Abstract
Background
Reprogramming of fast-to-slow myofiber switch can improve endurance capacity and alleviate fatigue. Accumulating evidence suggests that a muscle-specific microRNA, miR-499 plays a crucial role in myofiber type transition. In this study, we assessed the effects of natural flavonoid myricetin on exercise endurance and muscle fiber constitution, and further investigated the underlying mechanism of myricetin in vivo and in vitro.
Methods
A total of 66 six-week-old male Sprague Dawley rats were divided into non-exercise or exercise groups with/without orally administered myricetin (50 or 150 mg/kg) for 2 or 4 weeks. Time-to-exhaustion, blood biochemical parameters, muscle fiber type proportion, the expression of muscle type decision related genes were measured. Mimic/ inhibitor of miR-499 were transfected into cultured L6 myotubes, the expressions of muscle type decision related genes and mitochondrial respiration capacity were investigated.
Results
Myricetin treatment significantly improved the time-to-exhaustion in trained rats. The enhancement of endurance capacity was associated with an increase of the proportion of slow-twitch myofiber in both soleus and gastrocnemius muscles. Importantly, myricetin treatment amplified the expression of miR-499 and suppressed the expression of Sox6, the down-stream target gene of miR-499, both in vivo and in vitro. Furthermore, inhibition of miR-499 overturned the effects of myricetin on down-regulating Sox6.
Conclusions
Myricetin promoted the reprogramming of fast-to-slow muscle fiber type switch and reinforced the exercise endurance capacity. The precise mechanisms responsible for the effects of myricetin are not resolved but likely involve regulating miR-499/Sox6 axis.
Keywords: Myricetin, Endurance, Muscle fiber type, Myosin heavy chain, miR-499
Introduction
Skeletal muscle is composed of myofibers with different contractile and metabolic properties. The efficiency of muscle contraction depends on gene expression of two isoforms of myosin heavy chain (MHC), α-MHC and β-MHC (also known as Myh6 and Myh7, respectively). Slow-twitch fibers, also known as type I myofiber, predominantly express β-MHC, display oxidative metabolism and high endurance [1]. Evidences have shown that the proportion of type I myofiber correlated with insulin sensitivity [2, 3]. Type I fibers have higher glucose-handling capacity compared with type II fibers, due to higher expressions of insulin receptor, GLUT4, hexokinase II and glycogen synthase [4]. Under certain physiological or pathological stimuli, such as exercise or obesity, the constitution of myofibers swifts from fast-to-slow or slow-to-fast, which are referred to as skeletal muscle plasticity [5].
MicroRNAs (miRNAs) represent a family of noncoding RNA of 21–22 nucleotides in length that regulate the expression of gene involved in developmental and cellular processes, such as skeletal muscle plasticity. Myosin-encoded miRNAs, also known as MyomiRs, have the ability to regulate myosin switching and myofiber identity [6]. Encoded by intron 19 of Myh7b gene, miR-499 is co-expressed with β-MHC and almost exclusively expressed in slow-twitch myofiber. miR-499 played a crucial role in skeletal muscle fast-to-slow reprogramming via suppressing the expression of transcriptional repressors of slow-twitch myofiber gene, such as Sox6 [7, 8]. Research found that the 3′ UTR of Sox6 mRNA contained four conserved target sites for miR-499, which meant that Sox6 was one of the down-stream target genes of miR-499 to modulate muscle fiber type specification and muscle performance [1].
Undoubtedly, exercise is beneficial to enhance physical performance and improve metabolic status. Evidences showed that endurance exercise promoted skeletal muscle fast-to-slow shift while disuse led to slow-to-fast shift [9, 10]. However, exercise may not be practical under certain circumstances, such as physical limitations. Therefore, it’s essential to explore exercise substitutes/mimetics that can achieve analogous health benefits of regular exercise [11, 12]. Recently, synthetic drugs, such as AMPK and PPARβ agonists (AICAR and GW501516, respectively) were found to enhance the proportion of slow-twitch myofiber and amplify mitochondrial function. Although, these drugs are indeed potent to improve endurance capacity, they cannot be applied to human trials due to the uncertainty of their safety [13].
