Abstract
Background
Diarrheal disease still remains a major public health threat and is often associated with fatal outcome especially in children with shigellosis mostly in developing countries. This study aimed to determine the presence of any associations between drinking shallow tube well (STW) water and childhood shigellosis. A total of 1394 children aged 0–59 months who presented with moderate-to-severe diarrhea (MSD) in Kumudini Women’s Medical College and Hospital, Bangladesh, from December 2007 to March 2011 were enrolled into the study.
Results
Among the study children, STW users often represented poor families (44% vs. 37%, p = 0.010); less often had household electricity (60% vs. 68%, p = 0.001) and cemented floor material (12% vs. 21%, p < 0.001); washed hand before eating (79% vs. 84%, p = 0.020); and had Shigella sonnei infections (7.8% vs. 13.1, p = 0.002) compared to deep tube well (DTW) water families (in bivariate analysis). After adjusting for covariates, a significant negative association was observed between childhood MSD episodes due to Shigella sonnei infections and the use of STW water (aOR 0.53, 95% CI 0.36, 0.79).
Conclusions
An emergence of less severe Shigella sonnei has replaced relatively more severe Shigella flexneri among the MSD children from DTW-user families. However, more monitoring in terms of disease surveillance for changes in the distribution of Shigella serogroups and serotypes and its upsurges and antimicrobial susceptibility is essential.
Keywords: Bangladesh, Shigella, Shigellosis, Tube well water, Under-five children
Background
Recently, global estimates attribute that in 2016, diarrhea accounted for 9% of 5.6 million deaths in children less than 5 years of age (U5s) worldwide making it the second leading cause of child mortality [1]. The highest rates of child mortality are in sub-Saharan Africa and Southeast Asia [1]. In 2013, shigellosis was responsible for 28,000–48,000 deaths annually among those under 5 years [2, 3].
Shigella transmission occurs via the fecal-oral route, person-to-person contact, household flies, infected water, and inanimate objects [4]. The minimal infectious dose can be transmitted directly from contaminated fingers since intermediate bacterial replication is not required to achieve the low infectious dose [2]. Shigellosis occurs predominantly in developing countries due to overcrowding and poor sanitation [4]. According to the Bangladesh Demographic and Health Survey (BDHS) report (2014), rural Bangladesh enjoys universal access to an improved source of drinking water (97%). A tube well is virtually the only source of drinking water (94%). Other infrequent sources of drinking water in rural Bangladesh are protected well (< 1%), rain water (< 1%), bottled water (< 1%), water piped into dwelling (< 1%), water piped to yard/plot (< 1%), and public tap/standpipe (< 1%) [5]. In the case of 74% of households, the drinking water source is located within the household premise, costs are affordable, and typically less than 45 m deep, it is known to be a shallow tube well (STW). In rural areas, because of distance and other contextual factors, more time is needed in collecting water for drinking purposes than urban Bangladesh. According to the BDHS (2014) report, one in five households spends less than 30 min on foot in two-way trips in collecting water for drinking purposes [5]. Other tube wells are found in agriculture fields, and available water is used for irrigation and household consumption by family members and called deep tube wells (DTW; ≥ 45 m deep). Studies have indicated that about 50% of the water samples collected from STW in Bangladesh was contaminated with human fecal organisms [6–8]. Due to their shallowness, STW water is prone to have contamination from leakage of neighborhood polluted water [6]. More generally, fecal contamination of shallow groundwater could possibly be one of the important reasons for the prevalence of diarrheal disease in Bangladesh [7, 8]. In many cases, immediate environmental conditions are unfavorable, e.g., the distance of tube wells from latrines or sewage-contaminated ponds or tanks may be insufficient to avoid the contamination of the well water with human-pathogenic bacteria. Tube wells have failed to protect against gastrointestinal diseases in Bangladesh, despite regular use of tube well water for drinking [9]. Recent studies in Bangladesh have demonstrated that up to 65% of tube wells can contain indicators of fecal contamination such as fecal/thermotolerant coliforms and Escherichia coli (E. coli); the level of contamination, however, is typically low [6–8, 10–14]. Fecal pathogens including rotavirus, adenovirus, Shigella, Vibrio cholerae, and enterotoxigenic E. coli have also been detected in tube well water [10–13]. Among the microbiological contamination, Bangladesh has the highest rates of shigellosis reported by two recent multi-country studies [15].
The species distribution of Shigella [Shigella flexneri (S. flexneri), Shigella boydii (S. boydii), Shigella dysenteriae (S. dysenteriae), and Shigella sonnei (S. sonnei)] varies globally. The first three are often prevalent in developing countries while S. sonnei (least virulent Shigella bacterium) is common in developed countries and usually causes a self-limiting febrile watery diarrhea [8, 16–18]. Shigella boydii causes disease of intermediate severity and is least common of the four. S. flexneri is less virulent than S. dysenteriae type 1 (the epidemic strain that causes severe life-threatening disease) but can also cause bloody diarrhea and abdominal cramps [17–19], whereas S. flexneri cases have been observed to report to the facility with increased number of days of the illness at home, higher numbers of episodes of diarrhea, and longer duration of mucoid/bloody diarrhea as well as hospitalization than S. sonnei [20].
