Abstract
Butyrate is a major SCFA produced by microbial fermentation of dietary fiber in the gastrointestinal tract. Butyrate is widely thought to mediate the benefits of fiber and resistant starch consumption to colon health in humans. Besides serving as a substrate for energy production, butyrate has many regulatory effects in animals. Little is known about the signaling mechanisms underlying the regulatory effects of butyrate and other SCFA. In this study, we determined whether butyrate can activate cAMP-protein kinase A (PKA)- cAMP response element (CRE)-binding protein (CREB) signaling in Caco-2 cells, a model of intestinal epithelial cells. Butyrate promoted luciferase expression from a CRE-reporter construct, induced phosphorylation of CREB, increased the activity of PKA, and elevated the levels of cAMP in Caco-2 cells. These data suggest that butyrate activates cAMP-PKA-CREB signaling in Caco-2 cells. Butyrate, however, had no effect on the activities of adenylyl cyclase (AC) and phosphodiesterase (PDE), two enzymes that determine the production and degradation of intracellular cAMP, respectively. Because the activities of AC and PDE are primarily regulated by G protein-coupled receptor (GPR)-mediated intracellular signaling, lack of an effect of butyrate on these two enzymes suggests that butyrate does not activate cAMP-PKA-CREB signaling through GPR. Butyrate-treated Caco-2 cells had greater concentrations of ATP than untreated cells. Because ATP is the substrate for cAMP production, this difference suggests that butyrate may activate cAMP-PKA-CREB signaling in Caco-2 cells through increased ATP production. Overall, this study raises the possibility that some of the regulatory effects of butyrate in animals, including those on the colonocytes, may be mediated by the cAMP-PKA-CREB signaling pathway at the cellular level.
Introduction
Butyrate, together with acetate and propionate, are major SCFA, also known as volatile fatty acids, produced by microbial fermentation in the gastrointestinal tract (1). In monogastric animals such as humans, microbial fermentation mainly takes place in the colon, and the produced SCFA are the major source of metabolic energy for the colonocytes (1, 2). In ruminants such as cattle, microbial fermentation occurs primarily in the rumen, and the SCFA serve as the principal source of energy for the entire animal (1).
Besides being used as substrates for energy production, SCFA, in particular butyrate, also promote proliferation and functional maturation of intestinal and ruminal epithelial cells, pancreatic secretion of insulin and glucagon, and blood flow and motility of the gastrointestinal wall (1, 3, 4). Interestingly, butyrate is also known to induce apoptosis and differentiation in tumor cell lines, including those derived from colorectal cancer (2, 5–7). More recent studies suggest that butyrate and other SCFA may mediate the effects of diet and gut microbiota on host appetite, metabolism, adiposity, and immunity (8–11).
The mechanisms underlying these apparently signaling effects of butyrate and other SCFA have been largely unknown. Recently, GPR415 and GPR43 were identified as receptors for SCFA (12–15). This raises the possibility that SCFA exert their effects on cell growth, hormonal secretion, and gastrointestinal physiology through GPR41 and GPR43. Indeed, these GPR have been demonstrated to mediate the effects of SCFA on leptin production (16), adipogenesis (17), and lipolysis (18), and the effects of gut microbiota on host energy balance and immunity (10, 19).
Both GPR41 and GPR43 are coupled to Gαi/o and their binding by SCFA leads to a reduced production of intracellular cAMP (13, 20). However, in a previous study, we unexpectedly observed that SCFA stimulated reporter gene expression from a CRE-reporter plasmid in the absence of GPR41 or GPR43 (21). In the study reported here, we determined that butyrate can activate the entire cAMP-PKA-CREB signaling pathway in the human cell line Caco-2, a widely used model of intestinal epithelial cells (22). Furthermore, we explored how butyrate stimulates this signaling pathway. Our results suggest a novel signaling mechanism by which the regulatory effects of butyrate on cells and in animals may be mediated.
Materials and Methods
Cell culture, transfection, and luciferase assay.
