Abstract
Background
There is an ongoing outbreak of Mycobacterium chimaera infections among patients exposed to contaminated heater-cooler devices used during cardiac surgery. Recognition of M. chimaera infection is hampered by its long latency and non-specific symptoms. Standard diagnostic methods using acid-fast bacilli (AFB) culture often require invasive sampling, have low sensitivity, and can take weeks to result. We describe the performance of a plasma-based next-generation sequencing test (plasma NGS) for the diagnosis of M. chimaera infection.
Methods
We conducted a retrospective study of 10 patients with a history of cardiac surgery who developed invasive M. chimaera infection and underwent testing by plasma NGS between February 2017 and April 2018.
Results
Plasma NGS detected M. chimaera in 9 of 10 patients (90%) with invasive disease in a median of 4 days from specimen collection, including all 8 patients with disseminated infection. In 7 of these 9 cases (78%), plasma NGS was the first test to provide microbiologic confirmation of M. chimaera infection. In contrast, AFB cultures required a median of 20 days to turn positive, and the median time for confirmation of M. chimaera was 41 days. Of 24 AFB blood cultures obtained in this cohort, only 4 (17%) were positive. Invasive procedures were performed in 90% of cases, and in 5 patients (50%), mycobacterial growth was achieved only by culture of these deep sites.
Conclusions
Plasma NGS can accurately detect M. chimaera noninvasively and significantly faster than AFB culture, making it a promising new diagnostic tool.
Electronic supplementary material
The online version of this article (10.1186/s12879-019-4001-8) contains supplementary material, which is available to authorized users.
Keywords: Mycobacterium chimaera infection, Heater-cooler devices, Cell-free DNA, Next-generation sequencing
Background
Mycobacterium chimaera, a nontuberculous mycobacterium belonging to the Mycobacterium avium complex (MAC), is an opportunistic human pathogen that is ubiquitous in the environment, particularly in water sources [1]. M. chimaera contamination of the LivaNova 3 T (LivaNova, London, UK) heater-cooler device (HCD) used for thermoregulation during cardiothoracic surgery has been linked to an ongoing global outbreak of serious infections, including in Europe [2–7], North America [7–10], Australia and New Zealand [11, 12]. During HCD operation, the organism has been demonstrated to aerosolize via the exhaust of contaminated devices, resulting in airborne inoculation of the surgical site [13]. Whole genome sequencing of M. chimaera isolates from infected patients and HCD water samples worldwide has demonstrated sequence similarity, suggesting point source contamination at the time of device manufacturing [7, 14, 15]. The potential for widespread exposure to infection is significant; in the US alone, LivaNova 3 T HCDs have been in use since 2006, comprise over 60% of the HCD market, and are utilized for over 250,000 cardiac surgeries each year [16, 17]. While infection can be localized to the sternal wound, disseminated disease involving the liver, spleen, bone marrow, kidney, bones, joints or eyes has been typical among cardiac surgery patients with implanted prosthetic material [4, 8, 16]. Reported mortality has been as high as 50% [16].
There are several challenges inherent in the diagnosis of M. chimaera infection. Clinical symptoms, including fatigue, fever, sweats, cough, dyspnea and weight loss, are non-specific and indolent, manifesting as long as 6 years after surgery [16]. Standard confirmatory testing utilizing acid-fast bacilli (AFB) culture often requires invasive sampling, has limited sensitivity, and can take up to 8 weeks for mycobacterial growth. While some clinical laboratories can identify MAC isolates from AFB culture, the specialized molecular methodologies required for differentiation of M. chimaera are typically available only at reference or research laboratories, further delaying confirmation of the causative pathogen [2–4].
Given these challenges, there is an urgent need for innovative diagnostic approaches. Advances in next-generation sequencing (NGS) and bioinformatics have been utilized for the direct detection of pathogen nucleic acid and hold great promise, but invasive procedures are generally necessary to obtain appropriate clinical specimens for testing [18]. The application of NGS to a blood sample is one way to overcome this hurdle and has been used successfully in other areas of medicine for noninvasive diagnosis [19–23]. This approach takes advantage of the cell-free DNA (cfDNA) present in plasma that can be derived from potentially any source within the body, including pathogens at deep sites of infection [20, 24–26]. In this report, we describe the application of a plasma-based NGS test for the diagnosis of invasive M. chimaera infection.