Changing patterns in the distribution of Shigella serogroups and serotypes have been reported in Bangladesh [21]. In recent years, changes in Shigella serogroups, their geographical diversity, and emergence of S. sonnei have been reported in Bangladesh [21]. These changes have been indicated by researchers to be due to improved sanitary and hygienic practices, better living environment, improved nutritional status of children, and better access to safe drinking water. As shigellosis is a leading spectrum of diarrhea among the children presenting to the study sentinel health facility (SHC) in the present rural study community, this study aimed to determine any association between drinking STW water and childhood shigellosis [22] after adjusting for potential confounding impact of improved sanitary and hygienic practices, better living environment, improved nutritional status of children, and better access to safe drinking water. We hypothesized that because of the high proportion of households using STW water, there is an association between prevalence of childhood shigellosis particularly due to S. sonnei serogroup and STW water use in the Mirzapur community of rural Bangladesh.
Methods
Study site
For this secondary data analysis, relevant data were extracted from the database of the Global Enteric Multicenter Study (GEMS), Bangladesh site [22]. For this comparative study, all under-5 children from the STW water-using families comprised the study group and children from DTW-user households represented the comparison group [23]. The GEMS Bangladesh site was in a rural community of Bangladesh, located in Mirzapur sub-district of Tangail district, 60 km north of Dhaka, the capital city. The study had a backup of an ongoing demographic surveillance system (DSS). The sentinel health facility of the study was known as Kumudini Women’s Medical College and Hospital (KWMCH) (750 beds) which was situated in the middle of the study DSS area. All study participants (under-5 children with moderate-to-severe diarrhea (MSD) from the DSS area) were enrolled in the study in a sentinel health facility [22].
Study design and enrollment of study participants
The GEMS study during December 2007 to February 2011 followed a case-control cohort design. Under-5 children, residents of the DSS catchment area, presenting with MSD (fulfilling at least one of the following criteria: sunken eyes, loss of skin turgor, intravenous rehydration administered or prescribed, dysentery—visible blood in loose stools, or admission to hospital with diarrhea or dysentery) within 7 days of onset of an acute illness (onset after ≥ 7 diarrhea-free days) constituted as GEMS cases. In this study, parents or primary caretakers of patients only with MSD underwent standardized interviews to solicit demographic, epidemiological, and clinical information at enrollment. Nutritional assessments (weight, length/height, and midupper arm circumference (MUAC)) were performed at the time of enrollment (before rehydration) and after rehydration, and z-scores were calculated and categorized as underweight (weight-for-age z-score < − 2), stunting (height-for-age z-score < − 2), and wasting (weight-for-height z-score < − 2) following the WHO guideline [2].
Specimen collection and laboratory procedure
A single fresh stool (minimum of 3 g) was collected from each enrolled case child in the facility which within 1 h of passage was placed in a cold storage until delivery to the laboratory. Additionally, two rectal swabs for bacterial culture pending passage of the whole stool were obtained only when antibiotics were given to patients before stool was produced. All stool samples were shifted to the Clinical Microbiology Laboratory of International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, as per standard guidelines. Bacterial pathogens [Salmonella, Shigella, Campylobacter, Aeromonas spp., Vibrio cholerae, and Escherichia coli (enterotoxigenic, enteropathogenic, and enteroaggregative)], viruses (rotavirus, norovirus, sapovirus, astrovirus, and adenovirus), and protozoa (Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp.) were detected following standard laboratory methods [16].
Data analysis
Statistical Package for Social Sciences (SPSS) Windows (Version 20, Chicago, IL) was used for data analysis, and Epi Info (Version 7.0) was used to calculate unadjusted odds ratios. Statistical analyses included descriptive as well as analytic methods. For the categorical variable of interest, the significance of differences was evaluated by chi-square (χ2) test. Odds ratios (ORs) were calculated to assess the association between STW water use and the independent variables of interest. ORs also indicated the strength of association; in addition to ORs, their 95% confidence intervals (CIs) were also estimated. Principal component was categorized and performed to determine wealth quintiles (by using household assets), assuming that factor loadings for certain household assets may vary through the years. Variables that were studied are construction material of the wall, roof, and floor of the house and household assets like radio, television, cell phone, and table. The wealth index was used as a measure of socio-economic status (SES) using information on household possessions. A weight was attached to each item from the first principal component. The households were classified into SES quintiles based on the wealth index: quintile (poor, lower middle, middle, upper middle, and rich).