Caco-2 cells were purchased from ATCC and cultured as suggested by the supplier. At ∼50% confluence, the cells were transfected with the cAMP-response reporter plasmid pGL3C/CRE (Clontech) or the corresponding basal vector pGL3C by using FuGene 6 (Roche). Transfection efficiency was controlled by cotransfection of pRL-SV40 (Promega). Twenty-four hours after the transfection, the medium was replaced with serum-free medium and the cells were cultured for another 8 h. The cells were then treated with sodium butyrate for 16 h before being lysed for dual-luciferase assay (Promega).
In cotransfection assays to determine the effect of GPR41 or GPR43 on butyrate induction of luciferase expression from pGL3C/CRE, the cells were additionally transfected with GPR41 or GPR43 expression plasmid, which were prepared and validated in a previous study (21), or their corresponding empty vector pcDNA3 (Invitrogen). The GPR41 and GPR43 expression plasmids contained a C-terminal FLAG tag (21), and expression of GPR41-FLAG or GPR43-FLAG fusion protein in the transfected cells was confirmed by immunocytochemistry using an anti-FLAG antibody (Sigma-Aldrich). Untransfected Caco-2 cells did not express GPR41 or GPR43 based on RT-PCR of GPR41 or GPR43 mRNA.
Intracellular cAMP assay.
Caco-2 cells were seeded into 24-well plates at 1 × 105 cells/well. Twenty-four hours later, the culture medium was changed to Krebs-Ringer buffer and the cells were incubated at 37°C for 30 min. The cells were then treated with different concentrations of sodium butyrate or 10 μmol/L forskolin (as a positive control) at 37°C for 20 min before being lysed with 0.1 mol/L HCl. cAMP in the lysates was quantified by a direct cAMP enzyme immunoassay kit essentially according to the manufacturer's instructions (Assay Designs). Protein concentrations of the cell lysates were determined using a BCA protein assay kit (Pierce). The concentration of cAMP was normalized to that of protein in the same cell lysates.
Western-blot analysis.
Caco-2 cells in Krebs-Ringer buffer were treated with different concentrations of sodium butyrate or 10 μmol/L forskolin at 37°C for 30 min. The cells were lysed with 1% NP-40 supplemented with protease and phosphatase inhibitors (Roche). A total of 50 μg of whole cell lysate protein was separated by 10% SDS-PAGE and subsequently transferred to a nitrocellulose membrane. After being blocked with 5% milk in Tris buffered saline containing 0.1% Tween-20 for 1 h at room temperature, the membrane was incubated with a Phospho-CREB antibody (Cell Signaling Technology) or a total CREB antibody (Cell Signaling Technology) at a dilution of 1:1000 overnight at 4°C. After being washed in Tris buffered saline containing 0.1% Tween-20 three times, the membrane was incubated with an HRP-conjugated donkey anti-rabbit IgG antibody (Santa Cruz Biotechnology) at a 1:5000 dilution for 1 h at room temperature. The secondary antibody was detected by chemiluminescence (Pierce Biotechnology).
PKA assay.
Caco-2 cells in Krebs-Ringer buffer were treated with different concentrations of sodium butyrate or 10 μmol/L forskolin at 37°C for 30 min. The cells were lysed with 1% NP-40, and the PKA kinase activity in the total cell lysates was determined using a PepTag Non-Radioactive Protein Kinase Assays kit (Promega) essentially as previously described (23).
Membrane-associated AC assay.
This assay was performed as previously described (23, 24). Total lysates of Caco-2 cells, prepared as described above, were centrifuged at 1000 × g for 5 min and the supernatant was centrifuged again at 40,000 × g for 20 min at 4°C. The pelleted membrane protein was dissolved in elution buffer (23). A total of 30 μg of membrane protein was incubated in reaction buffer (23) in the presence of sodium butyrate or forskolin for 15 min at 37°C. The reaction was stopped by addition of 0.1 mol/L HCl. The cAMP converted from ATP in the supernatant of the sample was quantified as described above. The AC activity was represented by the rate of cAMP conversion from ATP.
cAMP-specific PDE assay.
This assay was performed as previously described (23). In brief, 30 μg of Caco-2 lysates was incubated with 100 nmol/L cAMP in the presence of various concentrations of sodium butyrate or 0.2 mmol/L IBMX for 30 min at 30°C. The reaction was terminated by addition of 0.1 mol/L HCl and followed by centrifugation. The cAMP remaining in the supernatant was quantified as described above. The PDE activity was represented by the amount of cAMP hydrolyzed during the reaction time.