Natural flavonoids possess a wide range of health benefits including promoting physical performance, anti-diabetic and anti-obesity effects [14, 15]. Flavonoid myricetin, derived from vegetables and fruits [16, 17], possesses numerous bioactivities, including anti-inflammatory, anti-diabetic and anti-carcinogen effects [18, 19]. Our previous study revealed that myricetin improved physical performance under hypoxic environment via maintaining mitochondrial biogenesis [20]. Recently myricetin was found to enhance mitochondrial activity to improve physical endurance [21]. However, the precise mechanism for endurance enhancement by myricetin has yet to be fully elucidated.
Here, we investigated the effects of myricetin on endurance capacity and myofiber type transition in SD rats, and explored the underlying mechanisms for these effects in rat L6 myotubes. We illustrated the role of miR-499/Sox6 pathway in myricetin-induced skeletal muscle reprograming and endurance enhancement.
Materials and methods
Chemicals and reagents
Myricetin (DY0103, HPLC ≥98%) was purchased from MUST Biotechnology Co., Ltd. (Chengdu, China) for animal study. For cell experiments, myricetin (70050) and DMSO (D2650) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle medium (DMEM) and horse serum (16050130) were bought from Gibco (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Hyclone Laboratories, Inc. (Logan, UT, USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo (Kumamoto, Japan). mirVana™ miRNA Inhibitors (rno-miR-499-5p, MH11352), mirVanaTM miRNA mimics (rno-miR-499-5p, MC11352), Lipofectamine RNAiMAX Transfection Reagent (13778083) and antibody against PGC-1α (PA5–38021) were bought from Invitrogen (Massachusetts, USA). Antibodies against slow skeletal myosin heavy chain (ab11083), fast skeletal myosin heavy chain (ab91506), Sox6 (ab30455), tnni1 (ab231720) and myoglobin (ab77232) were purchased from Abcam (Cambridge, UK). Antibody against Cytochrome C (Cyt C, sc-13,561) and β-actin (sc-47778) were purchased from Santa Cruz Biotechnology (CA, USA).
Animals and experimental design
A total of 66 six-week-old male Sprague Dawley (SD) rats were purchased from and housed three per cage under controlled conditions of temperature (22 ± 2 °C), humidity (60 ± 5%) and 12 h light/dark cycle in the specific pathogen-free grade room of the Experimental Animal Center of the Army Medical University (Chongqing, China). Food and water were freely available. All protocols involving animals and their care were performed according to institutional guidelines with the approval of the Institutional Animal Care and Use Committee of the Army Medical University.
After one week of acclimatization, 66 male rats were randomized into eleven groups (n = 6 per group): non-exercise group (NE), 50 mg/kg myricetin for 2 weeks (NE-2-MYR50), 150 mg/kg myricetin for 2 weeks (NE-2-MYR150), 50 mg/kg myricetin for 4 weeks (NE-4-MYR50), 150 mg/kg myricetin for 4 weeks (NE-4-MYR150), exercise training for 2 weeks (Ex-2), exercise training for 4 weeks (Ex-4), exercise training for 2 weeks with 50 mg/kg myricetin (Ex-2-MYR50), exercise training for 2 weeks with 150 mg/kg myricetin (Ex-2-MYR150), exercise training for 4 weeks with 50 mg/kg myricetin (Ex-4-MYR50), exercise training for 4 weeks with 150 mg/kg myricetin (Ex-4-MYR150). After dissolved in distilled water (2 mL), myricetin was given by orally gavage once a day, while the other groups were treated with the equal volume of distilled water.