Before performing logistic regression model, we also checked multicollinearity between independent variables using variance inflation factor (VIF). In the final model, the VIF values of all independent variables were less than 2 and the mean VIF was 1.17. To estimate ORs of several variables (selected on the basis of either statistical significance or biological importance), we used a multiple logistic regression model using forward elimination and taking tube well water use status as a dependent variable (coded as 1 = shallow tube well water use and 0 = deep tube well water use) and primary caretaker education (illiterate, i.e., no formal schooling = 1, literate = 0); number of living room in the household (1–4 = 1, > 4 = 0); floor material (earth/soil/non-cemented = 1, cemented = 0); household electricity (no = 1, yes = 0); treatment of drinking water (no = 1, yes = 0); treatment method (1 = filter through cloth, 0 = use of water filter); container observed to be covered (no = 1, yes = 0); hand wash use material (water only = 1, water and soap = 0); hand washing before eating (no = 1, yes = 0); cooking (no = 1, yes = 0); nursing (no = 1, yes = 0); after defecation (no = 1, yes = 0); place of feces disposal (traditional pit toilet = 1, pour flush = 0); nutritional status (stunting = < − 2.00 = 1, no stunting = 0; underweight = < − 2.00 = 1, no underweight = 0; wasting < − 2.00 = 1, no wasting = 0); wealth quintile (poor = 1, rich = 0); age (12–59 months = 1, 0–11 months = 0); and sex (girl = 1, boy = 0) as independent variables. The P value cutoff of 0.1 (the significance level of variables for inclusion) was considered adequate to prevent residual confounding in the forward step-wise logistic regression model [23–25]. Statistical significance to remain in the final multivariable model was set at < 0.05.
Results
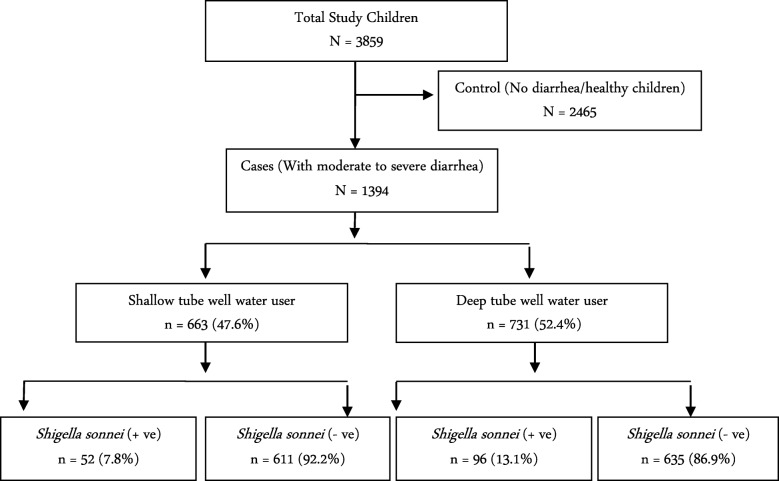
Of the overall 3859 children enrolled during the study period, 1394 had MSD (and their data were analyzed in this study) and 2465 had no diarrhea. Among the MSD children, 47.6% families (n = 663) were a user of STW, while 52.4% (n = 731) represented DTW water-user families (Fig. 1).
Fig. 1.
Study profile of enrolled children (cases with moderate-to-severe diarrhea)
Severity of diarrheal illness and dehydration status were found similar in STW- and DTW-user families (Table 1). The study classified all the children into three age groups: 550 children aged 0–11 months, 476 children aged 12–23 months, and 368 children aged 24–59 months. Age and nutritional status of study children were identical in two groups. Among the MSD children, 814 were boys and 580 were girls. We did not find any association between gender and tube well water use. STW users often represented poor families than DTW users. The number of sleeping rooms in the house, presence of household electricity, cemented floor material, and practice of washing hands before eating and nursing were observed significantly less often among the STW-using household members than their counterparts from DTW-user families (Table 2). Shigella sonnei infection was detected less frequently in stool specimens of children from STW-user families than DTW-user family (7.8% vs. 13.1%; p = 0.002) (Table 3).
Table 1.
Enrollment features of study children
| Variables | Shallow tube well water user (N = 663), n (%) | Deep tube well water user (N = 731), n (%) | p value* |
|---|---|---|---|
| Sunken eye | 108 (16.3) | 112 (15.3) | 0.673 |
| Loss of skin turgor | 35 (5.3) | 26 (3.6) | 0.150 |
| Intravenous rehydration | 77 (11.6) | 88 (12.0) | 0.871 |
| Dysentery | 478 (72.1) | 551 (75.4) | 0.183 |
| Hospitalized | 145 (21.9) | 138 (18.9) | 0.186 |
*p value stands for chi-square tests which were performed to examine the presence of significant association
Table 2.