ATP assay.
Caco-2 cells in Krebs-Ringer buffer were treated with different concentrations of sodium butyrate at 37°C for 20 min. The cells were lysed with 0.1 mol/L HCl. The concentrations of ATP in the whole cell lysates were measured using an ATP determination kit (Molecular Probes), essentially according to the manufacturer's instructions. The concentration of ATP was normalized to that of protein in the same cell lysates.
Statistical analysis and data presentation.
The experimental unit was the cell culture, and each effect of butyrate was examined in three or four repeated cell cultures. Two-group comparisons were analyzed using t test, assuming equal variances. Multiple groups were compared using ANOVA and Tukey's post hoc test. All statistical analyses were performed using the General Linear Model of SAS (SAS Institute). A difference of P < 0.05 was considered significant. In presenting the luciferase, CREB, PKA, AC, and PDE data, the values of the controls were set to 1.0 and those of treatments normalized to the control; the data are presented using arbitrary units.
Results
CRE-mediated reporter gene expression.
Butyrate at the concentrations of 1 and 10 mmol/L induced 7- and 9-fold increases in luciferase activity, respectively, from a transfected CRE-reporter plasmid in Caco-2 cells (P < 0.01) (Fig. 1A), although it had no effect at 0.01 and 0.1 mmol/L (P > 0.1) (Fig. 1A). Cotransfection of GPR41 or GPR43 had either no effect or diminished butyrate-induced luciferase activity from the CRE-reporter plasmid (Fig. 1B), indicating that the stimulatory effect of butyrate on CRE-mediated reporter gene expression was independent of GPR41 or GPR43.
FIGURE 1.
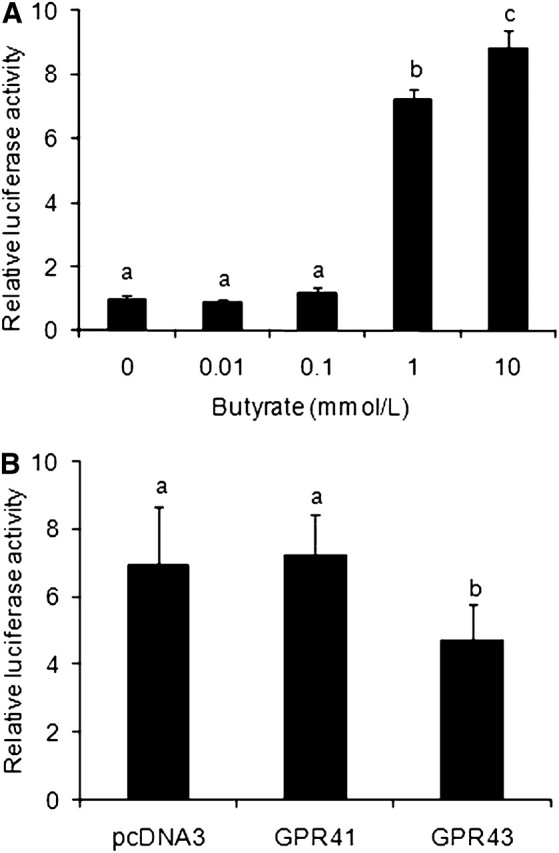
Effect of butyrate on CRE-mediated reporter gene expression in Caco-2 cells. (A) Butyrate stimulated CRE-mediated luciferase expression. Caco-2 cells were transfected with pGL3C/CRE and treated with sodium butyrate for 16 h before luciferase assay. (B) Effect of GPR41 or GPR43 on butyrate-stimulated luciferase activity. Caco-2 cells were transfected with GPR41 or GPR43 expression plasmid or its corresponding empty vector pcDNA3 in addition to pGL3C/CRE and were treated with 1 mmol/L butyrate for 16 h. Values are mean ± SEM, n = 4. Labeled means without a common letter differ, P < 0.05. CRE, cAMP-response element; GPR, G protein-coupled receptor.