Exercise training and run-to-fatigue experiment
One hour after vehicle or myricetin administration, animals in the Ex-2, Ex-4, Ex-2-MYR50, Ex-2-MYR150, Ex-4-MYR50, Ex-4-MYR150 groups performed a regular running on a motorized treadmill at speed of 20 m/min. They were trained 20 min on the first day, and then increased by 10 min each day, it will not increase when it reached to 60 min, 5 days/week.
At the end of intervention period, all groups performed a strenuous running on the treadmill at a speed of 25 m/min. Exhaustion was determined as the inability to run despite continuous electronic shocking for 20 s. The time from beginning to exhaustion is defined as the time-to-exhaustion (TTE) that is used to assess endurance capacity.
Immediately after the run-to-fatigue exercise, venous blood was collected from the tail of the rats to monitor blood glucose and lactate by Onetouch Ultra (Johnson, USA) and Lactate Scout (EKF, Germany), respectively. After euthanized, soleus (SOL), gastrocnemius (GAS) muscles and liver were quickly removed, washed with saline, and stored at − 80 °C for further analyses.
Immunofluorescence staining
Fresh soleus and gastrocnemius muscles were fixed in 4% paraformaldehyde at 4 °C 24 h, dehydrated with 30% sucrose solution, embedded in paraffin, and then sectioned with 5-μM thickness for immunofluorescence.
Sections were successively immerged into xylene for 15 min, replacing the xylene for another 15 min, anhydrous ethanol for 5 min, replacing the anhydrous ethanol for another 5 min, 85% ethanol for 5 min, and 75% ethanol for 5 min. Then sections were placed in a repairing kit filled with EDTA antigen retrieval buffer (pH 8.0). After blocking in BSA for 30 min, sections were incubated at 4 °C overnight in primary antibodies against slow skeletal myosin heavy chain (1:100) and fast myosin skeletal heavy chain (1:100). The next day, sections were washed with PBS three times followed by incubation with fluorescent-labeled secondary antibodies for 1 h. Then sections were incubated in DAPI Staining Solution for 10 min, washed with PBS for three times, mounted with Antifade Mounting Medium and photographed under MultiPhoto Laser Scanning Microscopy (Zeiss LSM780NLO).
RNA extraction, reverse transcription and real-time PCR
Total RNA was extracted from soleus, gastrocnemius muscles, or L6 myotubes using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. For real-time PCR, RNA was retrotranscribed using PrimeScript RT Master Mix (Takara) according to the manufacturer’s directions. Gene-specifc primers were designed by Sangon Biotech (Shanghai) Co., Ltd. Real-time PCR was conducted with TB Green Premix Ex Taq II (Takara) according to manufacturer’s instructions. The mRNA expressions were normalized to those of GAPDH used as endogenous control. Gene specifc primer sequences are listed in Table 1.
Table 1.
Primers used for Real-time PCR
| Gene | Primer sequence | Ta(°C) | |
|---|---|---|---|
| Myh1 | Forward | GCGACAGACACCTCCTTCAAGAAC | 60 |
| Reverse | CCAGCCAGCCAGCGATGTTG | ||
| Myh4 | Forward | CCATCACTGACGCCGCCATG | 60 |
| Reverse | GTTCTTCTTCATCCGCTCCAGGTG | ||
| Myh7 | Forward | GAGCAGGAGCTGATCGAGAC | 60 |
| Reverse | ATGGCCTTCTTGGCCTTCTC | ||
| Myh7b | Forward | CAACCTGGCTAAGTACCGCA | 60 |
| Reverse | AATAAGGACAGGACAGCGGC | ||
| Myoglobin | Forward | CACCATGGGGCTCAGTGATG | 60 |
| Reverse | CTCAGCCCTGGAAGCCTAGC | ||
| PGC-1α | Forward | GCAGCGGTCTTAGCACTCA | 60 |
| Reverse | GACTGCGGTTGTGTATGGGA | ||
| Sox6 | Forward | TCCATGGCTTTGTCACTTTCA | 60 |
| Reverse | ATACACGAGGATCACCCCAG | ||
| GAPDH | Forward | ACGGGAAGCTCACTGGCATGG | 60 |
| Reverse | CGCCTGCTTCACCACCTTCTT | ||
TaqMan miRNA
Total RNA was extracted from soleus, gastrocnemius muscles or L6 myotubes using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. TaqMan methods were used to assess miR-499 expression. 1 μg RNA was reverse transcribed to cDNA with the TaqMan® MicroRNA RT Kit (Applied Biosystems™). Thermal-cycling conditions of reverse transcription: 16 °C 5 min, 42 °C 50 min, 85 °C 5 min. Product from RT reaction was used to Quantitative Real-time PCR with the TaqMan MicroRNA Assays (Applied Biosystems™) specific for miR-499 (Assay ID: 472838_mat) and miR-U6. Thermal Cycling Conditions of qPCR: 94.5 °C 10 min for 1 cycles, 97 °C 30s and 59.7 °C 1 min for 40 cycles. The relative miR-499 level was normalized to miR-U6.
