Abstract
Tick‐borne anaplasmosis and ehrlichiosis are clinically important emerging zoonoses usually overlooked by veterinarians and physicians alike. This study aimed at detecting and genetically characterizing Ehrlichia and Anaplasma species in ixodid ticks and their animal hosts from the West Bank, Palestine. A total of 723 ixodid ticks belonging to three genera (Rhipicephalus, Hyalomma, Haemaphysalis) were collected from dogs, sheep, goats and camels. In addition, 189 blood samples were collected from dogs, sheep, camels, horses and a goat from the West Bank, Palestine. All tick and blood samples were investigated for the presence of Anaplasma and Ehrlichia targeting a 345 bp fragment of the 16S rRNA gene followed by sequence analysis. The infection rate of Anaplasma spp. in ticks was 6.5% (47/723). Anaplasma platys was identified in 28% (13/47) of them. Whereas, based on a partial sequence (851 bp) of msp4 gene, 38% (18/47) were identified as A. ovis. The species of the remaining 16 positive samples (16/47, 34%) could not be identified. Simultaneously, the infection rate of Ehrlichia spp. in the ticks was 0.6% (4/723). Three of which were E. canis and one was Ehrlichia spp. The infection rate of A. platys in dogs’ blood samples was 10% (13/135), while it was 1.5% (2/135) for E. canis. The infection rate of Anaplasma in sheep blood samples was 40% (19/47), out of which 26% (5/19) were caused by A. ovis as revealed by msp4‐PCR. Implementation of purely‐spatial analysis by saTScan for all cases of Anaplasma revealed two statistically significant clusters in two districts; Tubas town and Majdal‐Bani‐Fadil village on the western hills of the Jordan Valley. Most cases of Anaplasma (83%) were from rural areas where life cycle components (vector, host and reservoir) abundantly interact. This study is the first in Palestine to reveal the presence of Anaplasma and Ehrlichia in ticks, dogs and sheep providing crucial platform for future epidemiological surveys and control strategies in the country and region.
Keywords: Anaplasma, dogs, Ehrlichia, Ixodid ticks, Palestine, sheep
Impacts.
Among neglected tick‐borne bacterial zoonoses in Palestine, ehrlichiosis and anaplasmosis are considered emerging diseases worldwide with increasing number of human cases and substantial economic burden.
In Palestine and for the first time, anaplasmosis and ehrlichiosis have been detected and genetically characterized in ixodid ticks and their animal hosts.
The study broached the possibility of cross‐border spill out of infection to neighboring countries across Jordanian and Israeli borders which should trigger regional cooperation to control the diseases.
Introduction
In Palestine and elsewhere in the world, ehrlichiosis and anaplasmosis are considered neglected tick‐borne bacterial zoonoses caused by Ehrlichia and Anaplasma. The two genera belonging to the family Anaplasmataceae encompass groups of obligatory intracellular Gram‐negative bacteria invading blood cells of mammals including leukocytes, erythrocytes and thrombocytes (Yang et al. 2015). The reservoir hosts include numerous wild and domesticated animals (Dumler et al. 2001; Ismail et al. 2010). Hard ticks from the Ixodidae family usually transmit these pathogens to mammals; in addition, they can be transmitted directly to both human and animals by blood transfusion (Fine et al. 2016; Marenzoni et al. 2017). The genus Ehrlichia contains six recognized species: Ehrlichia canis, E. chaffeensis, E. ewingii, E. muris, E. ruminantium and E. minasensis (Cabezas‐Cruz et al. 2016). The currently recognized six species in the genus Anaplasma are Anaplasma phagocytophilum which cause human granulocytic anaplasmosis (HGA), A. platys, A. marginale, A. bovis, A. ovis, A. capra and A. odocoilei (Dumler et al. 2001; Ndip et al. 2010; Tate et al. 2013; Li et al. 2015; Silaghi et al. 2017). Ixodid ticks maintain different Anaplasma species in nature. Various species of Ixodes, Rhipicephalus, Amblyomma and Dermacentor serve as vectors for Anaplasma spp. worldwide including neighboring countries as Egypt and Israel (Loftis et al. 2006a,b; Harrus et al. 2011). R. sanguineus s.l. is a recognized vector of different pathogenic agents including bacteria, protozoa, nematodes and viruses that affect dogs and infrequently humans (Dantas‐Torres & Otranto 2015).
The clinical manifestations of ehrlichiosis and anaplasmosis are similar in both human and animals. The consequences of infection vary from asymptomatic infections or mild symptoms to a severe, potentially, fatal illness. In human, these diseases are characterized by fever, headache, chills and muscle aches within two weeks of the tick bite which is often accompanied by thrombocytopenia, leukopenia and elevated levels of hepatic enzymes in the blood (Ismail et al. 2010). Cattle can be infected by several Anaplasma species, like A. marginale, A. phagocytophilum, A. centrale and A. bovis (Silaghi et al. 2017). A. marginale is known to be highly pathogenic in cattle, whereas A. centrale is less virulent and is being used for immunization against anaplasmosis (Aubry & Geale 2011). A. ovis is moderately pathogenic in sheep, goats, and wild ruminants and causes acute disease in animals exposed to stress, hot weather, deworming and animal movement (Kuttler 1984; Friedhoff 1997). In dogs, different pathogenic Anaplasma and Ehrlichia species have been reported with ehrlichiosis showing generally more severe symptoms than anaplasmosis (Ismail et al. 2010; Sainz et al. 2015). Canine monocytic ehrlichiosis (CME) is a systemic infection in dogs caused by E. canis. Its clinical symptoms may vary but include fever, weight loss, lethargy, lymphadenopathy, splenomegaly, hepatomegaly, thrombocytopenia, bleeding disorders, bone marrow failure and may lead to death in dogs and other canids (Ismail et al. 2010; Sainz et al. 2015).