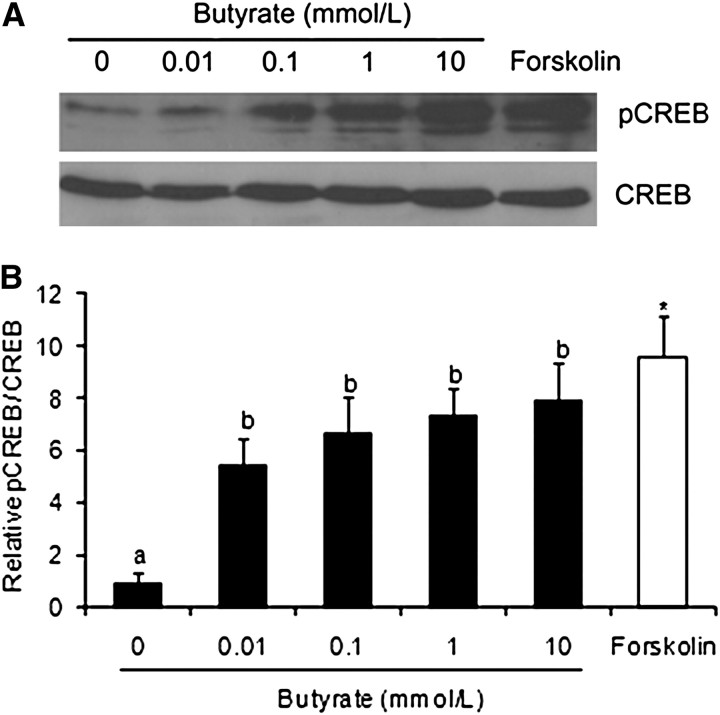
CREB phosphorylation.
The butyrate-increased luciferase expression from the CRE-containing plasmid was possibly due to increased phosphorylation and activation of CREB, the transcription factor that binds the CRE. To test this possibility, we determined the effect of butyrate on phosphorylation of CREB. The cells treated with butyrate from 0.01 to 10 mmol/L had greater levels of phosphorylated CREB than untreated cells (P < 0.05) (Fig. 2) and these increases were not due to increased expression of CREB protein, because the latter was not affected (Fig. 2A). As expected, forskolin, an activator of AC, also induced phosphorylation of CREB in Caco-2 cells (Fig. 2).
FIGURE 2.
Effect of butyrate on CREB phosphorylation in Caco-2 cells. The cells were treated with indicated concentrations of butyrate or 10 μmol/L forskolin for 30 min. pCREB and total CREB protein in total cell lysates were quantified by Western-blot analyses. (A) Representative photographs of Western blots. (B) Relative ratio of pCREB:total CREB. Values are mean ± SEM, n = 3. Labeled means without a common letter differ, P < 0.05. *Different from 0 mmol/L butyrate, P < 0.05. pCREB, phosphorylated CREB.
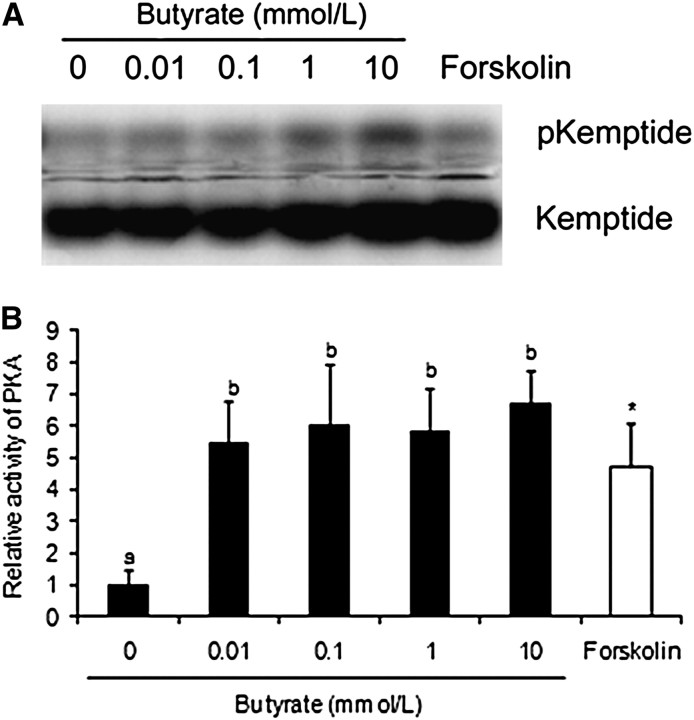
PKA activity.