Western blot
The protein was extracted from soleus, gastrocnemius muscles, and L6 myotubes. Briefly, protein samples were electrophoretically separated by 10% SDS-PAGE, then transferred to PVDF membranes, blocked for 1 h at room temperature using 5% blocking solution, incubated with primary antibodies: anti-Myh7 (1:1000), anti-Sox6 (1:1000), anti-tnni1 (1:1000), anti-myoglobin (1:1000), anti-cytochrome c (1:1500), and anti-PGC-1α (1:1500) overnight at 4 °C. After incubation with relative secondary antibodies for 1 h at room temperature, signal development was detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore). Images were aquired using fusion fx5 molecular imager (Vilber Lourmat).
Cell culture and treatments
Rat skeletal muscle L6 myoblasts were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and were grown in DMEM containing 10% FBS and 2% penicillin-streptomycin in 5% CO2 at 37 °C. Cells were induced to L6 myotubes by the differentiation medium containing DMEM and 2% horse serum. When the myoblasts were fully differentiated to myotubes, they were treated with myricetin (2 μM, 10 μM, and 50 μM) or vehicle (DMSO) for 24 h.
Cell proliferation assay
CCK-8 kits were used to measure cell proliferation following the manufacturer’s protocol. Briefly, L6 myoblasts were seeded in 96-well plates (5000 cells/well) and differentiated to myotubes. After fully differentiated, myotubes were incubating with myricetin (1-64 μM) or 0.1% DMSO as control for 24 h. Subsequently, CCK-8 solution (10 μL/well) was added to each well of the plates, and the plates were incubated for 1 h in the incubator. Cell viability was detected by the absorbance at 450 nm using an Infinite™ M200 Microplate Reader (Tecan, Mannedorf, Switzerland).
Manipulation of miR-499 in L6 myotubes
To modulate the expression of miR-499, inhibitor and mimic were transfected into L6 myotubes at the final concentration of 10 nM using Lipofectamine™ RNAiMAX Transfection Reagent according to the manufacturer’s instructions. Twenty-four hours after transfection, myotubes were treated with 10 μM myricetin or vehicle (DMSO) for 24 h.
Seahorse XFp cell mito stress test
Cell Mito Stress Test was measured using XFp Analyzer (Seahorse Bioscience, Agilent Technologies, USA). L6 myoblasts were seeded and differentiated in XFp Cell Culture Microplates. After differentiation and treatment, L6 myotubes were cultured in a 37 °C non-CO2 incubator for 1 h with assay medium, which contained 10 mM glucose, 1 mM pyruvate, and 2 mM glutamine, and were adjusted to pH 7.4 at 37 °C. Oligomycin, FCCP, and rotenone/antimycin were loaded in sensor cartridge for Cell Mito Stress Test. All steps were in accordance with the manufacturer’s instructions.
