Abstract
Background
In the Kingdom of Saudi Arabia (KSA), Leishmania major and L. tropica are the main causative agents of Old World cutaneous leishmaniasis (CL). The national CL treatment regimen consists of topical 1% clotrimazole/2% fusidic acid cream followed by 1–2 courses of intralesional sodium stibogluconate (SSG); however, treatment efficacy is highly variable and the reasons for this are not well understood. In this study, we present a complete epidemiological map of CL and determined the efficacy of the standard CL treatment regime in several endemic regions of KSA.
Results
Overall, three quarters of patients in all CL-endemic areas studied responded satisfactorily to the current treatment regime, with the remaining requiring only an extra course of SSG. The majority of unresponsive cases were infected with L. tropica. Furthermore, the development of secondary infections (SI) around or within the CL lesion significantly favoured the treatment response of L. major patients but had no effect on L. tropica cases.
Conclusions
The response of CL patients to a national treatment protocol appears to depend on several factors, including Leishmania parasite species, geographical location and occurrences of SI. Our findings suggest there is a need to implement alternative CL treatment protocols based on these parameters.
Electronic supplementary material
The online version of this article (10.1186/s13071-019-3453-4) contains supplementary material, which is available to authorized users.
Keywords: Cutaneous leishmaniasis, Saudi Arabia, Epidemiology, Treatment response, Secondary infections
Background
Old World cutaneous leishmaniasis (CL) is endemic throughout the East Mediterranean Region (EMR) [1]. According to the World Health Organization, in recent years, over 150,000 human CL cases were reported in 16 EMR countries [2]. Various factors contribute to the spread of CL in this region, including uncontrolled urbanization, irrigation, governmental sector integration, socio-economic factors and lack of health education [3]. War is another important factor responsible for CL outbreaks in the region, with over 200,000 people infected during the military conflict in Afghanistan [4] and a similar number affected since the start of the Syrian civil war [5–9].
CL is the second most important vector-borne disease in the Kingdom of Saudi Arabia (KSA) after dengue fever. Most of the reported CL cases are concentrated in the regions of Al Ahsa, Al Qassem, Riyadh, Asir, Hail and Al Madinah [10–22]. Since the early 1980’, KSA has implemented a national CL control programme consisting not only in carrying out case detection in focus areas, but also involving vector and reservoir control. Due to this well-designed national control programme, the number of formally registered CL cases in KSA has dropped since 1987, from ~17,000 to ~2000 cases per year in 2015 [14]. However, these official surveillance figures are likely to underestimate the prevalence and disease burden due to the number of unreported cases [14]. In addition to other non-communicable diseases, CL control in KSA is particularly important because of the millions of visitors that this country receives annually due to religious activities. Furthermore, around 35% of the country’s work force consists of visitors arriving from other leishmaniasis-endemic countries. Both anthroponotic (caused by L. tropica) [10, 17, 22] and zoonotic (caused by L. major) [6, 15, 23, 24] CL have been reported in KSA, but very little is currently known about the national distribution of these parasite species.
Current drug treatment protocols for patients with Old World CL vary between EMR countries. Intralesional pentostam or sodium stibogluconate (SSG) is commonly used in this region, despite their high toxicity and the increasing number of unresponsive individuals. Currently, SSG is the second-line treatment choice for CL in KSA. In most cases, it is administered if the patient remains unresponsive (i.e. lack of re-epithelisation) after treatment with topical broad-spectrum antimicrobials (azoles and/or antibiotics; first line treatment). Topical paromomycin, either alone or in combination with gentamicin, was found to be effective in treatment of L. major cases in Tunisia [25], although it has not been implemented in KSA.
In this study, we present a complete epidemiological map of CL in KSA, including the clinical features associated with the two main parasite species, L. major and L. tropica. Furthermore, we show evidence that the efficacy of the current CL treatment protocol is highly dependent on the parasite species associated with the infection, geographical location and possibly also to the development of secondary infections in or around the lesion.
Methods
Sample collection and clinical data
Skin aspirate samples from a total of 104 adult CL-patients were collected from several cities or towns in KSA. Overall, this represented around 7% of all reported cases of CL in KSA in 2012. Case record studies and information sheets were obtained for all patients. This information was anonymised and re-labelled with the appropriate study code. The recorded information included all relevant clinical data (i.e. lesion size, number(s) and location(s) on the body, clinical features and treatment response, patient age and sex) and is provided in Additional file 1: Table S1.
Parasite isolation and in vitro culture
Parasite samples were collected by wound aspiration from Leishmania-infected patients after one month of lesion appearance using 200–300 µl of sterile PBS. Wound aspirates were transferred to plastic flasks (in triplicate) containing Leishmania culture medium M199 (Gibco, Grand Island, NY, USA) supplemented with 15% heat-inactivated foetal bovine serum (Invitrogen), 1.5% BME vitamins (Sigma-Aldrich, Saint Louis, MO, USA) and 25 µg/ml gentamycin sulphate (Sigma-Aldrich, Saint Louis, MO, USA). Leishmania cultures were maintained for several days at 27 °C and parasite DNA was extracted from logarithmic phase cultures using the DNeasy Blood and Tissue kit (Qiagen, Germantown, MD, USA) following the manufacturer’s instructions.