PKA is widely known to phosphorylate CREB (25); thus, it is possible that butyrate increases phosphorylation of CREB through PKA activation. To test this possibility, we determined the effect of butyrate on the activity of PKA in Caco-2 cells. Based on its ability to phosphorylate kemptide, a synthetic substrate, PKA activity in Caco-2 cells treated with 0.01 to 10 mmol/L butyrate was 5 times that in untreated cells (P < 0.01) (Fig. 3B). Not surprisingly, Caco-2 cells treated with 10 μmol/L forskolin also had greater PKA activity than untreated cells (Fig. 3).
FIGURE 3.
Effect of butyrate on PKA activity in Caco-2 cells. The cells were treated with indicated concentrations of sodium butyrate or 10 μmol/L forskolin for 30 min. PKA activity in total cell lysates was determined by measuring the phosphorylation of PKA-specific substrate, kemptide. (A) Representative photograph of pKemptide and unphosphorylated kemptide. (B) Average PKA activity. Values are mean ± SEM, n = 3. Labeled means without a common letter differ, P < 0.05. *Different from 0 mmol/L butyrate, P < 0.05. PKA, protein kinase A; pKemptide, phosphorylated kemptide.
Intracellular cAMP.
The activity of PKA in the cells is mainly controlled by cAMP. We therefore determined whether butyrate increases cAMP levels in Caco-2 cells. Caco-2 cells treated with 0.1, 1, and 10 mmol/L butyrate had more cAMP than those untreated (P < 0.05) (Fig. 4A). The cells treated with 0.01 mmol/L butyrate tended to have greater amounts of cAMP than untreated Caco-2 cells (P = 0.09) (Fig. 4A).
FIGURE 4.

Effects of butyrate on cAMP (A) and ATP (B) concentrations in Caco-2 cells. The cells were treated with indicated concentrations of sodium butyrate at 37°C for 20 min. Concentrations of cAMP and ATP in total cell lysates were measured and normalized to protein concentrations. Values are mean ± SEM, n = 4. Labeled means without a common letter differ, P < 0.05.
AC and PDE activities.
To explore whether butyrate increases the levels of cAMP in Caco-2 cells by stimulating cAMP production or by inhibiting cAMP hydrolysis, we determined the effects of butyrate on the activities of AC and PDE, enzymes that are responsible for production and hydrolysis of intracellular cAMP, respectively. Butyrate at any of the tested concentrations (0.01, 0.1, 1, or 10 mmol/L) had no effect on AC activity in Caco-2 cells (P > 0.1) (data not shown). Similarly, butyrate at any of these concentrations did not alter PDE activity (P > 0.1) (data not shown). As positive controls, 10 μmol/L forskolin elicited a near 10-fold increase in the activity of AC (P < 0.05) (data not shown), whereas 0.2 mmol/L IBMX caused an ∼10-fold decrease in the activity of PDE (P < 0.05) (data not shown).
Intracellular ATP.
Because butyrate had no effect on AC or PDE activity in Caco-2 cells, we investigated the possibility that butyrate increases cAMP accumulation in Caco-2 cells by increasing the intracellular amount of ATP, the substrate for cAMP production. The cells treated with 0.1, 1, and 10 mmol/L butyrate had more ATP than untreated cells (P < 0.05); the cells treated with 0.01 mmol/L butyrate tended to have more ATP than untreated Caco-2 cells (P = 0.09) (Fig. 4B).