Statistics
Data are represented as mean ± SD. Statistical analyses were conducted by one-way ANOVA, followed by Tukey’s test using GraphPad prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). P-values less than 0.05 were considered to be statistically significant. All experiments were repeated a minimum of three times.
Results
Myricetin improved the aerobic capacity in rats
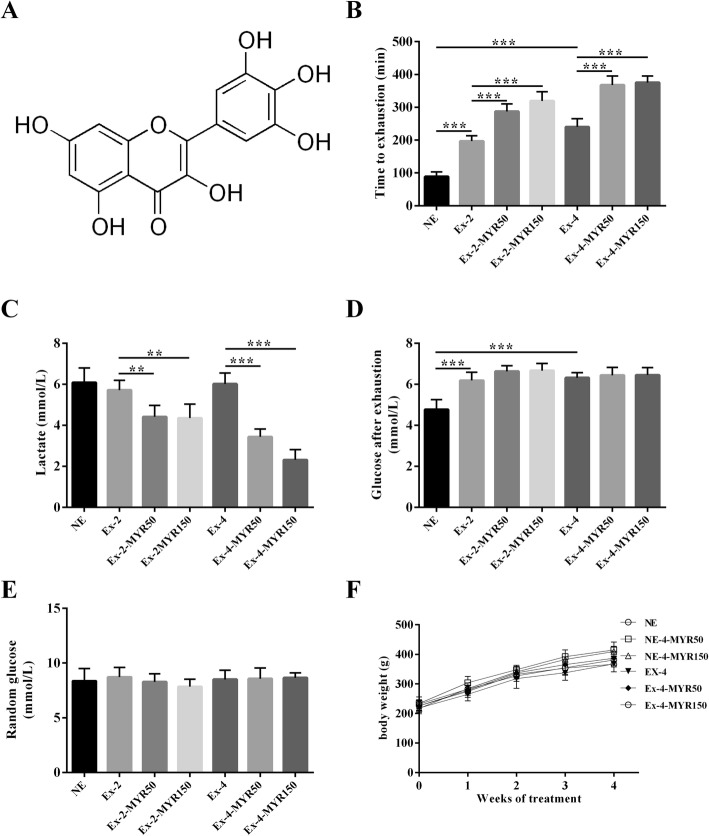
The chemical structure of myricetin, a natural flavonoid, was showed in Fig. 1a. Exercise indeed remarkably enhanced endurance capacity. As shown in Fig. 1b, TTE of both 2-week- and 4-week-exercise groups were significantly boosted, in a time-dependent way, compared with non-exercise group (P < 0.001). Intriguingly, both 2-week- and 4-week-treatment of myricetin exerted dramatic influence on the increase of TTE compared with exercise group (P < 0.001). Two weeks of treatments with low-dose (50 mg/kg) and high dose (150 mg/kg) myricetin were found to elevate TTE by 1.5-fold and 1.6-fold, respectively (P < 0.001), while four weeks of treatments with low-dose and high-dose myricetin increased TTE by 1.5-fold and 1.6-fold, respectively (P < 0.001).
Fig. 1.
The effect of myricetin on endurance capacity in rats. a Chemical structures of myricetin. After the run-to-fatigue experiments, time to exhaustion (b) was recorded, while blood lactate (c) and glucose (d) levels were determined. Random glucose (e) and body weight (f) was monitored weekly. Data are presented as mean ± SD. **P<0.01, ***P<0.001
Blood samples were collected after exhaustion. Exercise showed no significant effects on the level of lactate (P > 0.05 vs. non-exercise group, Fig. 1c), while both low-dose (50 mg/kg) and high-dose (150 mg/kg) of myricetin treatments remarkably reduced the levels of lactate (P < 0.01, Ex-2-MYR50 vs. Ex-2, Ex-2-MYR150 vs. Ex-2; P < 0.001, Ex-4-MYR50 vs. Ex-4, Ex-4-MYR150 vs. Ex-4, Fig. 1c). Interestingly, exercise significantly elevated plasma glucose after exhaustion compared with non-exercise group (P < 0.001, Fig. 1d), while myricetin treatments showed non-significant growth in levels of glucose after exhaustion compared with exercise groups (P > 0.05, Fig. 1d). Random plasma glucose among all groups was unchanged (P > 0.05, Fig. 1e), indicating that myricetin was nontoxic to glucose metabolism. These results, together with TTE data, suggested that myricetin enhanced endurance capacity in rats. The beneficial effects of myricetin on TTE did not result from decreased animal weight, since body weight did not differ among groups during both 4-week-experiment (Fig. 1f) and 2-week-experiment (data were not shown).