Characteristics of under-5 moderate-to-severe diarrheal children from the two types of tube well water-user families
| Variables | Shallow tube well (N = 663), n (%) | Deep tube well (N = 731), n (%) | Unadjusted OR (95% CI) | p value |
|---|---|---|---|---|
| Age (month) | ||||
| 0–11 | 264 (39.8) | 286 (39.1) | 1.03 [0.83, 1.27] | 0.957 |
| 12–23 | 226 (34.1) | 250 (34.2) | 0.99 [0.79, 1.24] | |
| 24–59 | 173 (26.1) | 195 (26.7) | 0.97 [0.76, 1.23] | |
| Gender | ||||
| Girl | 277 (41.8) | 303 (41.5) | 1.01 [0.81, 1.25] | 0.944 |
| Boy | 386 (58.2) | 428 (58.5) | ||
| Maternal education | ||||
| Illiterate | 79 (11.9) | 78 (10.7) | 1.13 [0.81, 1.57] | 0.516 |
| Literate | 584 (88.1) | 653 (89.3) | ||
| Sleeping room | ||||
| 1–4 | 630 (95.0) | 666 (91.1) | 1.86 [1.21, 2.87] | 0.005* |
| 5–10 | 33 (5.0) | 65 (8.9) | ||
| Floor | ||||
| Earth | 582 (87.8) | 578 (79.1) | 1.90 [1.41, 2.54] | < 0.001* |
| Cemented | 81 (12.2) | 153 (20.9) | ||
| Electricity | ||||
| No | 268 (40.4) | 233 (31.9) | 1.45 [1.16, 1.80] | 0.001* |
| Yes | 395 (59.6) | 498 (68.1) | ||
| Water trips daily | ||||
| 1–4 | 348 (53.0) | 386 (52.8) | 0.99 [0.80, 1.22] | 0.994 |
| 5–26 | 309 (47.0) | 345 (47.2) | ||
| Treating method | ||||
| Through cloth | 628 (94.7) | 721 (98.6) | 0.24 [0.12, 0.50] | < 0.001* |
| Filter | 35 (5.3) | 10 (1.4) | ||
| Treat drinking water | ||||
| No | 615 (92.8) | 718 (98.2) | 0.23 [0.12, 0.43] | < 0.001* |
| Yes | 48 (7.2) | 13 (1.8) | ||
| Type of container | ||||
| Wide mouth container | 633 (99.5) | 700 (99.4) | 1.20 [0.26, 5.40] | 1.000 |
| Narrow mouth container | 3 (0.5) | 4 (0.6) | ||
| Container covered | ||||
| No | 363 (57.1) | 384 (54.5) | 1.10 [0.89, 1.37] | 0.381 |
| Yes | 273 (42.9) | 320 (45.5) | ||
| Hand wash use | ||||
| Water with ash and mud | 88 (13.3) | 84 (11.5) | 1.17 [0.86, 1.62] | 0.353 |
| Water and soap only | 575 (86.7) | 647 (88.5) | ||
| Hand washing practice | ||||
| Before eating | ||||
| No | 139 (21.0) | 117 (16.0) | 1.39 [1.06, 1.82] | 0.020* |
| Yes | 524 (79.0) | 614 (84.0) | ||
| Before cooking | ||||
| No | 292 (44.0) | 328 (44.9) | 0.96 [0.78, 1.19] | 0.797 |
| Yes | 371 (56.0) | 403 (55.1) | ||
| Before nursing | ||||
| No | 506 (76.3) | 521 (71.3) | 1.29 [1.02, 1.65] | 0.038* |
| Yes | 157 (23.7) | 210 (28.7) | ||
| After defecation | ||||
| No | 108 (16.3) | 122 (16.7) | 0.97 [0.73, 1.29] | 0.897 |
| Yes | 555 (83.7) | 609 (83.3) | ||
| Fecal dispose | ||||
| Traditional pit toilet | 368 (55.5) | 438 (59.9) | 0.83 [0.67, 1.03] | 0.107 |
| Pour flush toilet | 295 (44.5) | 293 (40.1) | ||
| Nutritional status | ||||
| % stunting | 156 (23.5) | 179 (24.5) | 0.95 [0.74, 1.21] | 0.722 |
| % underweight | 221 (33.3) | 232 (31.7) | 1.07 [0.86, 1.35] | 0.563 |
| % wasting | 141 (21.3) | 154 (21.1) | 1.01 [0.78, 1.31] | 0.980 |
| Wealth quintile | ||||
| Poor | 291 (43.9) | 270 (36.9) | 1.33 [1.08, 1.66] | 0.010* |
| Rich | 372 (56.1) | 461 (63.1) | ||
OR odds ratio, CI confidence interval, *p value statistically significant at < 0.05
Table 3.
Pathogen distribution among under-5 moderate-to-severe diarrheal children from the two types of tube well water user families
| Pathogen variables | Shallow tube well water user (N = 663), n (%) | Deep tube well water user (N = 731), n (%) | Unadjusted ORs (95% CI) | p value |
|---|---|---|---|---|
| Shigella spp. | ||||
| Yes | 266 (40.1) | 325 (44.5) | 0.84 [0.68, 1.04] | 0.113 |
| No | 397 (59.9) | 406 (55.5) | ||
| S. flexneri | ||||
| Yes | 195 (29.3) | 206 (27.8) | 1.06 [0.84, 1.34] | 0.654 |
| No | 468 (70.7) | 525 (72.2) | ||
| S. sonnei | ||||
| Yes | 52 (7.8) | 96 (13.1) | 0.56 [0.39, 0.80] | 0.002* |
| No | 611 (92.2) | 635 (86.9) | ||
| S. boydii | ||||
| Yes | 7 (1.1) | 15 (2.1) | 0.43 [0.17, 1.05] | 0.364 |
| No | 656 (98.9) | 716 (97.9) | ||
| S. dysenteriae | ||||
| Yes | 4 (0.6) | 10 (1.4) | 0.44 [0.14, 1.46] | 0.246 |
| No | 659 (99.4) | 721 (98.6) | ||
| V. cholerae | ||||
| Yes | 10 | 9 | 0.99 [0.40, 2.46] | 1.000 |
| No | 721 | 654 | ||
| Salmonella | ||||
| Yes | 17 | 25 | 1.65 [0.88, 3.07] | 0.120 |
| No | 714 | 638 | ||
| ETEC | ||||
| Yes | 27 (4.1) | 38 (5.2) | 0.77 [0.47, 1.28] | 0.385 |
| No | 693 (94.8) | 636 (95.9) | ||
| EPEC | ||||
| Yes | 69 | 65 | 1.19 [0.83, 1.69] | 0.385 |
| No | 594 | 666 | ||
| EAEC | ||||
| Yes | 155 (23.4) | 178 (24.4) | 0.95 [0.74, 1.21] | 0.717 |
| No | 508 (76.6) | 553 (75.6) | ||
| Aeromonas | ||||
| Yes | 170 (25.6) | 186 (25.4) | 1.01 [0.79, 1.28] | 0.982 |