Methods
Patient identification and specimens
This was a retrospective study of patients with a history of cardiac surgery performed at a Southern California hospital with known M. chimaera exposure risk who developed invasive M. chimaera infection and who had testing of cell-free plasma by NGS (plasma NGS) between February 2017 and April 2018. Cases were defined as patients with M. chimaera identified from AFB culture. AFB cultures of blood, urine, sputum, and biopsy samples and identification of isolates to the level of MAC were performed at the hospital’s regional laboratory. Speciation by partial 16S rRNA gene sequencing was performed at the University of Texas at Tyler.
Plasma NGS testing was performed at Karius, Inc. (Redwood City, CA), a reference laboratory with Clinical Laboratory Improvement Amendments certification and College of American Pathologists accreditation. A broad plasma NGS was validated to detect over 1000 microorganisms, including bacteria (including M. chimaera), DNA viruses, and eukaryotic pathogens including yeasts, mold, and protozoa, and was used for patients 1–6. In May 2017, an M. chimaera plasma NGS test was validated and was used for patients 7–10.
For plasma NGS, whole blood (minimum of 4 mL) was collected in BD Vacutainer™ K2EDTA Blood Collection tubes (Becton, Dickinson and Company, New Jersey, US) or in plasma preparation tubes (PPT) via a peripheral blood draw. Samples in K2EDTA tubes were centrifuged at 1600 rcf for 10 min within 72 h of collection, and separated plasma was aliquoted into a sterile polypropylene tube. Samples in PPTs were centrifuged at 1100 rcf for 10 min within 6 h of collection to separate the plasma. Processed specimens were then shipped at ambient temperature to Karius, Inc.
Sequencing
The plasma sample was centrifuged at 16,000 rcf for 10 min at room temperature and spiked with a known concentration of synthetic DNA molecules for quality control purposes. Cell-free DNA was extracted from 0.5 mL plasma using a magnetic bead-based method (Omega Biotek, Norcross, GA), typically yielding 0.1 ng to 10 ng to be used for sequencing. DNA libraries for sequencing were constructed using a modified Ovation® Ultralow System V2 library preparation kit (NuGEN, San Carlos, CA). Negative controls (buffer only instead of plasma) and positive controls (healthy human plasma spiked with a known mixture of microbial DNA fragments) were processed alongside patient samples in every batch. Samples were multiplexed and sequenced on the Illumina NextSeq® 500 (Illumina, Inc., San Diego, CA).
Bioinformatics analysis pipeline
Primary sequencing output files were processed using bcl2fastq (v2.17.1.14) to generate the demultiplexed sequencing reads files. Reads were filtered based on sequencing quality and trimmed based on partial or full adapter sequence. The bowtie2 (version 2.2.4) method was used to align the high-quality reads against human and synthetic-molecules references. Sequencing reads that aligned to the human or synthetic molecule references were removed from further analysis. Remaining reads were aligned against Karius’ proprietary microorganism reference database using NCBI-blast (version 2.2.30). Only taxa present at statistically significant levels above background were reported. The entire process from DNA extraction through analysis is typically completed within 28 h. For full analysis details and evaluation of the bioinformatics analysis pipeline for the detection of M. chimaera, see Additional file 1: Supplementary Methods Appendix.
Results
Illustrative case (Fig. 1 & Table 1, patient 1)
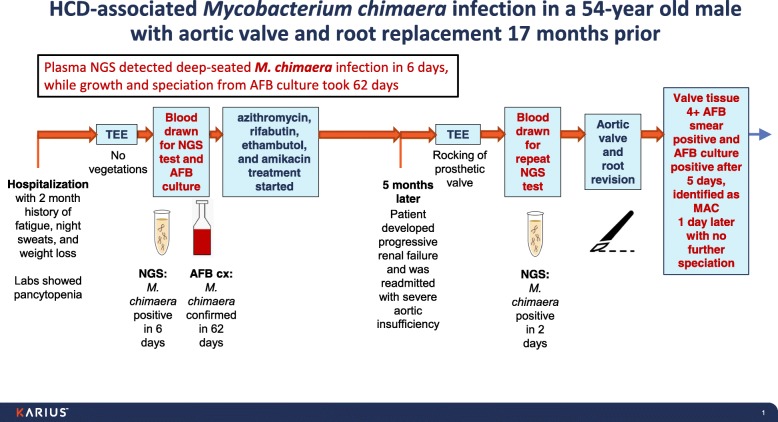
Fig. 1.