Drug treatment
Clinically diagnosed patients were referred for treatment as recommended by the current KSA leishmaniasis treatment policy. This begins with the application of topical clotrimazole (1%) and/or fusidic acid (2%) for the treatment of secondary infections (i.e. bacterial, fungal or both). If after one week of treatment healing (re-epithelisation) was not initiated, the patient then received one course (of 14 injections each) of intralesional SSG (20 mg/kg/day) (Additional file 2: Figure S1). All patients receiving SSG treatment were evaluated before and after treatment for complete blood counts and levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, amylase and gamma glutamyltransferase. A few patients (n = 16) from the unsatisfactory responsive group (i.e. those with lesion extension, formation of satellite lesions or recurrence) were referred for a 3rd course of intramuscular SSG (20 mg/kg/day for up to 2 weeks) after clinical assessment (blood count, liver and renal function analyses).
Identification of secondary infection species and antibiotic susceptibility testing
Secondary infections were identified by clinical assessment. Nutrient agar, blood agar, MacConkey agar and Sabouraud dextrose agar were used for bacterial and fungal culture. Microscopy and biochemical assays were carried out to identify microbial species. Antibiotic susceptibility testing from identified species were performed using the semi-automatic analyser Microscan Autoscan-4 system (Beckman Coulter, Indianapolis, IN, USA). The antibiotics tested were ampicillin, azithromycin, cefoxitin, ciprofloxacin, erythromycin, fosfomycin, fusidic acid and gentamicin. All the laboratory analyses for microbial identification were carried out at King Abdulaziz Centre for Science and Technology (KACST).
Species identification of Leishmania isolates
Identification was performed based on a modified PCR-restriction fragment length polymorphism (PCR-RFLP) method previously described (26). A first PCR reaction of 25 µl was set using the Q5 High-Fidelity 2X Mastermix (New England Biolabs, Ipswich, MA, USA) and the primers OL1853 and OL1854 at 200 nM. PCR conditions were as follows: 98 °C for 1 min, 35 cycles of 10 s at 98 °C, 30 s at 57 °C and 20 s at 72 °C, and a final step at 72 °C for 1 min. To increase the sensitivity, a second identical PCR was performed using 1 µl of PCR product as template. Then, 20 µl of the PCR product was incubated with HaeIII (New England Biolabs) in a 50 µl reaction at 37 °C for 1 hour and inactivated at 80 °C for 20 min. For the analysis, 5 µl of unrestricted PCR product and 20 µl of restricted product were run in a 2.5% agarose gel at 100 V. Leishmania species for each patient sample was determined by comparing its restriction pattern with L. major and L. tropica reference reactions. Unrestricted PCR products were also sequenced (Sanger sequencing; Source Biosciences, UK) to confirm product specificity.
Data analysis
All factors affecting the treatment responses were included in our analyses (i.e. clinical features, number and sites of the lesions, geographical area and parasite species). Fisher’s exact test and Chi-square test were used to validate the statistical significance among the different factors. IMB SPSS Statistics 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) was employed for all data analysis. Software ArcGIS 10 (ESRI 2011, ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute) was used to map the distribution of parasite species and patient response to anti-leishmanial drug treatment across the regions, and in relation to elevation based on gridded Global Relief Data (ETOPO2) data, based on the geographical coordinates (latitude and longitude) of each site.
Results
Identification of Leishmania species
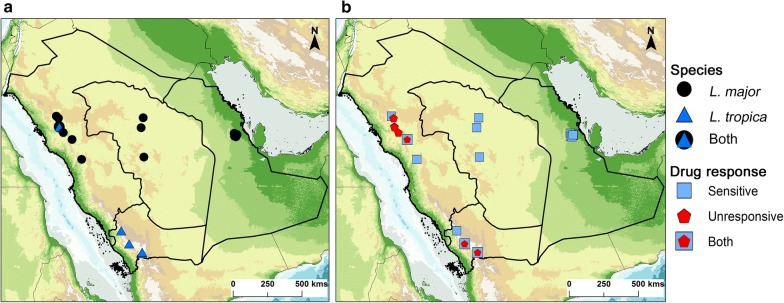
In KSA, Old World CL is mainly diagnosed by clinical assessment and microscopic examination, but not molecularly [10]. The infective Leishmania species is inferred based on the appearance, number of lesions and potential geographical location where infection may have occurred. Using a well-established PCR-RFLP analysis of the ITS1 region [10, 26, 27] we identified the Leishmania species present in 104 CL patients (Additional file 2: Figures S2–S5). All 104 CL patients were confirmed to be infected with either L. major or L. tropica. Leishmania major was the main species responsible for CL in KSA and predominately found in the regions of Al Ahsa (East), Al Qassem and Riyadh (Central), and Al Madinah (Northwest) (Fig. 1 and Additional file 2: Figures S6-S7). Furthermore, L. tropica was the only species found in the Southwest regions of Asir and Jazan (Fig. 1 and Additional file 2: Figure S8). Interestingly, L. tropica was also detected in a few cases from the Al Madinah region, with one particular village reporting the presence of both L. major and L. tropica infections (Fig. 1 and Additional file 2: Figure S3).
Fig. 1.
Distribution of Leishmania species (a) and patient response to anti-leishmanial treatment (b) within the main CL endemic regions of Saudi Arabia. Map was created using software ArcGIS 10 (ESRI 2011, ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute). See also detailed maps in Additional file 2: Figures S6, S7 and S8
Clinical presentations of CL patients vary depending on parasite species
Several clinical features of the CL lesions, along with the infecting parasite species, were measured. These parameters included size, clinical presentations (i.e. papular, nodular or ulcerated nodular), location on the body, numbers, and whether satellite lesions were present (Table 1). Leishmania major lesions tended to be smaller (16.5 mm) than L. tropica ones (22.2 mm). The clinical presentations were significantly associated with the infecting species (Fisher’s exact test of association; P = 0.001745), with relatively similar numbers of each lesion type in L. major infections, but a notable skew towards ulcerated nodular lesions in L. tropica infections.