| No | 493 (74.4) | 545 (74.6) | ||
| Campylobacter | ||||
| Yes | 115 (17.3) | 131 (17.9) | 0.96 [0.73, 1.26] | 0.833 |
| No | 548 (82.7) | 600 (82.1) | ||
| Rotavirus | ||||
| Yes | 113 (17.0) | 106 (14.5) | 1.21 [0.91, 1.62] | 0.219 |
| No | 550 (83.0) | 625 (85.5) | ||
| Norovirus | ||||
| Yes | 44 (6.6) | 59 (8.1) | 0.81 [0.540, 1.21] | 0.358 |
| No | 619 (93.4) | 672 (91.9) | ||
| Adenovirus | ||||
| Yes | 20 (3.0) | 30 (4.0) | 0.73 [0.41, 1.29] | 0.344 |
| No | 643 (97.0) | 701 (96.0) | ||
| Astrovirus | ||||
| Yes | 8 (1.2) | 9 (1.2) | 0.98 [0.37, 2.55] | 1.000 |
| No | 655 (98.8) | 722 (98.8) | ||
| Sapovirus | ||||
| Yes | 10 (1.5) | 9 (1.2) | 1.22 [0.49, 3.04] | 0.830 |
| No | 653 (98.5) | 722 (98.8) | ||
| Cryptosporidium | ||||
| Yes | 49 (7.4) | 49 (6.7) | 1.11 [0.74, 1.67] | 0.692 |
| No | 614 (92.6) | 682 (93.3) | ||
| Giardia | ||||
| Yes | 51 (7.7) | 55 (7.7) | 1.02 [0.69, 1.52] | 0.986 |
| No | 612 (92.3) | 676 (92.5) | ||
| E. histolytica | ||||
| Yes | 51 (7.7) | 42 (5.7) | 1.37 [0.89, 2.08] | 0.178 |
| No | 612 (92.3) | 689 (94.3) | ||
OR odds ratio, CI confidence interval, *p value statistically significant at < 0.05
The association of S. sonnei infection in STW water user group was further ascertained by regression analysis controlling for other variables. And we observed that children with S. sonnei infection were negatively associated with STW water use (aOR 0.55, 95% CI 0.37, 0.80) (Table 4). S. sonnei infection was also found positively associated with older children (age 12–59 months) (aOR 2.34, 95% CI 1.84, 2.97), not covering drinking water container (aOR 1.45, 95% CI 1.00, 2.10), and non-use of soap during hand washing (aOR 2.17, 95% CI 1.34, 3.49) (Table 4).
Table 4.
Association between Shigella sonnei infection, tube well use, and other factors in rural Mirzapur (number of subjects: S. sonnei + ve, n = 148; S. sonnei − ve, n = 1246, total, N = 1394)
| Variables | Unadjusted ORs | 95% CI | Adjusted ORs | 95% CI | p value* |
|---|---|---|---|---|---|
| Shallow tube well | 0.56 | 0.39–0.80 | 0.55 | 0.37–0.80 | 0.002* |
| Age (12–59 months) | 5.04 | 3.07–8.27 | 2.34 | 1.84–2.97 | < 0.001* |
| Gender (girl) | 0.93 | 0.65–1.32 | 0.89 | 0.62–1.28 | 0.553 |
| Wealth index (poor) | 0.57 | 0.39–0.83 | 0.80 | 0.47–1.36 | 0.419 |
| No maternal education | 0.42 | 0.20–0.87 | 0.51 | 0.24–1.16 | 0.093 |
| Household sleeping room (< 4) | 0.58 | 0.33–1.02 | 0.75 | 0.40–1.40 | 0.370 |
| Household floor (earth/mud) | 0.73 | 0.48–1.12 | 0.94 | 0.58–1.53 | 0.819 |
| No household electricity | 0.56 | 0.38–0.82 | 0.70 | 0.41–1.18 | 0.188 |
| No treatment of drinking water | 0.77 | 0.36–1.66 | 0.67 | 0.30–1.52 | 0.344 |
| Did not cover drinking water container | 1.28 | 0.90–1.82 | 1.45 | 1.00–2.10 | 0.048* |
| Did not use soap during hand washing | 1.78 | 1.14–2.79 | 2.17 | 1.34–3.49 | 0.001* |
| Did not wash hand before eating | 0.89 | 0.56–1.40 | 1.04 | 0.64–1.68 | 0.852 |
| Did not wash hand before cooking | 0.94 | 0.67–1.33 | 0.90 | 0.62–1.29 | 0.569 |
| Did not wash hand before nursing | 0.92 | 0.63–1.35 | 0.88 | 0.58–1.33 | 0.551 |
| Did not wash hand after defecation | 0.67 | 0.40–1.12 | 0.58 | 0.34–1.01 | 0.057 |
| Child feces disposal (pour flush toilet) | 1.26 | 0.88–1.79 | 1.06 | 0.71–1.58 | 0.751 |
| Weight for height z-score (wasting) | 1.07 | 0.71–1.62 | 1.02 | 0.57–1.74 | 0.995 |
| Height for age z-score (stunted) | 0.85 | 0.56–1.29 | 0.89 | 0.53–1.47 | 0.654 |
| Weight for age z-score (underweight) | 0.89 | 0.62–1.29 | 0.78 | 0.44–1.38 | 0.406 |
OR odds ratio, CI confidence interval
*p values are of adjusted ORs and statistically significant at < 0.05
Discussion
We hypothesized that because of higher proportion households using STW water, there would be an association between greater prevalence of childhood shigellosis particularly due to the S. sonnei serogroup among STW water-user families in the Mirzapur community of rural Bangladesh. However, our results refuted the hypothesis. We know that shigellosis is typically associated with poverty, poor hygiene, and crowded living conditions in developing countries, as well as an important contributor to childhood malnutrition. Shigellosis can occur even after ingestion of low inoculums (10–100 organisms) [26]. Usually, it is transmitted by fecal-oral route due to contamination of the hands, food, and water with infected feces. Incidence is higher in children 1–5 years of age, presumably as good personal