrapolation in toxicological risk assessment–empirical investigations. Regul Toxicol Pharmacol. 2004;39:334–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Body weights of young and old mice fed control or T3 diet for 6 wk. Values are mean ± SE, n = 13. There was a significant age effect and significant age × time interaction by repeated measures ANOVA (P < 0.0001) while no diet effect was detected. Y/C: young control; Y/T3: young T3; O/C: old control; O/T3: old T3.
Effect of dietary supplementation with T3 on spleen cell phenotype of young and old mice1,2