Myricetin enhanced proportions of slow-twitch fibers in vivo
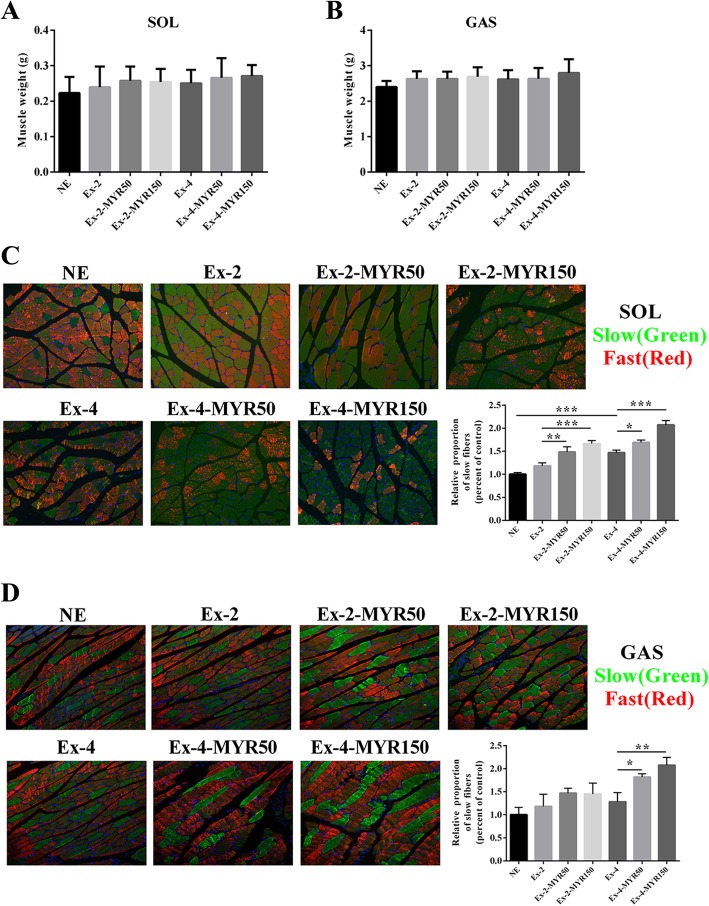
To investigate whether myricetin improved exercise performance of trained rats through enlarging the proportion of slow-twitch myofibers, we first evaluated the effect of myricetin on skeletal muscle mass and there were no differences between these groups (P > 0.05, Fig. 2a and b). Then we analyzed skeletal muscle fiber composition of soleus and gastrocnemius muscles. The results of immunofluorescence showed that myricetin dose-dependently increased the proportion of slow-twitch myofibers in both soleus (P < 0.01, Ex-2-MYR50 vs. Ex-2; P < 0.001, Ex-2-MYR150 vs. Ex-2; P < 0.05, Ex-4-MYR50 vs. Ex-4; P < 0.001, Ex-4-MYR150 vs. Ex-4, Fig. 2c) and gastrocnemius (P < 0.05, Ex-4-MYR50 vs. Ex-4; P < 0.01, Ex-4-MYR150 vs. Ex-4, Fig. 2d) muscles.