A. platys infection in dogs is reported to be either with few or no clinical signs or more virulent, while dogs infected with A. phagocytophilum may remain healthy or manifest clinical signs including fever, lameness, lethargy and anorexia (Ismail et al. 2010; Sainz et al. 2015). In camels, recent studies reported the presence of Anaplasma and Ehrlichia spp. (Sudan et al. 2014; Bastos et al. 2015). Although decision for treatment can be based on clinical signs and symptoms, yet microscopic examination of Giemsa‐stained thin peripheral blood smears was used to demonstrate cytoplasmic morula for diagnosis of ehrlichiosis and anaplasmosis. However, this method is only useful for detecting clinically suspected animals during the acute phase of the disease thus reducing sensitivity (Paddock & Childs 2003; Ismail et al. 2010; Bakken & Dumler 2015). Currently, serological methods with Immunofluorescence antibody (IFA) assay as the gold standard and enzyme‐linked immunosorbent assay (ELISA) method are used to diagnose both infections (CDC, 2008). However, cross‐reactivity between genera and species has been reported (Al‐Adhami et al. 2011). Therefore, molecular‐based methods such as polymerase chain reaction (PCR) and real‐time PCR, targeting different genes have been developed to detect and identify Anaplasma spp. and Ehrlichia spp. with fairly higher sensitivity and specificity (Parola et al. 2000; Dong et al. 2013). Several Anaplasma strains have been detected using the small‐subunit rRNA (16S rRNA) which has proven to be a sensitive molecular tool to confirm the presence of these pathogens’ DNA in the investigated ticks and/or animal hosts. However, 16S rRNA gene is highly conserved with few polymorphic positions, therefore, closely related species and strains cannot be distinguished (Mongruel et al. 2017). On the other hand, it has been reported that the major surface protein 4 (msp 4)‐that is encoded by msp4 gene‐is associated with faster evolution than other nuclear genes and involved in interactions with host cells (Yang et al. 2015). Thus, the genetic diversity of msp4 sequences is useful to reveal intraspecies variation and phylogenetic studies of several Anaplasma strains obtained from different hosts (Paulauskas et al. 2012).
Despite being emerging diseases worldwide with increasing number of human cases and the substantial economic burden with livestock infection, anaplasmosis and ehrlichiosis have not been investigated and no previous data is available on any of these diseases in Palestine. Therefore, this study was conducted to (i) detect and genetically characterize Anaplasma and Ehrlichia spp. in hard ticks and blood samples collected from domestic dogs, sheep and camels (ii) determine geographical distribution of Anaplasma and Ehrlichia spp. in the West Bank, Palestine.
Methods
Study design
A total of 723, partially engorged, hard ticks were collected during January to April, 2015 from 253 animals including dogs, camels, sheep and goats in nine districts (Jenin, Tubas, Tulkarm, Nablus, Jericho, Ramallah, Salfit, Bethlehem and Al‐Khalil) located in three zones in the central, northern and southern regions of the West Bank‐Palestine representing the overall tick population in the country (Fig. 1a, Table 1). At the time of tick sampling, blood samples were collected from different outdoor domestic dogs (n = 135 and camels (n = 4). In 2016, additional blood samples were taken from sheep (n = 47), horses (n = 2) and goats (n = 1) from Jericho and Bethlehem. Study animals were selected randomly; all of them were apparently healthy and did not show any clinical signs at the time of sampling.
Figure 1.
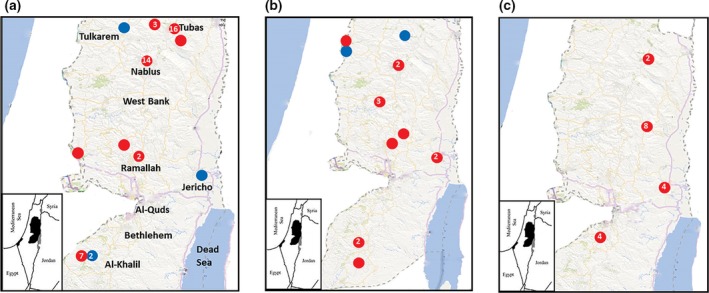
Spot maps of cases of Anaplasma (red circles) and Ehrlichia (blue circles) isolated from ticks (a), dogs (b) and sheep (c). The number within the circles indicates cases spotted in the area while those without numbers indicate one case.
Table 1.
Overall infection rate of Anaplasma and Ehrlichia infections in ticks using 16SrRNA PCR
| Ixodid tick species | # of tested ticks | Positives (%) | Life stage (n) | Animal hosts | District | Pathogen detected (n) |
|---|---|---|---|---|---|---|
| Rhipicephalus sanguineus | 508 | 26 (5.1) |
Female (16) Male (5) Nymph (5) |
Dogs, Sheep, Goats | Jenin, Ramallah, Al‐Khalil, Tubas, Nablus, Jericho, Tulkarm | Anaplasma platys (13), Anaplasma spp (10), Ehrlichia canis (3) |
| Rhipicephalus turanicus | 108 | 21 (19.4) |
Female (12) Male (9) |
Sheep, Dogs | Nablus, Tubas, Jenin, Ramallah, Jericho. | Anaplasma spp (21) |
| Rhipicephalus bursa | 11 | 0 | Sheep, Goat, Dogs | Tubas, Jenin, Ramallah | ||
| Rhipicephalus spp. | 32 | 3 (9.4) | Female (3) | Sheep, Dogs | Nablus, Jenin, Ramallah, Al‐Khalil, Tubas, Jericho | Anaplasma spp (3) |
| Hyalomma dromedarii | 32 | 0 | ||||
| Hyalomma impeltatum | 5 | 0 | Camels | Jericho | ||
| Hyalomma spp. | 6 | 0 | ||||
| Haemaphysalis parva | 16 | 1 (6.3) | Female (3) | Dogs | Ramallah, Jenin, Tulkarm | Ehrlichia spp (1) |
| Haemaphysalis adleri | 5 | 0 | Dogs | Jenin, Ramallah | ||
| Total | 723 | 51 (7.1) |
Ticks and blood samples
Two to five hard ticks were taken per animal host. All ticks were microscopically identified to the genus and species level using standard taxonomic keys (Guglielmone et al. 2009). Tick samples were separated into different micro‐centrifuge tubes containing 70% alcohol and kept at −20°C until DNA extraction. Blood samples (n = 189) were collected from different domestic animals including, dogs, sheep, camels, horses and a goat. All blood samples were collected in EDTA‐anticoagulant tubes and stored at −20°C until further use.
DNA extraction
Prior to DNA extraction, individual ticks were washed with phosphate‐buffered saline (PBS), air‐dried for 10 min on tissue paper and separately sliced into small pieces by a sterile scalpel, then manually homogenized with a sterile pestle and mortar. The sliced sample was re‐suspended in 200 μL of lysis buffer and 20 μL of proteinase K followed by DNA extraction using QIAamp animal blood and tissue Kit procedure (Qiagen GmbH, Hilden, Germany). DNA was extracted from whole blood samples (300 μL) using the same kit mentioned above. DNA concentration was measured by Nanodrop (Thermo Scientific NanoDrop 1000) and kept frozen at −20 until further use.