hygiene is much more difficult to achieve in young children particularly who have not yet acquired specific immunity [27]. In our study, we found poor hygiene practices especially covering of water container, use of hand washing substances, and children from poor families are highly vulnerable to childhood shigellosis. Alternatively, we did not find any significant association between S. flexneri, S. boydii, and S. dysenteriae infections and STW water use in children presenting with MSD episodes to the study sentinel health center. Moreover, we also did not find any noteworthy relationship between non-shigella species (Salmonella, Campylobacter, Aeromonas spp., V. cholerae, E.coli, rotavirus, astrovirus, adenovirus, norovirus, sapovirus, Giardia lamblia, Cryptosporidium, and E. histolytica) and STW water. Surprisingly, we observed a protective relationship between S. sonnei infections and STW water use for the first time in Bangladesh. Additionally, tube well water use impacted positively by demonstrating an emergence of less severe Shigella strain known to be as S. sonnei replacing a more severe Shigella strain called as S. flexneri among the MSD children from DTW-using families. Bangladesh has made adequate progress over recent years, and despite poverty, the economy is growing by about 6% each year. Such gross economic improvement has demonstrated concurrent positive behavioral changes like more access to safe drinking water.
Shigella spp. are dynamic and able to survive under diverse environmental conditions [28]. Shigellosis due to S. sonnei has been previously reported to occur less often among people living in developing countries. In this study, we revealed isolation of S. sonnei more commonly from MSD children reporting from DTW water-user families than STW water users. Infections caused by S. sonnei have become more common than those due to S. flexneri in a population that are reasonably well-off and living with improved water and sanitation practices. Such emergence of S. sonnei has also been reported even in Bangladesh in recent years [29, 30]. The relation between upsurge of S. sonnei infections among the MSD children and economic development may be explained by the less exposure of individuals from developing countries including Bangladesh to Plesiomonas shigelloides (P. shigelloides) in recent years [15].
P. shigelloides is a gram-negative bacterium often found in surface water that shares antigens with S. sonnei, and P. shigelloides has been a frequently isolated enteric pathogen from young diarrhea children aged less than 2 years old [31–33]. It is well recognized that exposure to P. shigelloides, through contaminated drinking water may immunize people to S. sonnei in the developing countries where people are often exposed to contaminated water drink [30]. With economic progress and accompanying water quality improvements (as a result, there is less exposure to P. shigelloides), susceptibility to Shigella sonnei in this population may have increased [28, 30]. The gross economic improvement in Bangladesh might have a positive impact with an increase in the use of DTW and bottled water, and the individuals in general have increasing access to improved piped water supply regularly in urban areas than in rural areas [21]. The changing trend as observed by the emergence of Shigella sonnei may be a reflection of this shifting phenomenon. However, such changes may require more monitoring in terms of disease surveillance for diversity in distribution of serogroups as well as serotypes, upsurges of cases of shigellosis, and antimicrobial susceptibility.
In this study, our strengths were the pre-set inclusion criteria for enrollment of children into the study which is undergoing fairly rapid economic development; identification of pathogens following standardized laboratory methods; and use of a large data set for this analysis. However, the study limitations were the lack of information like whether the areas of DTW were used to get often flooded, toilets of STW-using families were more hygienic, or garbage disposal areas were near the DTW than STW as well as water storing behavior of the family members of DTW users and so on. Moreover, our observations were based only on children with MSD and attending the KWMCH which might not represent the general population in a community including those with less severe disease and those who did not report to the sentinel health center.