Timeline of the clinical course for patient 1 (illustrative case). Plasma NGS detected M. chimaera infection in 6 days compared to 62 days for growth and speciation from AFB blood culture
Table 1.
Summary of patients with invasive M. chimaera disease
| Patient | Preceding cardiac surgery (months prior to presentation) | Presenting symptoms (duration in months) | Clinical scenario (disease type) | Specimen source | Days to positive AFB culture | Days to identify MAC from AFB culture | Days to speciate M. chimaera from AFB culture | Days to detect M. chimaera by plasma NGSa | Additional organisms detected by plasma NGS | Days on MAC therapy at time of plasma NGS (regimen) | Patient outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (illus-trative case) | AVR, ARR (17) | Fatigue, weight loss, night sweats (4) | Pancytopenia, renal failure, severe aortic insufficiency and rocking of prosthetic valve (disseminated) | Blood (1 of 3 positive) | 43 | 47 | 62 | 6c | None | 0 (azithromycin, ethambutol, rifabutin, amikacin) |
Alive (s/p redo surgery) |
| Valve | 5 | 6 | Not done | ||||||||
| 2 | AVR, ARR (14) | Fatigue, weight loss, cough, fever, night sweats (4) | Pancytopenia, granulomatous hepatitis and nephritis (disseminated) | Blood (4) | Negative | N/A | N/A | 3c | None | 12 (azithromycin, ethambutol, rifampin) |
Expired |
| Bone marrow | Negative | N/A | N/A | ||||||||
| Liver | 14 | 17 | 43 | ||||||||
| Urine | 18 | 20 | 41 | ||||||||
| 3 | AVR, MVR (22) | Nausea and chills (3) | Pancytopenia, large MV vegetation, granulomatous hepatitis and bone marrow granulomas (disseminated) | Blood (2) | Negative | N/A | N/A | 5c | EBV | 0 (azithromycin, ethambutol, rifabutin, amikacin) |
Expired |
| Bone marrow | 20 | 22 | Not done | ||||||||
| Valve | 6 | 8 | 23 | ||||||||
| 4 | AVR (17) | Lower back pain (2) | T11-T12 diskitis and vertebral osteomyelitis (disseminated) | Disk | 20 | 23 | 39 | 2 | EBV | 22 (azithromycin, ethambutol, rifabutin) |
Expired |
| 5 | AVR, ARR (21) | Fatigue, fever, night sweats (3) | Fluid collection surrounding aorta (localized) | Blood (3) | Negative | N/A | N/A | Negative | None | 0 (azithromycin, ethambutol, rifampin, amikacin, moxifloxacin) |
Alive (s/p redo surgery) |
| Graft/valve | 24 | 27 | 41 | ||||||||
| 6 | AVR (14) | Weakness, weight loss, fever, night sweats (3) | Thrombocyto-penia, granulomatous hepatitis (disseminated) | Liver | 26 | 35 | 92 | 6 | Actinomyces oris, Prevotella corporis, Prevotella buccalis, Gardnerella vaginalis, Acinetobacter baumannii | 103 (clarithromycin, ethambutol, rifabutin) |
Expired |
| 7 | AVR, ARR (27) | Dyspnea, lower extremity edema, weight loss (5) | Anemia, thrombocyto-penia, cholestatic hepatitis, aortic root abscess (disseminated) | Blood (1 of 5 positive) | 28 | 28 | Not done | 2c | N/Ab | 0 (azithromycin, ethambutol, rifampin, moxifloxacin) |
Expired |
| Sputum | 17 | 20 | 38 | ||||||||
| Bone marrow | 25 | 26 | 34 | ||||||||
| 8 | AVR (25) | Weakness, cough, fever (0.75) | Pancytopenia, cholestatic hepatitis (disseminated) | Blood (1) | Negative | N/A | N/A | 3c | N/Ab | 3 (azithromycin, ethambutol, rifampin, moxifloxacin, amikacin) |
Alive (s/p redo surgery) |
| Sputum | 23 | Not done | 42 | ||||||||
| Bone marrow | 27 | 29 | Not done | ||||||||
| Liver | 24 | 27 | Not done | ||||||||
| Valve | 6 | 10 | 34 | ||||||||
| 9 | AVR, ARR (12) | Chest pain, weight loss, fatigue (9) | Anemia, mediastinal mass (localized) | Blood (4) | Negative | N/A | N/A | 4c | N/Ab | 0 (azithromycin, ethambutol, rifampin, moxifloxacin) |
Alive (s/p redo surgery) |
| Urine (2) | Negative | N/A | N/A | ||||||||
| Mediastinal lymph node | 15 | 24 | 43 | ||||||||