PCR amplification and DNA sequence analysis
All DNA samples were screened by PCR using a primer pair, EHR16SR (5′‐ TAGCACTCATCGTTTACAGC‐3′) and EHR16SD (5′‐GGTACCYACAGAAGAAGTCC‐3′, targeting a 345 bp fragment of the 16S rRNA gene. These primers are specific for the family Anaplasmataceae, including the genera Anaplasma, Ehrlichia, Neorickettsia and Wolbachia (Parola et al. 2000). PCR was performed as described previously (Parola et al. 2000) with the following modifications; the PCR reactions were performed in a total volume of 25 μl using PCR ready mix (Thermo Fisher Scientific) containing 1 μmol/L of each set of primers and 5 μl of the extracted DNA.
Identification of Anaplasma spp. targeting msp4 gene
A fragment of 851 bp of the major surface protein gene (msp4) was amplified and sequenced to differentiate between A. marginale, A. centrale and A. ovis using the previously published primers (MSP45 (5′‐GGGAGCTCCTATGAATTACAGAGAATTGTTTAC‐3′) and MSP43 (5′‐CCGGATCCTTAGCTGAACAGGAATCTTGC‐3′)(de la Fuente et al. 2003). The msp4‐PCR reactions were performed in 25 μL PCR‐ready supreme mix (Syntezza Bioscience‐Jerusalem), containing 1 μmol/L of each set of primers and 5 μL of the extracted DNA. The thermal cycling procedure was as described previously (de la Fuente et al. 2003).
In all amplification reactions, negative controls (without DNA) were included. PCR amplifications were carried out in BiometraT Professional basic 96 gradient thermocycler. PCR products were visualized under UV illumination after electrophoresis on 2% agarose gels stained with ethidium bromide using different DNA ladders (100 bp and 1 kb) as molecular markers (Thermo Scientific GeneRuler). Gels were captured using Minilumi machine (DNR Bio Imaging Systems ltd).
DNA sequence analysis
Forward and reverse sequences of all amplified PCR products were obtained. The sequences were analyzed and evaluated with The Sequence Manipulation Suite program (Stothard 2000) and multiple sequence alignment with hierarchical clustering (http://multalin.toulouse.inra.fr/multalin/) (Corpet 1988). Species identity for DNA sequences was assessed based on the closest BLASTn match (identity ≥ 99%) using the MegaBLAST with homologous sequences deposited in NCBI database (National Center for Biotechnology Information, U.S. National Library of Medicine, 8600 Rockville Pike, Bethesda MD). CLUSTALW program (http://www.genome.jp/tools/clustalw/) was used for the multiple sequence alignment. Phylogenetic trees construction was carried out using the statistical method Maximum Likelihood (ML) with bootstrap of 1000 replications using MEGA X program (Kumar et al. 2018). Partial DNA sequences of 16S rRNA (345 bp) and msp4 genes (851 bp) were used to build the trees based on complete deletion option with gaps and missing data were eliminated. All based on Jukes‐Cantor model for nucleotide sequences. Initial trees for the heuristic search were automatically obtained by applying the Nearest‐Neighbor‐Interchange (NNI) algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach. The DNA sequence of Neorickettsia sennetsu (NR_044746.1) was used as an out‐group to produce a rooted tree.
Statistical analysis
Frequency tables, distributions and rates (positive/total tested) were calculated using EpiInfo™ statistical package (CDC free‐software). SaTScan™ v8.0 Freeware was used to detect statistical evidence for purely‐spatial clustering of cases caused by Anaplasma spp. Analysis was done on two levels, the first included segregation of cases based on the host, while the second was based on pooling of all cases regardless of host. It's based on a scanning window that moves across space. For each geographical location, a hypothetical window is drawn with observed and expected number of cases. The cases inside the window are compared to those outside. The window with the greatest observed‐to‐expected ratio is spotted on the map. The window identified as the least likely due to chance is subsequently evaluated by a maximum likelihood ratio test with a test decision based on a Monte‐Carlo simulated P‐value (999 simulations). The maximum proportion of the population that a cluster could contain was set at 50% of the cases. Circles were restricted to 1 km radius with no central overlap with other clusters. Input files included number of cases per locality, year of infection and total number of tested samples. Data were analysed based on discrete Poisson model with level of statistical significance considered at P‐value ≤ 0.05 (Kulldorff 1997). Significant clusters of cases were spotted on maps using Epi Info 7 based on exact longitude‐latitude coordinates of each location.
Results
Ticks identification
The ticks comprised three genera Rhipicephalus, Hyalomma and Haemaphysalis. Among which, 508 were R. sanguineus s.l (240 females, 210 males, 57 nymphs and one with undefined life stage), 108 R. turanicus (60 females, 43 males and five nymphs), 11 R. bursa (six females and five males), 32 Rhipicephalus spp. (27 females, three males, one nymph and one with undefined life stage), 32 Hy. dromedarii (nine females and 23 males), five Hy. impeltatum (one female and four males), six Hyalomma spp. (five females and one male), 16 H. parva (11 females, three males and one nymph) and five H. adleri (all of them were females).
Molecular detection of Ehrlichia and Anaplasma spp. in ticks
All samples (723 ticks) were screened for the presence of Anaplasma and Ehrlichia spp. DNA using 16S rRNA‐PCR. The sample was considered positive if a fragment of 345 bp was observed on 2% agarose gel. The infection rate for Anaplasma and Ehrlichia collectively in ticks was 7.1% (51/723). To identify the type bacteria; the amplified products were sequenced and subsequently matched with BLAST algorithm. Based on this, the infection rate of Ehrlichia spp. was 0.6% (4/723). The infection rate of Anaplasma spp was 6.5% (47 /723), of which 28% (13/47) were A. platys (as revealed by 16S rRNA PCR) and 38% (18/47) were A. ovis as revealed by msp‐4 PCR. The species of the remaining 16 positive samples (16/47, 34%) could not be identified. The 13 A. platys were detected in R. turanicus (n = 8) and R. sanguineus (n = 5). Three DNA sequences obtained from R. sanguineus s.l. were identified as E. canis, whereas one sequence from H. parva belonged to Ehrlichia spp. The tick species and their animal hosts are shown in (Table 1).