Table 1.
Clinical presentation of CL lesions according to infecting parasite species
| Parasite species | L. major | L. tropica | P-value | |
|---|---|---|---|---|
| Mean lesion size | 16.5 | 22.2 | ||
| Lesion feature | Papularb | 23 | 0 | 0.001745 |
| Nodular | 27 | 7 | ||
| Ulcerated nodularc | 25 | 14 | ||
| Lesion locationd | Hand, neck, head | 30 | 2 | < 0.00001 |
| Arm | 7 | 12 | ||
| Trunk, leg, foot | 19 | 0 | ||
| Face, nose, ear | 3 | 7 | ||
| Whole body | 16 | 0 | ||
| Lesion number | 1 | 23 | 10 | < 0.00001 |
| 2 | 9 | 3 | ||
| 3 | 5 | 8 | ||
| 4 | 4 | 0 | ||
| 5 | 7 | 0 | ||
| 6+ (max. 29) | 27 | 0 | ||
| Satellite lesions | Yes | 17 | 12 | 0.005813 |
| No | 58 | 9 |
aFisher’s exact test of association
bNodular lesions were found in L. tropica patients from Al Madinah (Al Jadidah) and Asir (Etoed)
cUlcerated nodular lesions were found in two thirds of CL patients. Lesions were mainly localized on the face, nose and/or ear
dThe criteria used by the Saudi MoH for categorizing the location of the lesions is related to the use of traditional outfits, which determines the area of the body exposed to sand fly bites
The location of lesions was also significantly associated with the infecting parasite species (P ≤ 0.00001), with L. tropica lesions appearing to be more common on the arms, face, nose or ear than L. major lesions, which are more common on other sites of the body (Table 1). Furthermore, the number of lesions was significantly associated with the parasite species (P = 7.201×10−5), with L. major infections displaying a bi-modal distribution of 1 lesion or > 6 lesions while L. tropica infections all showed ≤ 3 lesions. The presence of satellite lesions was also significantly associated with infecting species (P = 0.005813), with 57% of L. tropica infections displaying satellite lesions compared to 22% in L. major ulcers.
Efficacy of anti-leishmanial treatment varies depending on parasite species and geographical location
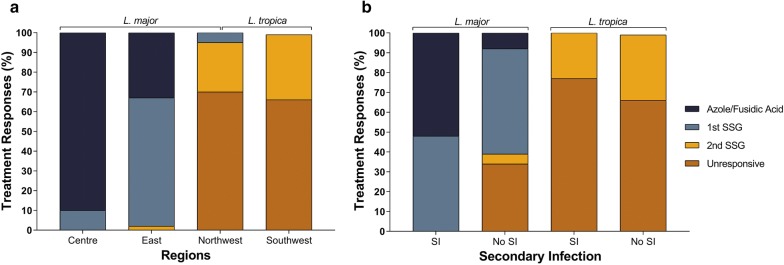
Confirmed (medically diagnosed) CL patients were referred for anti-leishmanial treatment starting with topical antifungals alone or in combination with antibiotics (first line of treatment) followed by 1–2 courses of IL SSG (as described in the Methods section). Overall, 30% of L. major-infected patients responded to the first line of treatment alone and 82% after SSG courses were completed (Fig. 2a). However, none of the L. tropica-infected patients responded to topical azoles/fusidic acid, and in fact 60% did not respond at all to any treatment regimen even after receiving 2 courses of SSG (Table 2). Most L. tropica unresponsive patients withdrew after receiving the second course of SSG. Interestingly, when the treatment responses of patients from different geographical locations were compared, 90% of the cases from Central (i.e. Riyadh; exclusively L. major-infected) responded favourably (within 1 week) to topical azoles/fusidic acid alone (Fisherʼs exact test, P = 0.001), whereas two-thirds of the cases from the Northwest (Al Madinah; mainly L. major-infected) and Southwest (Asir; exclusively L. tropica-infected) responded unsatisfactorily to both lines of treatment.
Fig. 2.
Correlation between responses to drug treatment and CL endemic regions (a), and development of secondary infections (SI) and treatment response (b). a Distribution by region of 104 patients with L. major or L. tropica infections confirmed by culture, PCR-RFLP or clinical features. Patients from the Central region (exclusively infected with L. major) had a significantly higher response (healing; P = 0.001, Fisher’s exact test) to the first line of treatment compared to other CL-endemic regions studied. In addition, patients from the Eastern region, responded significantly favorably (P = 0.001; Fisher’s exact test) to the first course of SSG compared to those from both Northwest and Southwest regions. b Data for 96 patients with L. major (n = 75) or L. tropica (n = 21) infections confirmed by culture or PCR-RFLP. A significant P = 0.0002 (Chi-square test) response to first line of treatment was observed only in L. major patients that had developed SI. However, there were no significant differences in L. tropica patients with or without SI. Abbreviations: SI, presence of secondary infections; No SI, absence of secondary infections
Table 2.