| 10 | AVR, ARR (21) | Weakness, fatigue, weight loss, fever (2) | Pancytopenia, bone marrow granulomas (disseminated) | Blood (2 of 2 positive) | 14 | 17 | 46 | 4c | N/Ab | 0 (azithromycin, ethambutol, rifabutin) |
Expired |
| 15 | 18 | Not done |
AVR = aortic valve replacement, ARR = aortic root replacement, MVR = mitral valve replacement, AFB = acid-fast bacilli, MAC = Mycobacterium avium complex, NGS = next-generation sequencing
aIncludes only the first positive plasma NGS
bDedicated M. chimaera plasma NGS
cNGS provided the first microbiological confirmation of M. chimaera infection. For patients 4 and 6, the plasma NGS was not performed until approximately 6 weeks and 4.5 months after AFB cultures had been collected
A 54-year old male, who had undergone aortic valve and root replacement 17 months prior, was admitted with a 2-month history of fatigue, night sweats, and weight loss. Laboratory studies revealed pancytopenia, but transesophageal echocardiogram (TEE) showed no vegetations. AFB blood cultures and plasma NGS were drawn upon admission, and the patient was empirically treated with azithromycin, rifabutin, ethambutol, and amikacin.
Plasma NGS detected M. chimaera 6 days later. The patient improved clinically and was discharged on oral therapy. One of three AFB blood cultures turned positive after 43 days. The isolate was identified as MAC 4 days later but speciation to M. chimaera required an additional 15 days, a total of 62 days from specimen collection.
Five months later, the patient developed progressive renal failure and was readmitted with severe aortic insufficiency and rocking of the prosthetic valve seen on TEE. Repeat plasma NGS was sent, and in 2 days, M. chimaera was again detected. The patient underwent aortic valve and root revision. Valve tissue was 4+ AFB smear positive and AFB culture was positive after 5 days, with identification as MAC one day later and no further speciation.
Case series overview
From February 2017 to April 2018, ten patients with invasive M. chimaera disease, all confirmed by standard AFB culture, who had undergone testing by plasma NGS were identified (Table 1). The median age of this cohort was 65.5 years (range: 51–79 years), and 80% were male. All had undergone prior aortic valve replacement and 60% had also had aortic root replacement. Initial clinical symptoms developed a median of 19 months (range: 12–27 months) after surgery and included weight loss (50%), fatigue (40%), fever (40%), night sweats (40%), and cough (20%). The median duration of symptoms prior to evaluation for mycobacterial infection was 3 months (range: 0.75–9 months). The majority of patients (80%) had evidence of disseminated disease involving the liver, kidney, bone marrow and/or vertebral disk and bone, whereas 2 patients had deep infection localized to the original surgical area (aorta and mediastinum). Despite antimycobacterial treatment, mortality was 60%.
One or more invasive procedures, such as biopsy of liver, bone marrow, disk, or lymph node or cardiac valve resection, were performed in 90% of cases in order to obtain specimens for AFB culture. In 5 cases (50%), growth of mycobacteria was achieved only by culture of these deep sites. In the remaining patients, blood, sputum, and/or urine for AFB culture was diagnostic. Of note, a total of 24 AFB blood cultures were obtained in this cohort, and only 4 (17%) were positive.
Overall, AFB cultures from any site were positive in a median of 20 days (range: 5–43 days); identification of MAC took a median of 3 additional days (range: 0–9 days), and further speciation to M. chimaera required a median of 19 additional days (range: 8–57 days). From specimen collection for AFB culture, the median total time required for confirmation of M. chimaera was 41 days (range: 23–92 days) (Fig. 2).
Fig. 2.