Ehrlichia and Anaplasma in blood samples
Of the 189 animal blood samples screened by 16S rRNA‐PCR, 34 samples (18%) were positive for Anaplasma or/and Ehrlichia (Table 2). None of the blood samples from camels (n = 4), horses (n = 2) and goats (n = 1) were positive (Table 2). Among the canine blood samples (n = 135), 11.1% (15/135) were positive (Table 2). The infection rate of A. platys in dogs’ blood was 10% (13/135) and 1.5% (2/135) for E. canis. The infection rate of Anaplasma in sheep blood samples was 40% (19/47) of which 26% (5/19) were caused by A. ovis as revealed by msp4‐PCR. Ehrlichia was not detected in sheep.
Table 2.
Overall infection rate of Anaplasma and Ehrlichia infections in animal blood samples
| Animal species | no. of animals | Positives (%) | District | Pathogens detected (n) |
|---|---|---|---|---|
| Dogs | 135 | 15 (11.1) | Jenin, Al‐Khalil, Ramallah, Jericho, Salfit, Tulkarm, Nablus | Ehrlichia canis (2), Anaplasma platys (13) |
| Sheep | 47 | 19 (40.4) | Jericho, Bethlehem | Anaplasma spp. (19) |
| Camels | 4 | 0 | Jericho | |
| Goats | 1 | 0 | Bethlehem | |
| Horses | 2 | 0 | Jericho | |
| Total | 189 | 34 (18) |
Phylogenetic analysis
Phylogenetic analysis based on partial sequences of 16S rRNA gene revealed two main clusters: Cluster I represented the strains of Ehrlichia (n = 6). The DNA sequences from ticks (n = 3) and those from dogs (n = 2) were identical to each other and to the E. canis strain deposited in the Genbank (KP182942.1). One sequence from H. parva tick formed a separate branch and showed 99% sequence identity to the strain of Ehrlichia spp. (KJ410253.1) (Fig. 2a). Cluster II representing the strains of Anaplasma spp. (n = 53), shared at least 99% sequence identity to each other and to the sequences of A. centrale, A. marginale and A. ovis (KC189842.1, KU686794.1 and KJ410246.1, respectively) (Fig. 2a). Cluster III, represents the strains of A. platys (n = 26) were obtained from dogs (n = 13) and ticks (n = 13) and showed 99–100% sequence identity to each other and to the sequence of A. platys deposited in GenBank (KU500914.1). Phylogenetic analysis based on the amplification of partial sequences of msp4 gene for different Anaplama spp. revealed all 18 Anaplasma ovis from Palestine grouping into one cluster (Fig. 2b). Representative sequences for 16S rRNA obtained in the course of this work were deposited into GenBank under the accession numbers of MK069487 and MK069495 for 16rRNA and under the accession numbers of MK087764 and MK087768 for msp4 gene.
Figure 2.
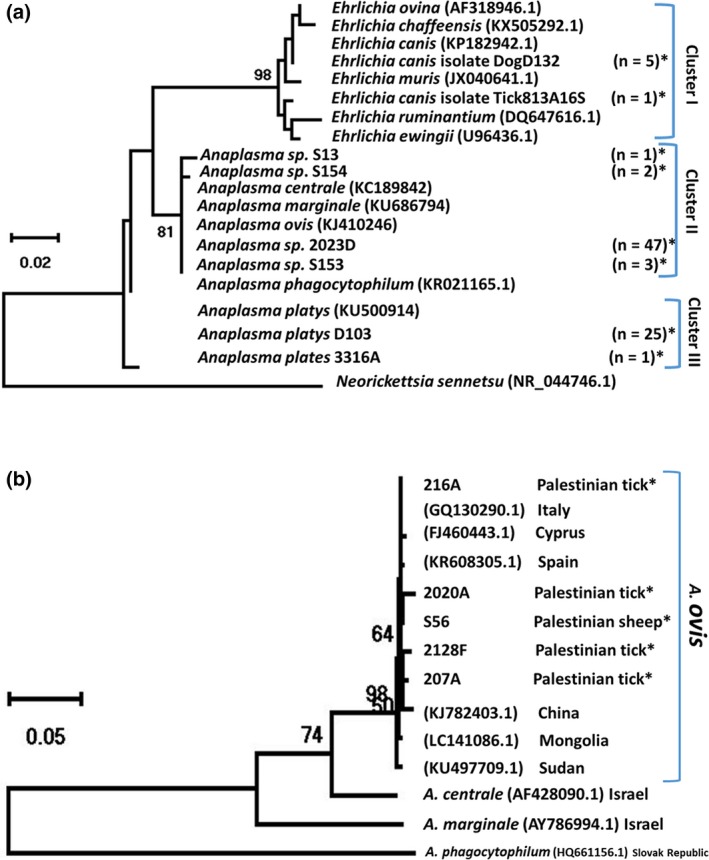
Phylogenetic analysis of Anaplasma and Ehrlichia based on partial sequences of (a) 16S rRNA and (b) msp4 genes. Phylogenetic analysis were constructed using the maximum likelihood method used on MEGA X program, with the complete deletion option, based on Jukes‐Cantor model for nucleotide sequences. Initial trees for the heuristic search were automatically obtained by applying the Nearest‐Neighbor‐Interchange (NNI) algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach. Statistical support for internal branches of the trees was evaluated using bootstrap of 1000 replications. (a) Based on 16srRNA sequences (345 bp): cluster I represents Ehrlichia strains. Cluster II represents Anaplasma spp. and Cluster III represents Anaplasma platys. The DNA sequence of Neorickettsia sennetsu (NR_044746.1) was used as an out‐group to produce rooted tree. (b): 851 bp msp4 DNA Anaplasma sequences detected in this study compared to Anaplasma reference sequences deposited in the NCBI GenBank. The Species, GenBank accession numbers and country of origin from which the sequences were derived are included for each sequence. Sequences derived from this study are marked by star (*). The number of identical sequences is in brackets. Selected reference Anaplasma spp. sequences from GenBank are also shown.