Correlation between location of CL lesions and patient response to drug treatment. Data for 96 patients with L. major or L. tropica infections confirmed by culture or PCR-RFLP
| Lesion location | % Patients responding to drug treatment (healing) | ||||
|---|---|---|---|---|---|
| 1st line (Azoles/fusidic acid) | 2nd line (SSG) 1st course | 2nd line (SSG) 2nd course | Unresponsive casesa | P-valueb | |
| Head, neck or hand | 41 (n = 13) | 37 (n = 12) | 5 (n = 2) | 16 (n = 15) | 0.0192 (n = 32) |
| Arms | 32 (n = 6) | 0 (n = 0) | 21 (n = 4) | 47 (n = 9) | 0.2 (n = 19) |
| Trunk, legs or feet | 32 (n = 6) | 63 (n = 12) | 0 (n = 0) | 5c (n = 1) | 0.02 (n = 19) |
| Face, nose or ear | 10 (n = 1) | 20d (n = 2) | 20 (n = 2) | 50e (n = 5) | 0.5 (n = 10) |
| Disseminated (whole body) | 13 (n = 2) | 31 (n = 5) | 13 (n = 2) | 43f (n = 7) | 0.7 (n = 16) |
a85% of patients that did not respond to drug treatment were infected with L. tropica
bFisher’s exact test
cUlcerated L. tropica cases with secondary infection
dAll cases were L. major patients with apparent secondary infections
eCases with satellite lesions
fCases with multiple lesions receiving more than two courses of stibogluconate (SSG)
SIs were primarily detected in patients from the Eastern (35%; exclusively L. major) and Southwest (40%; exclusively L. tropica) regions (Fig. 2). Interestingly, 52% of L. major patients presenting SIs responded favourably after treatment with just topical azoles/fusidic acid (Fisherʼs exact test, P = 0.002; Fig. 2b), with the remaining needing just one course of SSG to heal. Moreover, the group of L. major patients lacking SI responded poorly to azoles/fusidic acid treatment and many (~38%) remained unresponsive after a second SSG course. In contrast, the development of SIs did not have an effect upon the treatment response of L. tropica patients (Fig. 2b).
Staphylococcus species isolated from L. major lesions show resistance to a variety of antibiotics, including Fusidic Acid
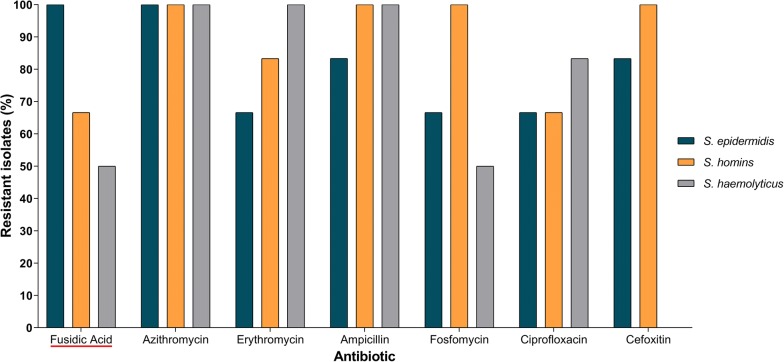
Staphylococcus epidermidis, S. haemolyticus and S. homins were isolated from 6 L. major patients from Al Ahsa region. The resistance profiles (Fig. 3) showed that 6/6 of S. epidermidis, 4/6 S. homins and 3/6 S. haemolyticus isolates are resistant to fusidic acid, which is part of the first line of CL treatment in KSA. From these 6 patients, 4 responded to the first line of treatment (azoles/fusidic acid) and 2 had to receive 1 course of SSG to complete healing. Furthermore, all the Staphylococcus species isolated from L. major-infected patients showed resistance to azithromycin, whereas S. homins strains were resistant to ampicillin, cefoxitin and fosfomycin, and S. haemolyticus strains resistant to ampicillin and erythromycin.
Fig. 3.
Percentage of resistant Staphylococcus species isolated from 6 volunteers with L. major lesion in Al Ahsa region of KSA. Only the antibiotics with at least one resistant isolate are shown. Fusidic acid is highlighted with a red line underneath the name
Discussion
Several studies have investigated the clinical profiles of CL in Saudi Arabia [15–17, 20, 21, 28]. However, to our knowledge, this study is the most comprehensive dataset of CL samples collected from patients across the country. One hundred and four parasite isolates were collected from infected CL patients from the main endemic regions of KSA and the species molecularly identified by PCR-RFLP. Each patient was medically assessed and referred for treatment according to the KSA national treatment policy.
Leishmania major was found in patients from Al Ahsa, Riyadh, Al-Qassim and Al-Madinah, all from arid or semi-arid areas at a low altitude (not sea level), while L. tropica was exclusively detected in patients from Asir, Jazan and Al-Jadidah (Al-Madinah) (high altitude) [29]. Interestingly, both L. major and L. tropica parasites were isolated in the Al-Jadidah village and from regions at similar altitude (~600 m above sea level). We speculate that this is likely due to L. tropica-infected patients migrating to this region, as the climatic conditions and ecology of Al-Jadidah do not favour transmission of L. tropica by its main vector, Phlebotomus sergenti [29]. Furthermore, we have recently characterized the sand fly vectors from the same Al-Madinah locations and found no evidence of Ph. sergenti in this region, although it has been previously reported in other areas [11, 15, 22, 29].
The clinical features of L. major patients appear to vary according to the endemic area [28]. Although we did not find statistically significant differences between the different regions sampled in this study, we observed that ulcerated nodular lesions were found predominantly in cases from Al Ahsa, whereas nodular and papular lesions were common in Al Madinah and Riyadh. Furthermore, multiple lesions were found in over half of L. major patients from Al Ahsa. This differs from previous reports where only 5% of the patients (presumably also infected with L. major) presented multiple lesions [28, 30]. These differences may be due in part to higher exposure of people to sand fly bites as most of the CL patients from Al Ahsa that took part in this study were migrant construction workers.