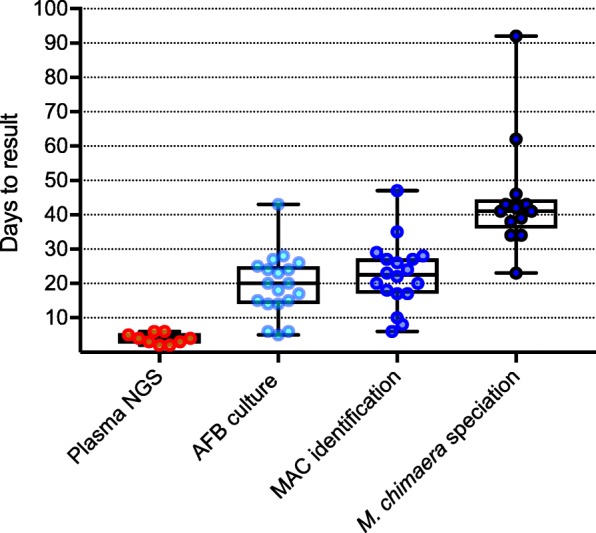
Number of days to positive test result (from sample collection): plasma NGS (9 samples): median of 4 days; AFB culture (19 samples): median of 20 days; MAC identification (18 samples): median of 22.5 days; M. chimaera speciation (13 samples): median of 41 days
Performance of plasma NGS
Plasma NGS detected M. chimaera in 90% of these patients with invasive disease in a median of 4 days from specimen collection (range: 2–6 days), including all 8 patients (100%) with disseminated disease and 1 of 2 patients (50%) with localized disease. In 7 of these 9 cases (78%), plasma NGS was the first test to provide microbiologic confirmation of M. chimaera infection (Table 1); in the other 2 cases, the plasma NGS was not performed until approximately 6 weeks and 4.5 months after AFB cultures had been collected. Five of the 9 positive tests (56%) were obtained prior to initiation of antimycobacterial therapy; the remaining 4 (44%) were collected while on treatment for a median of 17 days (range: 3–103 days).
Of the 6 positive broad plasma NGS tests performed in this cohort, three (50%) detected only M. chimaera while two (in patients 3 and 4) detected EBV and one (in patient 6) detected 5 microorganisms (Actinomyces oris, Prevotella corporis, Prevotella buccalis, Gardnerella vaginalis, and Acinetobacter baumannii) in addition to M. chimaera. Of note, EBV PCR of the blood drawn from patient 3 was positive at 600 copies/mL.
Discussion
To our knowledge, this is the first report describing the successful application of a plasma-based NGS test for the direct detection of M. chimaera, providing microbiologic confirmation of this fastidious organism noninvasively and over 1 month faster than standard AFB culture. Cases included in this series were typical of other patients with invasive M. chimaera reported in the literature with regard to the type of exposure, presence of prosthetic material, long incubation period, nonspecific presenting symptoms, disease manifestations, and mortality [2–12]. As in prior reports, confirmation of the diagnosis was typically delayed when reliant solely on AFB culture, and invasive biopsies were often necessary to grow the pathogen from deep-seated infections. The median time to AFB growth was 20 days with a median of 3 additional days to identify isolates as MAC, and a median of 19 additional days to speciate to M. chimaera. The median overall time from AFB culture collection to confirmation of M. chimaera was 41 days. In contrast, plasma NGS detected this pathogen in a median of 4 days, and in 78% of cases, this provided the first microbiologic confirmation. In four cases, plasma NGS detected M. chimaera weeks to months after initiation of antimycobacterial therapy, demonstrating that the pathogen cfDNA signal may persist even in the face of antibiotic pretreatment.
Although early diagnosis of M. chimaera infection has the potential to improve patient outcomes, this benefit was difficult to demonstrate in this small study. The mortality rate for our overall cohort was 60%, and this rate was similar even in the subset for whom plasma NGS was the first to provide microbiologic confirmation (7 patients) as well as the subset in whom M. chimaera was detected by plasma NGS prior to the start of antimycobacterial therapy (5 patients). Among the 4 patients who survived their infection, all underwent redo cardiac surgery, suggesting that removal of the infected prosthesis may be crucial. In 3 of these patients, plasma NGS was the first to provide microbiologic confirmation of M. chimaera infection. Thus, timely diagnosis could potentially improve mortality by informing early surgical intervention.
The application of NGS for the detection of cfDNA in plasma has been successfully employed for noninvasive diagnosis in other areas of medicine. Prenatal detection of fetal chromosomal defects can be accomplished by the sequencing of fetal cfDNA in maternal circulation, obviating the need for amniocentesis [19]. Organ rejection in solid organ transplant recipients has been monitored by sequencing of tumor-derived cfDNA in the recipient’s blood [21, 22]. In some cases, the diagnosis of cancer can be made without biopsy via sequencing of tumor-derived cfDNA in a blood sample [23].