Fig. 2.
The effects of myricetin on the formation of slow muscle fibers in vivo. Soleus (a) and gastrocnemius (b) muscle mass was detected at the end of experiment. Soleus (c) and gastrocnemius (d) muscle phenotypes were shown by immunofluorescent staining. Data are presented as mean ± SD. *P<0.05, **P<0.01, ***P<0.001
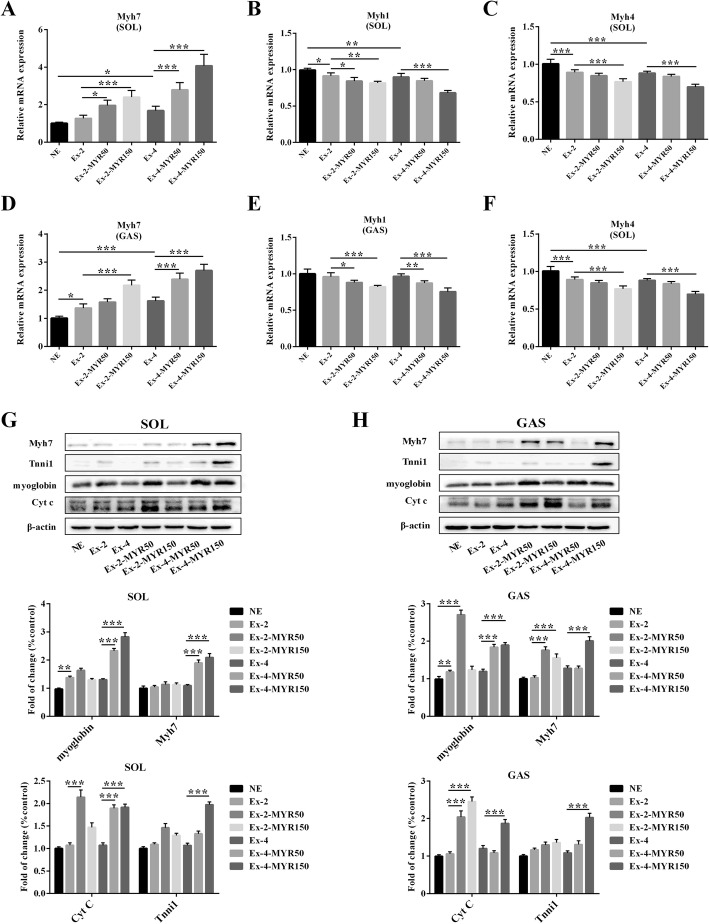
Further more, mRNA expression of Myh7 gene, predominantly expressed in slow-twitch myofibers, was increased in soleus and gastrocnemius muscles of myricetin-treated rats (Fig. 3a and d), concomitant with a decline in mRNA expression of Myh1, and Myh4 genes, predominantly expressed in fast-twitch myofibers (Fig. 3b, c and e, f). The results of immunoblotting further confirmed that myricetin significantly increased protein expression of Myh7. Moreover, protein expressions of myoglobin, tnni1, and Cyt C, which are molecular markers of slow-twitch myofibers, were also elevated in myricetin-treated rats’ soleus and gastrocnemius muscles (Fig. 3g and h). These results suggested that myricetin increases the proportion of slow-twitch myofibers in trained rats.
Fig. 3.