Several factors may account for differences in treatment response, including the possible presence of different drug-resistant parasites, the nutritional and immune status of the patients and their genetic backgrounds. In addition, sand fly saliva could also play an important role in disease outcome as saliva from Ph. papatasi (L. major vector) has been reported to trigger either a Type-I (regarded as protective against a Leishmania infection) or II delayed-type hypersensitivity in healthy individuals from CL endemic areas [31]. Furthermore, we recently showed that previous exposure to Ph. papatasi bites appears to influence the severity of CL [29]. As for Ph. sergenti saliva [32], nothing is known about its effect in the human immune system or in modulating CL pathology. The presence of ulcerated nodular lesions also showed a strong correlation with L. tropica infection in patients from all the CL endemic regions where this species was found. Therefore, the infection with each of the parasite species appears to favour the development of specific clinical feature(s), which vary depending on geography and may also impact treatment response. More research is needed to establish a correlation between treatment efficacy and the different clinical presentations of Old World CL patients.
Approximately 50% of the L. major patients (mainly from the Central or Eastern regions) who presented with ulcerated nodular lesions had detectable SI. Interestingly, there was a statistically significant (Fisher’s exact test, P = 0.0001) correlation with the response to re-epithelisation after treatment with azole/fusidic acid (without further administration of SSG) in patients from the Central region compared to patients from other endemic areas (Fig. 2). This suggests that elimination of the SI appears to favour healing of L. major patients from Riyadh and Al Ahsa, but has little effect in those from Al Madinah, although the lack of response of the latter may be also correlate with the time of residency in this region [27, 28]. In addition, we cannot rule out that first line of treatment could accelerate re-epithelization in patients who may have self-healed over time. However, this seems unlikely because most of the patients seek medical assistance after the lesion has worsened. Moreover, like pathogenic fungi, Leishmania parasites make ergosterol, an essential membrane lipid, the synthesis of which is inhibited by azoles [33]. Thus, treatment with topical azoles alone may possibly impact directly on L. major susceptible strains. Also, elimination of the fungal or bacterial infection may boost the immune system to fight the L. major infection in many cases, with the exception being those presenting papular lesions from the Al Madinah region. Alternatively, the indirect effect that the development of SIs may have in the treatment of L. major-infected patients could also be a result of the release of bacterial or fungal molecules after application of the first line of treatment. This could activate host immunity and contribute to the elimination of L. major infection and subsequent activation of wound healing mechanisms [34]. Currently, we are performing an extensive metagenomic analysis to characterise the microbiota present in lesions from both L. major and L. tropica patients. These results will be key to associate specific microbial groups with differences in treatment response.
It is worth mentioning that in some cases the first line of treatment is applied to increase efficacy of SSG regime. Regarding L. tropica-infected patients, most were unresponsive (60%) to the first course of anti-leishmanial treatment and healing only occurred in patients receiving a second (and sometimes a third) course of SSG. Unresponsive cases to SSG have been reported among L. tropica patients in neighbour countries like Iran [35]. Moreover, it remains to be determined whether L. tropica strains from KSA are less sensitive to azoles or alternatively, the drug may be more accessible to the parasite in L. major lesions than in L. tropica ones.
Novel therapies against CL need to be evaluated in clinical trials in KSA. For example, the oral administration of miltefosine, an alkylphosphocholine analogue that inhibits phospholipid and sterol biosynthesis, proved to be safer and more effective than SSG for the treatment of CL caused by L. braziliensis in Brazil [36]. Likewise, it would be interesting to test the efficacy of topical paromomycin for the treatment of L. tropica infections [25]. None of these drugs is used in the current CL treatment regime in KSA but could represent alternative approaches in unresponsive cases.
Taken together, differences in the susceptibility to either azoles or SSG among parasite strains and resistance against fusidic acid observed for the Staphylococcus species isolated from L. major CL lesions suggest that the current protocols for CL treatment need to consider not only the parasite species, but also the geographical endemicity of CL infections. Further research is required to understand whether treatment unresponsiveness in KSA is also due to the development of drug-resistant parasites. Knowing this will help determine if different anti-leishmanial treatment protocols need to be considered.
Conclusions
The findings of this study demonstrate that patient responses to current anti-leishmanial treatment vary between the different CL endemic areas and are also partially dependent on the development of SIs. These results have implications for the implementation of differential treatment regimens according to the CL-endemic area, which in turn will save financial resources and ensure patients are treated with the most efficacious anti-leishmanial therapies. It remains to be determined in CL-endemic areas from other EMR countries whether such profound differences in anti-Leishmania treatment responses are also observed among CL patients from different geographical locations.
Additional files
Additional file 1: Table S1. Cohort characteristics and relevant clinical features.