In contrast to these applications, which focus on human cfDNA, the plasma NGS test described in this report targets microbial cfDNA in the plasma for pathogen detection. This approach has previously been used to rapidly detect another fastidious bacterium, Capnocytophaga canimorsus, in an asplenic patient presenting with culture-negative sepsis [24]. In addition, the same plasma NGS technology was performed on nine patients with proven invasive fungal infection and was able to detect the same fungus identified from biopsy tissue in 7 cases (78%) [25]. Similar to the current study, this series demonstrated that pathogen cfDNA from deep-seated infections caused by difficult-to-culture organisms, including molds like Aspergillus, Rhizomucor, and Scedosporium species, can be detected by NGS applied to a cell-free plasma sample, potentially providing a more rapid diagnosis and obviating the need for invasive biopsies.
Despite the promise of NGS technology, some limitations remain [18]. The kinetics of how pathogen cfDNA enters the plasma space is poorly understood and may differ by pathogen and by site and severity of infection. In this small cohort, plasma NGS was positive in 100% of patients with disseminated infection but in only one of two patients with localized infection, suggesting that the abundance of pathogen cfDNA available in the plasma space may be lower with milder or limited disease. The sole case not detected by plasma NGS (patient 5) was found to have a focal fluid collection surrounding his aorta at the previous surgical site and had no other evidence of disease extension, including 3 negative AFB blood cultures. In addition, broad NGS tests may detect more than one microorganism, and discerning which represent co-pathogens, commensals, or contaminants can prove challenging. The detection of EBV in two of our patients (patients 3 and 4) may have represented viral reactivation; in the former, EBV PCR of the blood corroborated the plasma NGS result. In patient 6, we suspect that the mixed mucosal and environmental organisms detected were due to low-level mucosal translocation or sample contamination. Despite this concern, a broad NGS test could be useful in many clinical settings for the detection of unsuspected pathogens or co-pathogens. However, this breadth may not be necessary when a specific pathogen is suspected, and for this reason, a dedicated M. chimaera plasma NGS was validated and made available. Finally, this particular study was limited by its small sample size. To truly define test performance characteristics such as sensitivity, specificity, and positive and negative predictive values, larger studies are needed [27]. Nevertheless, this case series serves as a pilot study that demonstrates the potential of this novel technology.
Conclusions
Considering the widespread exposure to contaminated HCDs, the challenges with recognition and diagnosis of M. chimaera infection, and the high case fatality rate, there is an urgent need for sensitive and noninvasive tests to detect this pathogen in a clinically actionable time frame. This plasma NGS test rapidly and noninvasively detected M. chimaera in 90% of patients with deep-seated infection and is a promising new diagnostic tool for clinicians.
Additional file
Supplementary Methods Appendix. This document describes the analytical components of the plasma next-generation sequencing methods in greater detail. (DOCX 603 kb)
Acknowledgements
The authors thank the following employees of Karius Inc. for their important contributions to this manuscript: Martin Lindner, Liza Huijse, Gregory Wall, Sumedha Sinha, and Gillian Lounsbach.
Funding
Plasma NGS testing was conducted by Karius, Inc. Employees of Karius assisted in the analysis, interpretation, and writing of the manuscript as outlined in the Authors’ contribution section.
Availability of data and materials
The data that support the findings of this study are available from Karius, Inc. but restrictions apply to the availability of these data, which were used for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Karius, Inc.
Abbreviations
- AFB
Acid-fast bacilli
- cfDNA
cell-free DNA
- EBV
Epstein-Barr virus
- HCD
Heater-cooler device
- K2EDTA tube
K2- Ethylenediaminetetraacetic acid tube
- MAC
Mycobacterium avium complex
- NGS
Next-generation sequencing
- PCR
Polymerase chain reaction
- PPT
Plasma preparation tube
- TEE
transesophageal echocardiogram
Authors’ contributions
JN collected data. JN, DKH, and BPL analyzed the data and drafted the manuscript. SB performed the bioinformatics analysis. JN, GR, GB, TT, AL, and DT oversaw treatment and management of the patients. All authors have read and approved the manuscript.
Ethics approval and consent to participate
This retrospective case series was approved by the Institutional Review Board of Kaiser Permanente. The IRB did not require patient consent for this study.
Consent for publication
Written consent to publish was obtained from patient 1 (illustrative case).
Competing interests
D.K.H. and S.B. are employees of Karius, Inc. B.P.L. is a consultant for and previous employee of Karius, Inc. All other authors report no potential conflicts.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jim Nomura, Phone: 323-783-5867, Email: jim.h.nomura@kp.org.
Gunter Rieg, Email: gunter.k.rieg@kp.org.