Discussion
In a previous study (21), we accidently observed that SCFA could stimulate luciferase gene expression from a CRE-containing plasmid in CHO cells independent of their receptors GPR41 and GPR43. We confirmed this observation in Caco-2 cells in the present study. The CRE TGACGTCA mediates the effect of the transcription factor CREB on gene transcription (26). As implied by its name, CREB binds to CRE in response to cAMP in cells. When intracellular cAMP is produced, it activates PKA and the activated PKA translocates into the nucleus, where it phosphorylates CREB at serine 133. Phosphorylation at serine 133 enables CREB to recruit its coactivator, CREB-binding protein (CBP), and CBP in turn mediates transcriptional activation through association with RNA polymerase II (26). In this study, we observed that at the concentrations it stimulated CRE-mediated reporter gene expression, butyrate promoted CREB phosphorylation at serine 133, activated PKA, and increased the concentration of cAMP in Caco-2 cells. These changes support the possibility that butyrate stimulates CRE-mediated gene expression in Caco-2 cells by activating the cAMP-PKA-CREB pathway. Besides cAMP and PKA, other signals (e.g. calcium and fibroblast growth factor) and kinases (e.g. protein kinase C and calcium-calmodulin-dependent kinases II/IV) can also promote phosphorylation of CREB at serine 133 (26); therefore, we cannot rule out the possibility that butyrate stimulates CRE-mediated gene expression through additional signaling pathways.
AC and cAMP-specific PDE are two primary determinants of cellular levels of cAMP (27, 28). The former catalyzes the generation of cAMP from ATP, whereas the latter the degradation of cAMP into 5′-AMP. In the present study, neither the activity of membrane-associated AC nor that of cAMP-specific PDE was altered by butyrate in Caco-2 cells. This result suggests that butyrate did not increase the levels of cAMP in Caco-2 cells by activating the AC or inhibiting the PDE. The activity of membrane-associated AC is mainly controlled by G proteins in response to ligand binding to GPR (29). If the activated G protein is Gαs, it stimulates AC and subsequently leads to cAMP production; if the G protein is Gαi, it inhibits AC and cAMP generation (29). The inhibitory effect of butyrate and other SCFA on cAMP production and signaling is mediated by Gαi-coupled GPR41 and GPR43 (13, 20). The result that butyrate does not affect the activity of membrane-associated AC suggests that the stimulatory effect of butyrate on cAMP levels and cAMP-PKA-CREB signaling in Caco-2 cells is unlikely mediated through Gαs-coupled receptors. Besides the widely known membrane-associated AC, a recently identified soluble AC, present in cytosol and organelles, also can catalyze the production of cAMP in cells (30). Soluble AC is activated by bicarbonate and calcium, but not by G proteins or forskolin (30). Whether butyrate activates soluble AC, directly or indirectly, to increase cAMP production in Caco-2 cells remains to be determined in future studies.
In this study, we also tested the possibility that butyrate increases the concentration of cAMP in Caco-2 cells by increasing the production of intracellular ATP. The rationale behind this test was that cAMP is converted from ATP and that butyrate is a major source of ATP production in cells such as colonocytes and rumen epithelial cells (1, 2, 31). We observed that Caco-2 cells treated with butyrate had higher levels of ATP than untreated cells. This difference supports the possibility that butyrate increases the levels of ATP and then cAMP and then activates cAMP-PKA-CREB signaling in Caco-2 cells. It was recently reported that butyrate can prevent autophagy in colonocytes by increasing the production of ATP, which subsequently increases the ratio of ATP:AMP and inhibits the activity of AMP-activated protein kinase, a master regulator of cellular energy homeostasis (32, 33). The present study suggests that the increased ATP may also activate cAMP-PKA-CREB signaling.
Although the activation of butyrate on cAMP-PKA-CREB signaling is detected in Caco-2 cells in the present study, there is evidence suggesting that butyrate might stimulate the same pathway in other cell types. Butyrate increased cAMP levels and PKA activity in Jurkat T-cells (34). Butyrate stimulated the cAMP-dependent expression of the tyrosine hydroxylase gene in rat chromaffin cells PC12 (35, 36). Butyrate regulated the proliferation of porcine peripheral blood mononuclear cells in a cAMP-dependent manner (37). All these previous data suggest that butyrate activation of cAMP-PKA-CREB signaling may be not limited to Caco-2 cells.