Conclusions
Our findings suggest that the emergence of less severe Shigella sonnei has replaced relatively more severe Shigella flexneri among the moderate-to-severe diarrhea children from DTW-user families. These observations signify the impact of gross economic improvement in Bangladesh and concurrent behavioral changes—more access to safe drinking water which impacted positively by demonstrating the emergence of Shigella sonnei replacing the Shigella strain known as Shigella flexneri. Therefore, further interventions are needed in the domestic domain to reduce any spread of shigellosis by water treatment and hygiene practices.
Acknowledgements
This study was conducted in collaboration with the Center for Vaccine Development, University of Maryland School of Medicine, USA. For conducting this study, sincere thanks and acknowledgement are given to the generous support of Kumudini Women’s Medical College and Hospital. And finally, earnest thanks to the family members of the participating children.
Funding
This study received a grant from the Bill & Melinda Gates Foundation.
Availability of data and materials
The dataset and materials of this study are available through the corresponding author and can be accessed on reasonable request.
Abbreviations
- DSS
Demographic surveillance system
- DTW
Deep tube well
- GEMS
Global Enteric Multicenter Study
- icddr,b
International Centre For Diarrhoeal Disease Research, Bangladesh
- KWMCH
Kumudini Women’s Medical College and Hospital
- MSD
Moderate-to-severe diarrhea
- MUAC
Midupper arm circumference
- SHC
Sentinel health facility
- STW
Shallow tube well
- WHO
World Health Organization
Authors’ contributions
YJ conceptualized the study, contributed to the data preparation, interpreted the results, drafted the original manuscript, and revised the manuscript. SH and YJ prepared the data, interpreted the results, and revised the manuscript. MM, MMR, FF, SA, SKD, MIH, ASGF, and MJC provided advice on the data preparation and interpretation and critically revised the manuscript. All authors read and approved the final manuscript as submitted.
Ethics approval and consent to participate
The study protocol approval was given by the Institutional Review Board of the University of Maryland, USA, and also by the Research Review Committee and Ethical Review Committee of icddr,b. Prior to enrollment into the study, mothers/caregivers of eligible under-5 children were verbally informed about the study objectives as well as the protocol itself; only those who gave consent voluntarily were enrolled after providing stool specimen. Then, mothers/caregivers of study children were interviewed.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yasmin Jahan, Email: d160207@hiroshima-u.ac.jp.
Michiko Moriyama, Email: morimich@hiroshima-u.ac.jp.
Soroar Hossain, Email: ihossain@icddrb.org.
Md. Moshiur Rahman, Email: moshiur@hiroshima-u.ac.jp.
Shahnawaz Ahmed, Email: shahnawz@icddrb.org.
Sumon Kumar Das, Email: sumon.das@menzies.edu.au.
Md. Iqbal Hossain, Phone: (88-02) 9827104, Email: ihossain@icddrb.org.
Abu Syed Golam Faruque, Email: gfaruque@icddrb.org.
Mohammod Jobayer Chisti, Email: jhum01712@yahoo.com.
References
- 1.UNICEF. Diarrhoeal disease. 2018.
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet (London, England) 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8(9):e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village: American Academy of Pediatrics; 2015. Available on https://redbook.solutions.aap.org/DocumentLibrary/Red%20Book%202015%201.pdf. Accessed on Sept 2018.
- 5.Sankoh O, Welaga P, Debpuur C, Zandoh C, Gyaase S, Poma MA, Mutua MK, Hanifi SM, Martins C, Nebie E, Kagoné M, Emina JB, et al. Bangladesh Demographic and Health Survey, Dhaka. Nat Inst Popul Res Train (NIPORT) 2014;43(3):645–653. doi: 10.1093/ije/dyu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islam MS, Siddika A, Khan MN, et al. Microbiological analysis of tube-well water in a rural area of Bangladesh. Appl Environ Microbiol. 2001;67(7):3328–30. [DOI] [PMC free article] [PubMed]
- 7.Luby S, Islam MS, Johnston R. Chlorine spot treatment of flooded tube wells, an efficacy trial. J Appl Microbiol. 2006;100(5):1154–1158. doi: 10.1111/j.1365-2672.2006.02940.x. [DOI] [PubMed] [Google Scholar]
- 8.Luby SP, Gupta SK, Sheikh MA, Johnston RB, Ram PK, Islam MS. Tubewell water quality and predictors of contamination in three flood-prone areas in Bangladesh. J Appl Microbiol. 2008;105(4):1002–1008. doi: 10.1111/j.1365-2672.2008.03826.x. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Khan MR, D'Souza S, Nalin DR. Failure of sanitary wells to protect against cholera and other diarrhoeas in Bangladesh. Lancet (London, England) 1976;2(7976):86–89. doi: 10.1016/S0140-6736(76)92299-6. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson AS, Layton AC, Mailloux BJ, Culligan PJ, Williams DE, Smartt AE, et al. Comparison of fecal indicators with pathogenic bacteria and rotavirus in groundwater. Sci Total Environ. 2012;431:314–322. doi: 10.1016/j.scitotenv.2012.