Gary Bluestone, Email: gary.l.bluestone@kp.org.
Townson Tsai, Email: townson.x.tsai@kp.org.
Andrew Lai, Email: andrew.x.lai@kp.org.
Dawn Terashita, Email: dterashita@ph.lacounty.gov.
Sivan Bercovici, Email: bercovici@kariusdx.com.
David K. Hong, Email: david.hong@kariusdx.com
Brian P. Lee, Phone: 650-409-5007, Email: brian.lee@kariusdx.com
References
- 1.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210–220. doi: 10.3978/j.issn.2072-1439.2013.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achermann Y, Rossle M, Hoffmann M, Deggim V, Kuster S, Zimmermann DR, et al. Prosthetic valve endocarditis and bloodstream infection due to Mycobacterium chimaera. J Clin Microbiol. 2013;51:1769–1773. doi: 10.1128/JCM.00435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sax H, Bloemberg G, Hasse B, Sommerstein R, Kohler P, Achermann Y, et al. Prolonged outbreak of Mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis. 2015;61:67–75. doi: 10.1093/cid/civ198. [DOI] [PubMed] [Google Scholar]
- 4.Kohler P, Kuster SP, Bloemberg G, Schulthess B, Frank M, Tanner FC, et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur Heart J. 2015;36:2745–2753. doi: 10.1093/eurheartj/ehv342. [DOI] [PubMed] [Google Scholar]
- 5.Haller S, Höller C, Jacobshagen A, Hamouda O, Abu Sin M, Monnet DL, et al. Contamination during production of heater-cooler units by Mycobacterium chimaera potential cause for invasive cardiovascular infections: results of an outbreak investigation in Germany, April 2015 to February 2016. Euro Surveill. 2016;21(17) 10.2807/1560-7917.ES.2016.21.17.30215. [DOI] [PubMed]
- 6.Chand M, Lamagni T, Kranzer K, Hedge J, Moore G, Parks S, et al. Insidious risk of severe Mycobacterium chimaera infection in cardiac surgery patients. Clin Infect Dis. 2017;64:355–342. doi: 10.1093/cid/ciw754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svensson E, Jensen ET, Rasmussen EM, Folkvardsen DB, Norman A, Lillebaek T. Mycobacterium chimaera in heater–cooler units in Denmark related to isolates from the United States and United Kingdom. Emerg Infect Dis. 2017;23(3):507. doi: 10.3201/eid2303.161941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan Nicholas, Sampath Rahul, Abu Saleh Omar M., Tweet Marysia S., Jevremovic Dragan, Alniemi Saba, Wengenack Nancy L., Sampathkumar Priya, Badley Andrew D. DisseminatedMycobacterium chimaeraInfection After Cardiothoracic Surgery. Open Forum Infectious Diseases. 2016;3(3):ofw131. doi: 10.1093/ofid/ofw131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lymann MM, Grigg C, Kinsey CB, Keckler MS, Moulton-Meissner H, Cooper E, et al. Invasive nontuberculous mycobacterial infections among cardiothoracic surgical patients exposed to heater-cooler devices. Emerg Infect Dis. 2017;23:796–805. doi: 10.3201/eid2305.161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamad R, Noly P-E, Perrault LP, Pellerin M, Demers P. Mycobacterium chimaera infection after cardiac surgery: first Canadian outbreak. Ann Thorac Surg. 2017;104:e43–e45. doi: 10.1016/j.athoracsur.2017.01.115. [DOI] [PubMed] [Google Scholar]
- 11.Robinson JO, Coombs GW, Speers DJ, Keehner T, Keil AD, D’Abrera V, Boan P, Pang S. Mycobacterium chimaera colonisation of heater–cooler units (HCU) in Western Australia, 2015: investigation of possible iatrogenic infection using whole genome sequencing. Eurosurveillance. 2016;21(46). [DOI] [PMC free article] [PubMed]
- 12.Williamson D, Howden B, Stinear T. Mycobacterium chimaera spread from heating and cooling units in heart surgery. N Engl J Med. 2017;37:600–602. doi: 10.1056/NEJMc1612023. [DOI] [PubMed] [Google Scholar]
- 13.Sommerstein R, Rüegg C, Kohler P, Bloemberg G, Kuster SP, Sax H. Transmission of Mycobacterium chimaera from heater–cooler units during cardiac surgery despite an ultraclean air ventilation system. Emerg Infect Dis. 2016;22(6):1008. doi: 10.3201/eid2206.160045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Ingen J, Kohl TA, Kranzer K, Hasse B, Keller PM, Szafranska AK, et al. Global outbreak of severe Mycobacterium chimaera disease after cardiac surgery: a molecular epidemiological study. Lancet Infect Dis. 2017;17:1033–1041. doi: 10.1016/S1473-3099(17)30324-9. [DOI] [PubMed] [Google Scholar]