Can the other two major SCFA, acetate and propionate, also activate cAMP-PKA-CREB signaling? We did not include acetate and propionate in the present study, but the answer to this question seems to be “yes,” because acetate and propionate can also be oxidized to generate ATP in cells (1, 2) and because acetate and propionate can also stimulate CRE-mediated luciferase gene expression (21). However, the extent to which cAMP-PKA-CREB signaling in a cell is activated by acetate, propionate, and butyrate may vary with the type of the cell. This is because different cell types may have different activities of acetyl-CoA, propionyl-CoA, and butyryl-CoA synthetases, key enzymes that determine ATP generation from acetate, propionate, and butyrate, respectively (38–40). Ruminal and intestinal epithelial cells, including the colonocytes, have more butyryl-CoA than acetyl-CoA or propionyl-CoA synthetase activity (38–40). This difference may explain why butyrate has more effect than acetate and propionate on rumen and intestinal physiology and on colonic health (1, 2).
Epidemiological and experimental data in general support an association of increased consumption of fiber and resistant starch (i.e., indigestible starch) with reduced risk of colorectal cancer and reduced incidence of inflammatory bowel diseases such as ulcerative colitis (2). Increasing evidence suggests that the protective effects of dietary fiber and resistant starch on colon health are mediated through SCFA, especially butyrate (11, 41–44). The effects of butyrate and other SCFA on colon health and physiology have been attributed to their roles as inhibitors of histone deacetylases (45, 46) and as ligands for GPR41 and GPR43 (12, 13, 15). The cAMP-PKA-CREB signaling pathway plays a versatile role in development, physiology, and pathology (25–27). Activation of this pathway by butyrate suggests that some of the effects of dietary fiber and starch on colon health and rumen epithelial development may be mediated through cAMP-PKA-CREB signaling at the cellular level.
Acknowledgments
The authors thank Lee Johnson for technical assistance. A.W., D.L., and H.J. designed the research; A.W. and H.S. conducted the research; A.W. analyzed the data; A.W., D.L., and H.J. wrote the paper; and H.J. had primary responsibility for the final content. All authors read and approved the final manuscript.
Abbreviations
- AC
adenylyl cyclase
- CRE
cAMP response element
- GPR
G protein-coupled receptor
- PDE
phosphodiesterase
- PKA
protein kinase A
Footnotes
Supported in part by National Research Initiative Competitive Grant no. 2007-35206-17839 from the USDA Cooperative State Research, Education, and Extension Service.
Literature Cited
- 1. Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–90. [DOI] [PubMed] [Google Scholar]
- 2. Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64. [DOI] [PubMed] [Google Scholar]
- 3. Cherbut C. Motor effects of short-chain fatty acids and lactate in the gastrointestinal tract. Proc Nutr Soc. 2003;62:95–9. [DOI] [PubMed] [Google Scholar]
- 4. Marsman KE, McBurney MI. Dietary fiber and short-chain fatty acids affect cell proliferation and protein synthesis in isolated rat colonocytes. J Nutr. 1996;126:1429–37. [DOI] [PubMed] [Google Scholar]
- 5. Comalada M, Bailon E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Galvez J. The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol. 2006;132:487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakata T, Yajima T. Influence of short chain fatty acids on the epithelial cell division of digestive tract. Q J Exp Physiol. 1984;69:639–48. [DOI] [PubMed] [Google Scholar]
- 7. Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res. 1994;54:3288–93. [PubMed] [Google Scholar]
- 8. Sleeth ML, Thompson EL, Ford HE, Zac-Varghese SE, Frost G. Free fatty acid receptor 2 and nutrient sensing: a proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr Res Rev. 2010;23:135–45. [DOI] [PubMed] [Google Scholar]
- 9. Conterno L, Fava F, Viola R, Tuohy KM. Obesity and the gut microbiota: does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011;6:241–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meijer K, de Vos P, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care. 2010;13:715–21. [DOI] [PubMed] [Google Scholar]
- 12. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–9. [DOI] [PubMed] [Google Scholar]
- 13. Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–9. [DOI] [PubMed] [Google Scholar]