Spot mapping and statistically significant clusters
In the nine districts in the West Bank, 50 Palestinian localities targeted for the collection of samples. Cases of both infections in ticks, dogs and sheep in the study originated from 18 (36%) localities, which included villages (11/18 = 61%), cities (6/18 = 33%) and refugee camps (1/18 = 6%). Most of them (12/18 = 67%) were from rural areas. However, cities like Jericho, Tubas and Salfit are by standards considered towns rather than well‐established cities with rural activities on the margins. Anaplasma DNA was detected by 16S‐rRNA PCR in dogs in 10 (29%) out of 35 localities (Fig. 1b), while from ticks in eight (21%) localities out of 39 (Fig. 1a). At the same time, it was detected from sheep in all seven targeted localities (Fig. 1c). Ehrlichia DNA positive cases were from the districts of Al‐Khalil, Jericho, Tulkarem, and Jenin (Fig. 1a–c). Implementation of purely‐spatial analysis by saTScan for the pooled cases of Anaplasma from ticks, dogs and sheep revealed two statistically significant clusters (P < 0.05) (Fig. 3). However, implementing the same analysis on segregated bulks of cases did not reveal any significant clusters. Significant clusters were in Tubas town (P = 0.00005, Relative risk (RR) = 4.3) and Majdal‐Bani‐Fadil village near Ramallah (P = 0.0012, RR = 6.5) (Fig. 3). In Al‐Khalil district south of the West Bank, Hitta village close to the Green Line (1949 Armistice border between Palestinians and Israelis) had ten cases of Anaplasma infection which was close to forming a cluster (P = 0.08; RR = 3).
Figure 3.
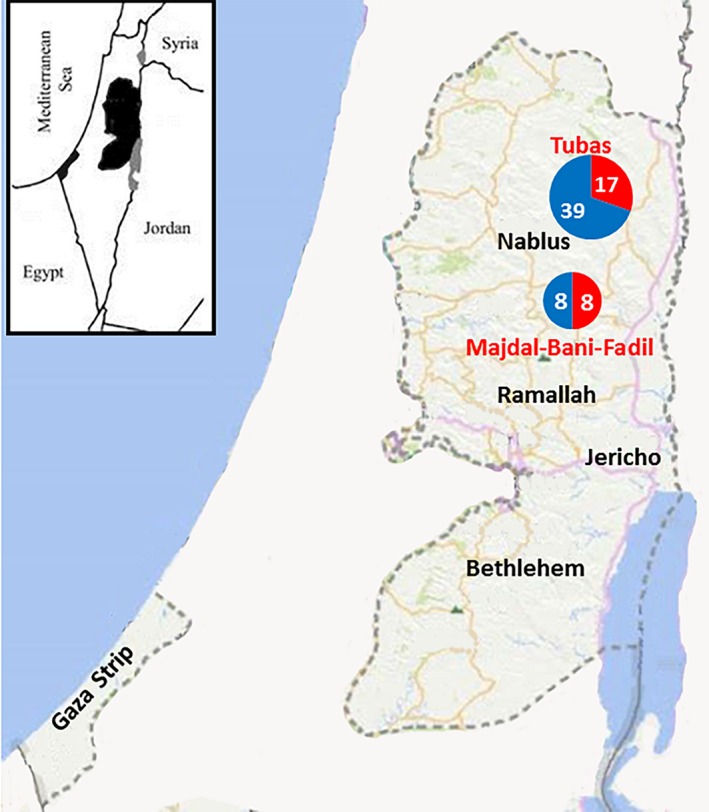
Geographical distribution of statistically significant foci of Anaplasma cases in Palestine (excluding Gaza strip) on implementing purely spatial analysis by SaTScan: red circles indicate positive cases while blue circles indicate negative cases. The numbers within the circles indicate the number of cases.
Discussion
Tick‐borne bacteria are important pathogens which affect the health of both humans and animals globally. In this study and for the first time in Palestine, we have reported the presence of Anaplasma and Ehrlichia in ixodid ticks and blood samples from different domestic animals. These pathogens have been reported from different neighboring countries including; Egypt, Israel and Iran (Loftis et al. 2006a,b; Harrus et al. 2011; Jafarbekloo et al. 2014). Two pathogens were detected in canine blood samples: A. platys and E. canis. The prevalence of A. platys determined in this study (10%) was lower than Kenya (12.5%) (Matei et al. 2016) and Brazil (19.4%) (da Silva et al. 2012).
In congruence with a study reported from Japan, E. canis was detected in 1.5% of the tested canine blood samples (Kubo et al. 2015). In contrast, two studies conducted in Brazil (da Silva et al. 2012) and Panama (Santamaria et al. 2014) reported much higher prevalence; 16.4% and 64.2%, respectively. Despite that E. canis is well‐known as a dog pathogen; it has been reported in domestic ruminants (Zhang et al. 2015). However, in this study, E. canis was not detected in sheep, camels, goats and horses.
Furthermore, A. platys and E. canis were identified in Ixodid ticks obtained from the same infected dogs. Although the blood and tick samples were collected from dogs simultaneously, our results showed that the prevalence of A. platys in ticks (1.8%) was lower than in dogs (9.6%). The discrepancy in the prevalence could be attributed to unequal burden of tick populations per animal host, different structure of the tick community which is derived from dogs from nine districts in Palestine and possibility of more than one tick species acting as vector in a given area. Our findings showed that A. platys‐infected ticks were mainly from the species of R. sanguineus s.l. which has been reported as the most prevalent dog tick in Palestine (Dantas‐Torres 2008a,b; Ereqat et al. 2016a,b; Harrus et al. 2011). However, the presence of A. platys in other species of Rhipicephalus, such as unengorged R. turanicus and R. bursa were reported in Turkey and Israel (Aktas et al. 2009; Harrus et al. 2011). Additionally, the prevalence of E. canis in ticks (0.6%) was lower than in dogs (1.5%). Higher infection rates of E. canis in tick were reported from Iran (16.7%) (Khazeni et al. 2013) and Israel (10%) (Harrus et al. 2011). However, in that study, questing ticks have been collected from vegetation by flagging while engorged ticks were collected from dogs in our study. Leschnik et al. (2012) demonstrated that sampling strategy for collecting ticks and the time of collection affect the species composition of the sample, developmental stages and the prevalence of their microbial pathogens (Leschnik et al. 2012). Moreover, co‐occurrence of infected nymphs and susceptible larvae on the same host and spatial clustering of ticks on the same host surfaces appear to be essential for transmission from one tick to another which may influence the prevalence of microbial pathogens in the ticks (Leschnik et al. 2012).