Additional file 2: Figure S1. Scheme representing the current leishmaniasis treatment policy in KSA. Figure S2. Leishmania spp. identification in Central Region by PCR-RFLP analysis of parasite ITS1 region. Lane Lt: L. tropica positive control; Lane Lm: L. major positive control; Lanes 1–10: different examples of Leishmania isolates from Rass, Dwadmi and Muzahmyyah. Figure S3. Leishmania spp identification in Al Madinah Province by PCR-RFLP analysis of parasite ITS1 region. Lanes 1–4: different examples of Leishmania isolates from Aljadaida and Sulailah, Al Madinah Province; Lanes 1 and 2: L. tropica samples; Lanes 3 and 4: L. major samples; Lane Lm: L. major positive control; Lane Lt: L. tropica positive control. Figure S4. Leishmania spp. identification in Al Ahsa Region by PCR-RFLP analysis of parasite ITS1 region. Lanes 1–13: different examples of Leishmania isolates from Al Ahsa Region; Lane Lt: L. tropica positive control; Lane Lm: L. major positive control. Figure S5. Leishmania spp identification in Asir Region by PCR-RFLP analysis of parasite ITS1 region. Lanes 1–5: different examples of Leishmania isolates from Asir Province. Figure S6. Distribution of Leishmania species (a) and patient response to anti-leishmanial treatment (b) within the Eastern region of Saudi Arabia. The map was created using software ArcGIS 10 (ESRI, Redlands, CA). Figure S7. Distribution of Leishmania species (a) and patient response to anti-leishmanial treatment (b) within the Northwest region of Saudi Arabia. The map was created using software ArcGIS 10 (ESRI, Redlands, CA). Figure S8. Distribution of Leishmania species (a) and patient response to anti-leishmanial treatment (b) within the southwest region of Saudi Arabia. The map was created using software ArcGIS 10 (ESRI, Redlands, CA).
Acknowledgements
We are indebted to colleagues from the Saudi MoH who helped in all aspects related to sample collection, especially to Dr Mohammed Adnan Aldahan and Mr Khalid Alsuhaibani (Riyadh), Mr Ahmed Yassin Almuhanaa (Al Ahsa), Mr Salim Alzubiani and Mr Alkhir Dafeallah (Al Madinah), and Mr Abdulaziz AL Jarallah (Asir). We thank Dr Turki Saud Mohammed Al-Saud (KACST president) for his kindness in allowing access to KACST culture facilities, Dr Lee Haines, Dr Deirdre Walshe and Dr Krishanthi Subramaniam for manuscript editing, Dr Ian Hasting for valuable statistical advice, and Mrs Rabab Al-Malki for artistic work.
Funding
This work was partially funded by a PhD studentship from the Saudi Cultural Bureau (awarded to WSAS); GlycoPar-EU FP7 Marie Curie Initial Training Network (GA. 608295 awarded to ACS and AAS; http://www.ec.europa.eu); Shefa Fund (awarded to AAS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Authorsʼ contributions
WSA contributed to conceiving, designing, conducting and analysing the experiments, design of the study and manuscript writing. CS contributed to conducting, analysing the experiments and manuscript writing. GDW, NAD, LKH, ACS, YA, EJA, AH contributed to conducting and analysing experiments. SMB, KSA, ZA, AMAS contributed to sample collection. MHA, AMA, ZM contributed in study design. AAS contributed to conceiving, designing and analysing experiments, design of the study and manuscript writing, with input from all authors. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethical approval was obtained from both from the Liverpool School of Tropical Medicine, UK (12.03RS) and the Saudi Ministry of Health (MoH) Ethical Committees. All patients provided written informed consent for the collection of biological samples and subsequent analyses. All patient data were anonymized. Furthermore, all research was conducted according to Declaration of Helsinki principles.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- KSA
Kingdom of Saudi Arabia
- CL
cutaneous leishmaniasis
- SSG
sodium stibogluconate
- SI
secondary infections
- EMR
East Mediterranean Region
- MoH
Ministry of Health
Contributor Information
Waleed S. Al-Salem, Email: walsalelm@moh.gov.sa
Carla Solórzano, Email: Carla.SolorzanoGonzalez@lstmed.ac.uk.
Gareth D. Weedall, Email: G.D.Weedall@ljmu.ac.uk
Naomi A. Dyer, naomi223@googlemail.com
Louise Kelly-Hope, Email: Louise.Kelly-Hope@lstmed.ac.uk.
Aitor Casas-Sánchez, Email: Aitor.Casas-Sanchez@lstmed.ac.uk.
Yasser Alraey, Email: Yasser.Alraey@lstmed.ac.uk.
Essam J. Alyamani, Email: eyamani@kacst.edu.sa
Alice Halliday, Email: alice.halliday@bristol.ac.uk.
Salah M. Balghonaim, Email: balghonaim@yahoo.com
Khalid S. Alsohibany, Email: k.b.m_2007@hotmail.com
Zeyad Alzeyadi, Email: bszaa@leeds.ac.uk.
Mohamed H. Alzahrani, Email: abuhassenm@yahoo.com
Ali M. Al-Shahrani, Email: alidoh@hotmail.com
Abdullah M. Assiri, Email: abdullahm.asiri@moh.gov.sa
Ziad Memish, Email: zmemish@yahoo.com.
Álvaro Acosta-Serrano, Email: alvaro.acosta-serrano@lstmed.ac.uk.