- 14. Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–52. [DOI] [PubMed] [Google Scholar]
- 15. Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11. [DOI] [PubMed] [Google Scholar]
- 16. Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–9. [DOI] [PubMed] [Google Scholar]
- 18. Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, Tian H, Li Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–26. [DOI] [PubMed] [Google Scholar]
- 19. Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yonezawa T, Kobayashi Y, Obara Y. Short-chain fatty acids induce acute phosphorylation of the p38 mitogen-activated protein kinase/heat shock protein 27 pathway via GPR43 in the MCF-7 human breast cancer cell line. Cell Signal. 2007;19:185–93. [DOI] [PubMed] [Google Scholar]
- 21. Wang A, Gu Z, Heid B, Akers RM, Jiang H. Identification and characterization of the bovine G protein-coupled receptor GPR41 and GPR43 genes. J Dairy Sci. 2009;92:2696–705. [DOI] [PubMed] [Google Scholar]
- 22. Hilgers AR, Conradi RA, Burton PS. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm Res. 1990;7:902–10. [DOI] [PubMed] [Google Scholar]
- 23. Liu D, Jiang H, Grange RW. Genistein activates the 3′,5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology. 2005;146:1312–20. [DOI] [PubMed] [Google Scholar]
- 24. Lohse MJ, Benovic JL, Caron MG, Lefkowitz RJ. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990;265:3202–11. [PubMed] [Google Scholar]
- 25. Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–61. [DOI] [PubMed] [Google Scholar]
- 26. Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–90. [DOI] [PubMed] [Google Scholar]
- 28. Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375:517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hepler JR, Gilman AG. G proteins. Trends Biochem Sci. 1992;17:383–7. [DOI] [PubMed] [Google Scholar]
- 30. Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006;362:623–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Britton R, Krehbiel C. Nutrient metabolism by gut tissues. J Dairy Sci. 1993;76:2125–31. [DOI] [PubMed] [Google Scholar]
- 32. Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dumas ME. The microbial-mammalian metabolic axis: beyond simple metabolism. Cell Metab. 2011;13:489–90. [DOI] [PubMed] [Google Scholar]
- 34. Diakos C, Prieschl EE, Saemann M, Novotny V, Bohmig G, Csonga R, Baumruker T, Zlabinger GJ. Novel mode of interference with nuclear factor of activated T-cells regulation in T-cells by the bacterial metabolite n-butyrate. J Biol Chem. 2002;277:24243–51. [DOI] [PubMed] [Google Scholar]
- 35. DeCastro M, Nankova BB, Shah P, Patel P, Mally PV, Mishra R, La Gamma EF. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res. 2005;142:28–38. [DOI] [PubMed] [Google Scholar]
- 36. Mally P, Mishra R, Gandhi S, Decastro MH, Nankova BB, Lagamma EF. Stereospecific regulation of tyrosine hydroxylase and proenkephalin genes by short-chain fatty acids in rat PC12 cells. Pediatr Res. 2004;55:847–54. [DOI] [PubMed] [Google Scholar]
- 37. Weber TE, Kerr BJ. Butyrate differentially regulates cytokines and proliferation in porcine peripheral blood mononuclear cells. Vet Immunol Immunopathol. 2006;113:139–47. [DOI] [PubMed] [Google Scholar]
- 38. Ash R, Baird GD. Activation of volatile fatty acids in bovine liver and rumen epithelium. Evidence for control by autoregulation. Biochem J. 1973;136:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cook RM, Liu SC, Quraishi S. Utilization of volatile fatty acids in ruminants. 3. Comparison of mitochondrial acyl coenzyme A synthetase activity and substrate specificity in different tissues. Biochemistry. 1969;8:2966–9. [DOI] [PubMed] [Google Scholar]
- 40. Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–9. [PubMed] [Google Scholar]
- 41. Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr Pharm Des. 2003;9:347–58. [DOI] [PubMed] [Google Scholar]
- 42. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. [DOI] [PubMed] [Google Scholar]
- 43. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–43. [DOI] [PubMed] [Google Scholar]
- 44. McOrist AL, Miller RB, Bird AR, Keogh JB, Noakes M, Topping DL, Conlon MA. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr. 2011;141:883–9. [DOI] [PubMed] [Google Scholar]
- 45. Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14:105–13. [DOI] [PubMed] [Google Scholar]
- 46. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:S2485–93. [DOI] [PubMed] [Google Scholar]