On the basis of 16SrRNA phylogenetic analysis, similarity was observed among the sequences of A. platys identified in this study. Furthermore, no heterogeneity was observed among E. canis group using 16S rRNA gene. However, distinct Ehrlichia sequence was found to be 99% similar to the corresponding sequence of a not well identified Ehrlichia spp. reported from China (Dong et al. 2014; Kang et al. 2014). Further characterization with additional genes is needed to reveal the species.
In this study, A. ovis, the agent of ovine anaplasmosis, was identified for the first time in Palestine. The overall prevalence of A. ovis in sheep (26%) and their corresponding ticks (38%) was lower than reported by other studies conducted in northwest China (Yang et al. 2015), Iraq, Sudan, Portugal and Turkey (Renneker et al. 2013). However, the blood samples were not taken at the same time as the ticks.
Our findings provide molecular evidence for the presence of A. ovis in R. turanicus and R. sanguineus s.l. ticks which was in line with previous studies, showing that Rhipicephalus spp. is one of the most important vectors of diseases in sheep (Renneker et al. 2013; Hosseini‐Vasoukolaei et al. 2014; Jafarbekloo et al. 2014).
Amplification of 16SrRNA gene is commonly used for the detection of Anaplasma/Ehrlichia DNA and thus further testing is required to investigate co‐infections by the two pathogens and for species identification of the same genus such as A. marginale and A. ovis. In the present study, and since 16SrRNA PCR was unable to definitively identify most of the Anaplasma species; A. ovis infections have been identified using msp4 primers (de la Fuente et al. 2003).
The presence of A. ovis is confirmed in Palestine as around the world, although this bacterium is supposed to cause only mild clinical symptoms, its adverse effect if the animals were under stress by different factors such as poor health conditions, hot weather, co‐infection, heavy tick infestation, vaccination or deworming is aggravated in infected ruminants (Hornok et al. 2007). Since the small ruminants are major source of meat, milk and wool in Palestine, ovine anaplasmosis, caused by A. ovis, may lead to economic burden if stress occurs at any time, so it is important to better understand this disease and further investigations are necessary.
Spot mapping of cases confirmed that most of the cases of anaplasmosis and ehrlichiosis (83%) were in rural areas where the vast majority of livestock and accompanying dogs are located and the optimal habitat of Ixodid ticks exist. Cases of Ehrlicha were in north, south and east of the West Bank, Palestine (Fig. 1a–c). This is inconsistent with the distribution of livestock and open wilderness with caves and wild vegetation. Kulldorf's saTScan revealed two main statistically significant foci for Ananplasma infection regardless of the host or vector (Fig. 3). These were in Tubas district north of the West Bank and in a village on the hills overlooking the western side of the Jordan Valley. The two statistically significant foci lie on the migration route of birds on the great Syria‐African rift valley extending from East Africa until Syria. The Jordan Valley lying in the middle of the rift is a major point of attraction for these birds to rest in on their way during the spring and autumn annual migration. Although not proved in Palestine, migrating birds have been found to carry infected ticks and transfer tick‐borne diseases from one area to another as found in Sweden (Kuo et al. 2017). Another plausible explanation for the two foci is the climate change. The vector‐borne diseases are climate‐sensitive and the vectors have been found to move north as far as Norway, Sweden and Russia as well as to higher altitudes (Jore et al. 2011; Andreassen et al. 2012; Ostfeld & Brunner 2015). Furthermore, the two areas are considered under‐privileged rural areas with low socioeconomic status and leading extensively active life style of farming and livestock‐raising which again could have contributed to the high disease rate (Campbell‐Lendrum et al. 2015). Anyhow, this is far from being a single factor event, but largely multifactorial with complex interactions of several variables such as climate change, environmental change and human behavior.
Conclusion
The results of this study highlight for the first time the presence of A. platys and E. canis infection in dogs from Palestine. A. ovis is also detected in sheep indicating a potential risk for adverse more serious disease. As part of a comprehensive control strategy, veterinarians in Palestine should put into consideration the presence of Anaplasma and Ehrlichia during clinical examination of sick animals particularly when clinical signs are compatible with anaplasmosis and ehrlichiosis. The possibility of cross‐border spill out of infection to neighboring countries should trigger regional cooperation to control the diseases. In addition, community and health professional awareness and surveillance system for neglected, yet emerging, zoonotic tick‐borne diseases are recommended to be undertaken at the official level.
Conflict of interest
The authors declare that the research was conducted without any conflict of interest.
Ethical statement
The animal owners were verbally informed about the goals of the study and the sampling protocol. All owners gave their verbal informed consent to collect ticks and blood samples from their animals. The study was approved by the ethics committee at the Faculty of Medicine in Al‐Quds University‐Palestine (EC number: ZA/196/013).
Acknowledgement
This research received financial support from the grant (2014.52146) funded by the Netherlands Ministry of Foreign Affairs, Hague, Netherlands.