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Geen A, Ahmed KM, Akita Y, Alam MJ, Culligan PJ, Emch M, et al. Fecal contamination of shallow tubewells in Bangladesh inversely related to arsenic. Environ Sci Technol. 2011;45(4):1199–1205. doi: 10.1021/es103192b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoque BA, Hallman K, Levy J, Bouis H, Ali N, Khan F, et al. Rural drinking water at supply and household levels: quality and management. Int J Hyg Environ Health. 2006;209(5):451–460. doi: 10.1016/j.ijheh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Knappett PS, McKay LD, Layton A, Williams DE, Alam MJ, Mailloux BJ, et al. Unsealed tubewells lead to increased fecal contamination of drinking water. J Water Health. 2012;10(4):565–578. doi: 10.2166/wh.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leber JRM, Ahmed KM, Mailloux B, Geen A. Ground water contrasting influence of geology on E. coli and arsenic in aquifers of Bangladesh. Ground Water Assoc. 2011;49:111–123. doi: 10.1111/j.1745-6584.2010.00689.x. [DOI] [PubMed] [Google Scholar]
- 15.von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006;3(9):e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard G, Ahmed MF, Shamsuddin AJ, Mahmud SG, Deere D. Risk assessment of arsenic mitigation options in Bangladesh. J Health Popul Nutr. 2006;24(3):346–355. [PMC free article] [PubMed] [Google Scholar]
- 17.Panchalingam S, Antonio M, Hossain A, Mandomando I, Ochieng B, Oundo J, et al. Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin Infect Dis. 2012;55(Suppl 4):S294–S302. doi: 10.1093/cid/cis754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farshad S, Sheikhi R, Japoni A, Basiri E, Alborzi A. Characterization of Shigella strains in Iran by plasmid profile analysis and PCR amplification of ipa genes. J Clin Microbiol. 2006;44(8):2879–2883. doi: 10.1128/JCM.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sur D, Ramamurthy T, Deen J, Bhattacharya SK. Shigellosis : challenges & management issues. Indian J Med Res. 2004;120(5):454–462. [PubMed] [Google Scholar]
- 20.Vinh H, Nhu NT, Nga TV, Duy PT, Campbell JT, et al. A changing picture of shigellosis in southern Vietnam: shifting species dominance, antimicrobial susceptibility and clinical presentation. BMC infectios disease. 2009;9:204. doi: 10.1186/1471-2334-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keusch GT, Bennish ML. Shigellosis: recent progress, persisting problems and research issues. Pediatr Infect Dis J. 1989;8:713–719. doi: 10.1097/00006454-198910000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Khatun F, Faruque AS, Koeck JL, Olliaro P, Millet P, Paris N, et al. Changing species distribution and antimicrobial susceptibility pattern of Shigella over a 29-year period (1980-2008) Epidemiol Infect. 2011;139(3):446–452. doi: 10.1017/S0950268810001093. [DOI] [PubMed] [Google Scholar]
- 23.Das SK, Ahmed S, Ferdous F, Farzana FD, Chisti MJ, Leung DT, Malek MA, Talukder KA, Bardhan PK, Salam MA, Faruque AS, et al. Changing emergence of Shigella sero-groups in Bangladesh: observation from four different diarrheal disease hospitals. PloS one. 2013;8(4):e62029. doi: 10.1371/journal.pone.0062029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55(Suppl 4):S232–S245. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow NE, Day NE. Statistical methods in cancer research. In: Volume I - The analysis of case-control studies. IARC Sci Publ. 1980;(32):5–338. [PubMed]
- 26.Niyogi SK. Shigellosis. J Microbiol (Seoul, Korea) 2005;43(2):133–143. [PubMed] [Google Scholar]
- 27.Torres AG. Current aspects of Shigella pathogenesis. Rev Latinoam Microbiol. 2004;46(3–4):89–97. [PubMed] [Google Scholar]
- 28.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 29.Budtz-Jorgensen E, Keiding N, Grandjean P, Weihe P. Confounder selection in environmental epidemiology: assessment of health effects of prenatal mercury exposure. Ann Epidemiol. 2007;17(1):27–35. doi: 10.1016/j.annepidem.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Holt KE, Baker S, Weill FX, Holmes EC, Kitchen A, Yu J, et al. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat Genet. 2012;44(9):1056–1059. doi: 10.1038/ng.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sack DA, Hoque AT, Huq A, Etheridge M. Is protection against shigellosis induced by natural infection with Plesiomonas shigelloides? Lancet (London, England) 1994;343(8910):1413–1415. doi: 10.1016/S0140-6736(94)92531-3. [DOI] [PubMed] [Google Scholar]
- 32.Khan AM, Faruque AS, Hossain MS, Sattar S, Fuchs GJ, Salam MA. Plesiomonas shigelloides-associated diarrhoea in Bangladeshi children: a hospital-based surveillance study. J Trop Ped. 2004;50:354–356. doi: 10.1093/tropej/50.6.354. [DOI] [PubMed] [Google Scholar]
- 33.Khan AM, Hossain MS, Khan AI, Chisti MJ, Chowdhury F, Faruque ASG, Salam MA. Bacterial enteropathogens of neonates admitted to an urban diarrhoeal hospital in Bangladesh. J Trop Ped. 2009;55:122–124. doi: 10.1093/tropej/fmn090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset and materials of this study are available through the corresponding author and can be accessed on reasonable request.