References
- 1.Postigo JAR. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int J Antimicrob Agents. 2010;36:S62–S65. doi: 10.1016/j.ijantimicag.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010;7–8:1–186. [PubMed] [Google Scholar]
- 4.Wallace MR, Hale BR, Utz GC, Olson PE, Earhart KC, Thornton SA, et al. Endemic infectious diseases of Afghanistan. Clin Infect Dis. 2002;34:S171–S207. doi: 10.1086/340704. [DOI] [PubMed] [Google Scholar]
- 5.Alasaad S. War diseases revealed by the social media: massive leishmaniasis outbreak in the Syrian Spring. Parasit Vectors. 2013;6:94. doi: 10.1186/1756-3305-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salam N, Al-Shaqha WM, Azzi A. Leishmaniasis in the Middle East: incidence and epidemiology. PLoS Negl Trop Dis. 2014;8:e3208. doi: 10.1371/journal.pntd.0003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saroufim M, Charafeddine K, Issa G, Khalifeh H, Habib RH, Berry A, et al. Ongoing epidemic of cutaneous leishmaniasis among Syrian refugees, Lebanon. Emerg Infect Dis. 2014;20:1712–1715. doi: 10.3201/eid2010.140288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharara SL, Kanj SS. War and infectious diseases: challenges of the Syrian civil war. PLoS Pathog. 2014;10:e1004438. doi: 10.1371/journal.ppat.1004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Salem WS, Pigott DM, Subramaniam K, Haines LR, Kelly-Hope L, Molyneux DH, et al. Cutaneous leishmaniasis and conflict in Syria. Emerg Infect Dis. 2016;22:931–933. doi: 10.3201/eid2205.160042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Salem WS, Ferreira FM, Dyer NA, Alyamani EJ, Balghonaim SM, Al-Mehna AY, et al. Detection of high levels of anti-α-galactosyl antibodies in sera of patients with Old World cutaneous leishmaniasis: a possible tool for diagnosis and biomarker for cure in an elimination setting. Parasitology. 2014;141:1898–1903. doi: 10.1017/S0031182014001607. [DOI] [PubMed] [Google Scholar]
- 11.El-Badry A, Al-Juhani A, Ibrahim E-K, Al-Zubiany S. Distribution of sand flies in El-Nekheil province, in Al-Madinah Al-Munawwarah region, western of Saudi Arabia. Parasitol Res. 2008;103:151–156. doi: 10.1007/s00436-008-0942-3. [DOI] [PubMed] [Google Scholar]
- 12.Al-Zahrani MA, Peters W, Evans DA, Chin C, Smith V, Lane RP. Phlebotomus sergenti, a vector of Leishmania tropica in Saudi Arabia. Trans R Soc Trop Med Hyg. 1988;82:416. doi: 10.1016/0035-9203(88)90142-3. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim EA, Al-Zahrani MA, Al-Tuwaigri AS, Al-Shammary FJ, Evans DA. Leishmania infecting man and wild animals in Saudi Arabia. 9. The black rat (Rattus rattus) a probable reservoir of visceral leishmaniasis in Gizan Province, south-west Saudi Arabia. Trans R Soc Trop Med Hyg. 2017;86:513–514. doi: 10.1016/0035-9203(92)90090-Y. [DOI] [PubMed] [Google Scholar]
- 14.Abuzaid AA, Abdoon AM, Aldahan MA, Alzahrani AG, Alhakeem RF, Asiri AM, et al. Cutaneous leishmaniasis in Saudi Arabia: a comprehensive overview. Vector Borne Zoonotic Dis. 2017;17:673–684. doi: 10.1089/vbz.2017.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Beshbishy HA, Al-Ali KH, El-Badry AA. Molecular characterization of cutaneous leishmaniasis in Al-Madinah Al-Munawarah Province, western Saudi Arabia. Int J Infect Dis. 2013;17:e334–e338. doi: 10.1016/j.ijid.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Dye C, Killick-Kendrick R, Ismail RB, Al-Gindan Y. Zoonotic cutaneous leishmaniasis in Saudi Arabia: results of a preliminary epidemiological survey in Al-Ahsa oasis. Trans R Soc Trop Med Hyg. 1989;83:493–498. doi: 10.1016/0035-9203(89)90265-4. [DOI] [PubMed] [Google Scholar]
- 17.Al-Zahrani MA, Peters W, Evans DA, Smith V, Ching Chin I. Leishmania infecting man and wild animals in Saudi Arabia. 6. Cutaneous leishmaniasis of man in the south-west. Trans R Soc Trop Med Hyg. 1989;83:621–628. doi: 10.1016/0035-9203(89)90376-3. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim EA, Mustafa MB, Al Amri SA, Al-Seghayer SM, Hussein SM, Gradoni L. Meriones libycus (Rodentia: Gerbillidae), a possible reservoir host of zoonotic cutaneous leishmaniasis in Riyadh Province, Saudi Arabia. Trans R Soc Trop Med Hyg. 1994;88:39. doi: 10.1016/0035-9203(94)90488-X. [DOI] [PubMed] [Google Scholar]
- 19.El Sibae MM, Eesa NM, Morsy TA. Rodents and cutaneous leishmaniasis in Qasim, Saudi Arabia. J Egypt Soc Parasitol. 1993;23:667–673. [PubMed] [Google Scholar]
- 20.Khan W, Zakai HA. Epidemiology, pathology and treatment of cutaneous leishmaniasis in Taif region of Saudi Arabia. Iran J Parasitol. 2014;9:365–373. [PMC free article] [PubMed] [Google Scholar]
- 21.El-Sibae MM, Eesa NM. A study on Phlebotomus species, the vectors of leishmaniasis in Gassim, Saudi Arabia. J Egypt Soc Parasitol. 1993;23:231–238. [PubMed] [Google Scholar]
- 22.El-Beshbishy HA, Al-Ali KH, El-Badry AA. Molecular characterization of Leishmania infection in sand flies from Al-Madinah Al-Munawarah Province, western Saudi Arabia. Exp Parasitol. 2013;134:211–215. doi: 10.1016/j.exppara.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Al-Gindan Y, Omer AHS, A-Humaidan Y, Peters W, Evans DA. A case of mucocutaneous leishmaniasis in Saudi Arabia caused by Leishmania major and its response to treatment. Clin Exp Dermatol. 1983;8:185–188. doi: 10.1111/j.1365-2230.1983.tb01763.x. [DOI] [PubMed] [Google Scholar]