References
- Aktas M., Altay K., Dumanli N. & Kalkan A. (2009) Molecular detection and identification of Ehrlichia and Anaplasma species in ixodid ticks. Parasitology Research 104, 1243–1248. [DOI] [PubMed] [Google Scholar]
- Al‐Adhami B., Scandrett W.B., Lobanov V.A. & Gajadhar A.A. (2011) Serological cross‐reactivity between Anaplasma marginale and an Ehrlichia species in naturally and experimentally infected cattle. Journal of Veterinary Diagnostic Investigation 23, 1181–1188. [DOI] [PubMed] [Google Scholar]
- Andreassen A., Jore S., Cuber P., Dudman S., Tengs T., Isaksen K. et al (2012) Prevalence of tick borne encephalitis virus in tick nymphs in relation to climatic factors on the southern coast of Norway. Parasit Vectors 5, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry P. & Geale D.W. (2011) A review of bovine anaplasmosis. Transboundary and Emerging Diseases 58, 1–30. [DOI] [PubMed] [Google Scholar]
- Bakken J.S. & Dumler J.S. (2015) Human granulocytic anaplasmosis. Infectious Disease Clinics of North America 29, 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos A.D., Mohammed O.B., Bennett N.C., Petevinos C. & Alagaili A.N. (2015) Molecular detection of novel Anaplasmataceae closely related to Anaplasma platys and Ehrlichia canis in the dromedary camel (Camelus dromedarius). Veterinary Microbiology 179, 310–314. [DOI] [PubMed] [Google Scholar]
- Cabezas‐Cruz A., Zweygarth E., Vancova M., Broniszewska M., Grubhoffer L., Passos L.M.F. et al (2016) Ehrlichia minasensis sp. nov., isolated from the tick Rhipicephalus microplus . International Journal of Systematic and Evolutionary Microbiology 66, 1426–1430. [DOI] [PubMed] [Google Scholar]
- Campbell‐Lendrum D., Manga L., Bagayoko M. & Sommerfeld J. (2015) Climate change and vector‐borne diseases: what are the implications for public health research and policy? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 370, 20130552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2008) Ehrlichiosis and Anaplasmosis: 2008 Case Definition. US Department and Health and Human Services, CDC, National Notifiable Diseases Surveillance System (NNDSS): Atlanta, GA. [Google Scholar]
- Corpet F. (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Research 16, 10881–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas‐Torres F. (2008a) The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Veterinary Parasitology 152, 173–185. [DOI] [PubMed] [Google Scholar]
- Dantas‐Torres F. (2008b) Canine vector‐borne diseases in Brazil. Parasit Vectors 1, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas‐Torres F. & Otranto D. (2015) Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Veterinary Parasitology 208, 9–13. [DOI] [PubMed] [Google Scholar]
- Dong T., Qu Z. & Zhang L. (2013) Detection of A. phagocytophilum and E. chaffeensis in patient and mouse blood and ticks by a duplex real‐time PCR assay. PLoS ONE 8, e74796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Chen X.P., Liu N., Dumler S.J. & Zhang Y.Z. (2014) Co‐circulation of multiple species of Rickettsiales bacteria in one single species of hard ticks in Shenyang, China. Ticks and Tick‐borne Diseases 5, 727–733. [DOI] [PubMed] [Google Scholar]
- Dumler J.S., Barbet A.F., Bekker C.P., Dasch G.A., Palmer G.H., Ray S.C. et al (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila . International Journal of Systematic and Evolutionary Microbiology 51, 2145–2165. [DOI] [PubMed] [Google Scholar]
- Ereqat S., Nasereddin A., Al‐Jawabreh A., Azmi K., Harrus S., Mumcuoglu K. et al (2016a) Molecular detection and identification of spotted fever group Rickettsiae in ticks collected from the West Bank, Palestinian Territories. PLoS Neglected Tropical Diseases 10, e0004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereqat S., Nasereddin A., Vayssier‐Taussat M., Abdelkader A., Al‐Jawabreh A., Zaid T. et al (2016b) Molecular evidence of Bartonella species in ixodid ticks and domestic animals in palestine. Frontiers in Microbiology 7, 1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine A.B., Sweeney J.D., Nixon C.P. & Knoll B.M. (2016) Transfusion‐transmitted anaplasmosis from a leukoreduced platelet pool. Transfusion 56, 699–704. [DOI] [PubMed] [Google Scholar]
- Friedhoff K.T. (1997) Tick‐borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 39, 99–109. [PubMed] [Google Scholar]
- de la Fuente J., Van Den Bussche R.A., Prado T.M. & Kocan K.M. (2003) Anaplasma marginale msp1α genotypes evolved under positive selection pressure but are not markers for geographic isolates. Journal of Clinical Microbiology 41, 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmone A.A., Robbins R.G., Apanaskevich D.A., Petney T.N., Estrada‐Pena A. & Horak I.G. (2009) Comments on controversial tick (Acari: Ixodida) species names and species described or resurrected from 2003 to 2008. Experimental and Applied Acarology 48, 311–327. [DOI] [PubMed] [Google Scholar]
- Harrus S., Perlman‐Avrahami A., Mumcuoglu K.Y., Morick D., Eyal O. & Baneth G. (2011) Molecular detection of Ehrlichia canis, Anaplasma bovis, Anaplasma platys, Candidatus Midichloria mitochondrii and Babesia canis vogeli in ticks from Israel. Clinical Microbiology & Infection 17, 459–463. [DOI] [PubMed] [Google Scholar]
- Hornok S., Elek V., de la Fuente J., Naranjo V., Farkas R., Majoros G. & Foldvari G. (2007) First serological and molecular evidence on the endemicity of Anaplasma ovis and A. marginale in Hungary. Veterinary Microbiology 122, 316–322. [DOI] [PubMed] [Google Scholar]
- Hosseini‐Vasoukolaei N., Oshaghi M.A., Shayan P., Vatandoost H., Babamahmoudi F., Yaghoobi‐Ershadi M.R. et al (2014) Anaplasma infection in ticks, livestock and human in Ghaemshahr, Mazandaran Province, Iran. Journal of Arthropod‐Borne Diseases 8, 204–211. [PMC free article] [PubMed] [Google Scholar]
- Ismail N., Bloch K.C. & McBride J.W. (2010) Human ehrlichiosis and anaplasmosis. Clinics in Laboratory Medicine 30, 261–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarbekloo A., Bakhshi H., Faghihi F., Telmadarraiy Z., Khazeni A., Oshaghi M.A. et al (2014) Molecular detection of Anaplasma and Ehrlichia infection in ticks in borderline of Iran‐Afghanistan. Journal of Biomedical Science and Engineering 7, 919. [Google Scholar]
- Jore S., Viljugrein H., Hofshagen M., Brun‐Hansen H., Kristoffersen A.B., Nygard K. et al (2011) Multi‐source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasites & Vectors 4, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.J., Diao X.N., Zhao G.Y., Chen M.H., Xiong Y., Shi M. et al (2014) Extensive diversity of Rickettsiales bacteria in two species of ticks from China and the evolution of the Rickettsiales. BMC Evolutionary Biology 14, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazeni A., Telmadarraiy Z., Oshaghi M., Mohebali M., Zarei Z. & Abtahi S. (2013) Molecular detection of Ehrlichia canis in ticks population collected on dogs in Meshkin‐Shahr, Ardebil Province, Iran. Journal of Biomedical Science and Engineering 6, 1–5. [Google Scholar]