- 15.Struelens MJ, Plachouras D. Mycobacterium chimaera infections associated with heater-cooler units (HCU): closing another loophole in patient safety. Eurosurveillance. 2016;21(46). [DOI] [PMC free article] [PubMed]
- 16.Marra AR, Diekema DJ, Edmond MB. Mycobacterium chimaera infections associated with contaminated heater-cooler devices for cardiac surgery: outbreak management. Clin Infect Dis. 2017;65:669–674. doi: 10.1093/cid/cix368. [DOI] [PubMed] [Google Scholar]
- 17.Perkins KM, Lawsin A, Hasan NA, Strong M, Halpin AL, Rodger RR, et al. Notes from the field: Mycobacterium chimaera contamination of heater-cooler devices used in cardiac surgery — United States. MMWR Morb Wkly Rep. 2016;65:1117–1118. doi: 10.15585/mmwr.mm6540a6. [DOI] [PubMed] [Google Scholar]
- 18.Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66(5):778–788. doi: 10.1093/cid/cix881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil MM, Quezada MS, Revello R, Akolekar R, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2015;45(3):249–266. doi: 10.1002/uog.14791. [DOI] [PubMed] [Google Scholar]
- 20.De Vlaminck I, Martin L, Kertesz M, Patel K, Kowarsky M, Strehl C, Cohen G, Luikart H, Neff NF, Okamoto J, Nicolls MR. Noninvasive monitoring of infection and rejection after lung transplantation. Proc Natl Acad Sci. 2015;112(43):13336–13341. doi: 10.1073/pnas.1517494112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder TM, et al. Universal noninvasive detection of solid organ transplant rejection. Proc Natl Acad Sci U S A. 2011;108(15):6229–6234. doi: 10.1073/pnas.1013924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vlaminck I, Valantine HA, Snyder TM, Strehl C, Cohen G, Luikart H, Neff NF, Okamoto J, Bernstein D, Weisshaar D, Quake SR. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6(241):241ra77. doi: 10.1126/scitranslmed.3007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro-Giner Francesc, Gkountela Sofia, Donato Cinzia, Alborelli Ilaria, Quagliata Luca, Ng Charlotte, Piscuoglio Salvatore, Aceto Nicola. Cancer Diagnosis Using a Liquid Biopsy: Challenges and Expectations. Diagnostics. 2018;8(2):31. doi: 10.3390/diagnostics8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abril MK, Barnett AS, Wegermann K, Fountain E, Strand A, Heyman BM, et al. Diagnosis of Capnocytophaga canimorsus sepsis by whole-genome next-generation sequencing. Open Forum Infect Dis. 2016;3:1–4. doi: 10.1093/ofid/ofw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong DK, Blauwkamp TA, Kertesz M, Bercovici S, Truong C, Banaei N. Liquid biopsy for infectious diseases: sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn Microbiol Infect Dis. 2018;92:210–213. doi: 10.1016/j.diagmicrobio.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Blauwkamp Timothy A., Thair Simone, Rosen Michael J., Blair Lily, Lindner Martin S., Vilfan Igor D., Kawli Trupti, Christians Fred C., Venkatasubrahmanyam Shivkumar, Wall Gregory D., Cheung Anita, Rogers Zoë N., Meshulam-Simon Galit, Huijse Liza, Balakrishnan Sanjeev, Quinn James V., Hollemon Desiree, Hong David K., Vaughn Marla Lay, Kertesz Mickey, Bercovici Sivan, Wilber Judith C., Yang Samuel. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nature Microbiology. 2019;4(4):663–674. doi: 10.1038/s41564-018-0349-6. [DOI] [PubMed] [Google Scholar]
- 27.Diagnostics Evaluation Expert Panel TDR. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2010;8:S17–S29. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods Appendix. This document describes the analytical components of the plasma next-generation sequencing methods in greater detail. (DOCX 603 kb)
Data Availability Statement
The data that support the findings of this study are available from Karius, Inc. but restrictions apply to the availability of these data, which were used for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Karius, Inc.