- 24.Elbihari S, Kawasmeh ZA, Al-Naiem A, Al-Atiya S. Leishmania infecting man and wild animals in Saudi Arabia. 3. Leishmaniasis in Psammomys obesus Cretzschmar in Al-Ahsa oasis. Trop Med Parasitol. 1987;38:89–92. [PubMed] [Google Scholar]
- 25.Ben Salah A, Ben Messaoud N, Guedri E, Zaatour A, Ben Alaya N, Bettaieb J, et al. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N Engl J Med. 2013;368:524–532. doi: 10.1056/NEJMoa1202657. [DOI] [PubMed] [Google Scholar]
- 26.Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HDF, Presber W, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349–358. doi: 10.1016/S0732-8893(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 27.El Tai NO, Osman OF, El Fari M, Presber W, Schönian G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans R Soc Trop Med Hyg. 2000;94:575–579. doi: 10.1016/S0035-9203(00)90093-2. [DOI] [PubMed] [Google Scholar]
- 28.Al-Tawfiq JA, AbuKhamsin A. Cutaneous leishmaniasis: a 46-year study of the epidemiology and clinical features in Saudi Arabia (1956–2002) Int J Infect Dis. 2004;8:244–250. doi: 10.1016/j.ijid.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Mondragon-Shem K, Al-Salem WS, Kelly-Hope L, Abdeladhim M, Al-Zahrani MH, Valenzuela JG, et al. Severity of Old World cutaneous leishmaniasis is influenced by previous exposure to sandfly bites in Saudi Arabia. PLoS Negl Trop Dis. 2015;9:e0003449. doi: 10.1371/journal.pntd.0003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Qurashi AR, Ghandour AM, Osman M, Al-Juma M. Dissemination in cutaneous leishmaniasis due to Leishmania major in different ethnic groups in Saudi Arabia. Int J Dermatol. 2000;39:832–836. doi: 10.1046/j.1365-4362.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira F, Traoré B, Gomes R, Faye O, Gilmore DC, Keita S, et al. Delayed-type hypersensitivity to sand fly saliva in humans from a leishmaniasis-endemic area of Mali is TH1-mediated and persists to midlife. J Invest Dermatol. 2013;133:452–459. doi: 10.1038/jid.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohoušová I, Subrahmanyam S, Volfová V, Mu J, Volf P, Valenzuela JG, et al. Salivary gland transcriptomes and proteomes of Phlebotomus tobbi and Phlebotomus sergenti, vectors of leishmaniasis. PLoS Negl Trop Dis. 2012;6:e1660. doi: 10.1371/journal.pntd.0001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanden Bossche H, Marichal P, Gorrens J, Coene MC, Willemsens G, Bellens D, et al. Biochemical approaches to selective antifungal activity. Focus on azole antifungals. Mycoses. 1989;32(Suppl. 1):35–52. doi: 10.1111/j.1439-0507.1989.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 34.Lopes MEM, Carneiro MBH, dos Santos LM, Vieira LQ. Indigenous microbiota and leishmaniasis. Parasite Immunol. 2016;38:37–44. doi: 10.1111/pim.12279. [DOI] [PubMed] [Google Scholar]
- 35.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3:e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machado PR, Ampuero J, Guimarães LH, Villasboas L, Rocha AT, Schriefer A, et al. Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl Trop Dis. 2010;4:e912. doi: 10.1371/journal.pntd.0000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Cohort characteristics and relevant clinical features.
Additional file 2: Figure S1. Scheme representing the current leishmaniasis treatment policy in KSA. Figure S2. Leishmania spp. identification in Central Region by PCR-RFLP analysis of parasite ITS1 region. Lane Lt: L. tropica positive control; Lane Lm: L. major positive control; Lanes 1–10: different examples of Leishmania isolates from Rass, Dwadmi and Muzahmyyah. Figure S3. Leishmania spp identification in Al Madinah Province by PCR-RFLP analysis of parasite ITS1 region. Lanes 1–4: different examples of Leishmania isolates from Aljadaida and Sulailah, Al Madinah Province; Lanes 1 and 2: L. tropica samples; Lanes 3 and 4: L. major samples; Lane Lm: L. major positive control; Lane Lt: L. tropica positive control. Figure S4. Leishmania spp. identification in Al Ahsa Region by PCR-RFLP analysis of parasite ITS1 region. Lanes 1–13: different examples of Leishmania isolates from Al Ahsa Region; Lane Lt: L. tropica positive control; Lane Lm: L. major positive control. Figure S5. Leishmania spp identification in Asir Region by PCR-RFLP analysis of parasite ITS1 region. Lanes 1–5: different examples of Leishmania isolates from Asir Province. Figure S6. Distribution of Leishmania species (a) and patient response to anti-leishmanial treatment (b) within the Eastern region of Saudi Arabia. The map was created using software ArcGIS 10 (ESRI, Redlands, CA). Figure S7. Distribution of Leishmania species (a) and patient response to anti-leishmanial treatment (b) within the Northwest region of Saudi Arabia. The map was created using software ArcGIS 10 (ESRI, Redlands, CA). Figure S8. Distribution of Leishmania species (a) and patient response to anti-leishmanial treatment (b) within the southwest region of Saudi Arabia. The map was created using software ArcGIS 10 (ESRI, Redlands, CA).
Data Availability Statement
All data generated or analysed during this study are included in this published article.