- Kubo S., Tateno M., Ichikawa Y. & Endo Y. (2015) A molecular epidemiological survey of Babesia, Hepatozoon, Ehrlichia and Anaplasma infections of dogs in Japan. Journal of Veterinary Medical Science 77, 1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulldorff M. (1997) A spatial scan statistic. Communications in Statistics ‐ Theory and Methods 26, 1481–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C. & Tamura K. (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35, 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.C., Lin Y.F., Yao C.T., Shih H.C., Chung L.H., Liao H.C. et al (2017) Tick‐borne pathogens in ticks collected from birds in Taiwan. Parasites & Vectors 10, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttler K.L. (1984) Anaplasma infections in wild and domestic ruminants: a review. Journal of Wildlife Diseases 20, 12–20. [DOI] [PubMed] [Google Scholar]
- Leschnik M.W., Khanakah G., Duscher G., Wille‐Piazzai W., Horweg C., Joachim A. & Stanek G. (2012) Species, developmental stage and infection with microbial pathogens of engorged ticks removed from dogs and questing ticks. Medical and Veterinary Entomology 26, 440–446. [DOI] [PubMed] [Google Scholar]
- Li H., Zheng Y.‐C., Ma L., Jia N., Jiang B.‐G., Jiang R.‐R. et al (2015) Human infection with a novel tick‐borne Anaplasma species in China: a surveillance study. The Lancet Infectious Diseases 15, 663–670. [DOI] [PubMed] [Google Scholar]
- Loftis A.D., Reeves W.K., Szumlas D.E., Abbassy M.M., Helmy I.M., Moriarity J.R. & Dasch G.A. (2006a) Population survey of Egyptian arthropods for rickettsial agents. Annals of the New York Academy of Sciences 1078, 364–367. [DOI] [PubMed] [Google Scholar]
- Loftis A.D., Reeves W.K., Szumlas D.E., Abbassy M.M., Helmy I.M., Moriarity J.R. & Dasch G.A. (2006b) Rickettsial agents in Egyptian ticks collected from domestic animals. Experimental and Applied Acarology 40, 67–81. [DOI] [PubMed] [Google Scholar]
- Marenzoni M.L., Lauzi S., Miglio A., Coletti M., Arbia A., Paltrinieri S. & Antognoni M.T. (2017) Comparison of three blood transfusion guidelines applied to 31 feline donors to minimise the risk of transfusion‐transmissible infections. Journal of Feline Medicine and Surgery 20, 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei I.A., D'Amico G., Yao P.K., Ionica A.M., Kanyari P.W., Daskalaki A.A. et al (2016) Molecular detection of Anaplasma platys infection in free‐roaming dogs and ticks from Kenya and Ivory Coast. Parasites & Vectors 9, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongruel A.C.B., Benevenute J.L., Ikeda P., Andre M.R., Machado R.Z., Carrasco A.O.T. & Seki M.C. (2017) Detection of Anaplasma sp. phylogenetically related to A. phagocytophilum in a free‐living bird in Brazil. Revista Brasileira de Parasitologia Veterinária 26, 505–510. [DOI] [PubMed] [Google Scholar]
- Ndip L.M., Ndip R.N., Esemu S.N., Walker D.H. & McBride J.W. (2010) Predominance of Ehrlichia chaffeensis in Rhipicephalus sanguineus ticks from kennel‐confined dogs in Limbe, Cameroon. Experimental and Applied Acarology 50, 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld R.S. & Brunner J.L. (2015) Climate change and Ixodes tick‐borne diseases of humans. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 370, 20140051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock C.D. & Childs J.E. (2003) Ehrlichia chaffeensis: a prototypical emerging pathogen. Clinical Microbiology Reviews 16, 37–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P., Roux V., Camicas J.L., Baradji I., Brouqui P. & Raoult D. (2000) Detection of ehrlichiae in African ticks by polymerase chain reaction. Transactions of the Royal Society of Tropical Medicine and Hygiene 94, 707–708. [DOI] [PubMed] [Google Scholar]
- Paulauskas A., Radzijevskaja J. & Rosef O. (2012) Molecular detection and characterization of Anaplasma phagocytophilum strains. Comparative Immunology, Microbiology and Infectious Diseases 35, 187–195. [DOI] [PubMed] [Google Scholar]
- Renneker S., Abdo J., Salih D.E., Karagenc T., Bilgic H., Torina A. et al (2013) Can Anaplasma ovis in small ruminants be neglected any longer? Transboundary and Emerging Diseases 60(Suppl 2), 105–112. [DOI] [PubMed] [Google Scholar]
- Sainz A., Roura X., Miro G., Estrada‐Pena A., Kohn B., Harrus S. & Solano‐Gallego L. (2015) Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasites & Vectors 8, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria A., Calzada J.E., Saldana A., Yabsley M.J. & Gottdenker N.L. (2014) Molecular diagnosis and species identification of Ehrlichia and Anaplasma infections in dogs from Panama, Central America. Vector‐Borne and Zoonotic Diseases 14, 368–370. [DOI] [PubMed] [Google Scholar]
- Silaghi C., Santos A.S., Gomes J., Christova I., Matei I.A., Walder G. et al (2017) Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector‐Borne and Zoonotic Diseases 17, 12–22. [DOI] [PubMed] [Google Scholar]
- da Silva G.C., Benitez Ado N., Girotto A., Taroda A., Vidotto M.C., Garcia J.L. et al (2012) Occurrence of Ehrlichia canis and Anaplasma platys in household dogs from northern Parana. Revista Brasileira de Parasitologia Veterinária 21, 379–385. [DOI] [PubMed] [Google Scholar]
- Stothard P. (2000) The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques 28(1102), 1104. [DOI] [PubMed] [Google Scholar]
- Sudan V., Sharma R.L. & Borah M.K. (2014) Subclinical anaplasmosis in camel (Camelus dromedarius) and its successful therapeutic management. Journal of Parasitic Diseases 38, 163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate C.M., Howerth E.W., Mead D.G., Dugan V.G., Luttrell M.P., Sahora A.I. et al (2013) Anaplasma odocoilei sp. nov. (family Anaplasmataceae) from white‐tailed deer (Odocoileus virginianus). Ticks and Tick‐borne Diseases 4, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Li Y., Liu Z., Liu J., Niu Q., Ren Q. et al (2015) Molecular detection and characterization of Anaplasma spp. in sheep and cattle from Xinjiang, northwest China. Parasites & Vectors 8, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kelly P., Guo W., Xu C., Wei L., Jongejan F. et al (2015) Development of a generic Ehrlichia FRET‐qPCR and investigation of ehrlichioses in domestic ruminants on five Caribbean islands. Parasites & Vectors 8, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]