The effects of myricetin on mRNA and protein expressions of slow twitch-specific in vivo. The mRNA levels of slow-twitch myosin Myh7 (a) and fast-twitch myosin Myh1 (b), Myh4 (c) in soleus and gastrocnemius (d-f) were determined by Real-time PCR. Protein levels of slow-twitch myosin Myh7 and slow-twitch fiber biomarkers in SOL (g) and GAS (h) were measured by immunoblotting. Data are presented as mean ± SD. *P<0.05, **P<0.01, ***P<0.001
Myricetin promotes fast-to-slow switch in vitro
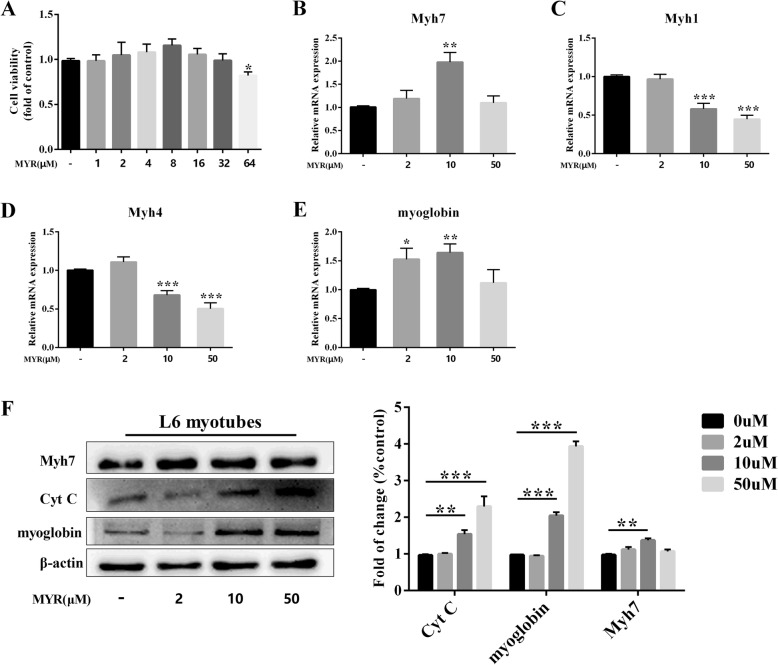
To further verify the role of myricetin in promoting the formation of slow-twitch myofibers, we next detected the effects of myricetin on fast-to-slow switch in rat L6 myotubes. Myricetin did not significantly suppress cell viability at concentrations lower than 64 μM (Fig. 4a). Myricetin at 10 μM significantly enhanced mRNA and protein expression of Myh7 in L6 myotubes (P < 0.01, Fig. 4b and f), while high-dose (50 μM) showed no significant effect on promoting Myh7 expression. Myricetin reduced mRNA expressions of fast-twitch myofiber genes, Myh1 and Myh4 (P < 0.001, Fig. 4c and d). Low- and middle-dose of myricetin treatments (2 μM and 10 μM, respectively) evidently increased mRNA expression of myoglobin, a molecular marker of slow-twitch myofiber (P < 0.05 and P < 0.01, respectively, Fig. 4e), although high-dose (50 μM) of myricetin showed no significant effect on elevating myoglobin expression. Protein levels of slow-twitch myofiber markers, myoglobin and Cyt C, were elevated by middle- and high-dose treatments of myricetin (10 μM and 50 μM, respectively), while low-dose treatment of myricetin (2 μM) showed no significant effect on protein levels of myoglobin and Cyt C (P > 0.05, Fig. 4f). Together with in vivo data, these results suggested that the effects of myricetin on fast-to-slow switch might contribute to enhancement of endurance capacity.
Fig. 4.
The effects of myricetin on muscle fiber-type composition in L6 myotubes. Cell viability (a) was measured using CCK-8 assay. The mRNA expressions of slow-twitch myosin Myh7 (b), fast-twitch myosin Myh1 (c), Myh4 (d), and slow-twitch fiber biomarker myoglobin (e) were determined by real-time PCR. Protein levels of slow-twitch myosin Myh7 and slow-twitch fiber biomarkers (f) in L6 myotubes were measured by immunoblotting. Data are presented as mean ± SD. *P<0.05, **P<0.01, ***P<0.001 compared with control
Myricetin regulated miR-499/Sox6 in vivo and in vitro
miR-499, encoded by Myh7b gene, plays a crucial role in reprogramming of fast-to-slow fiber type switch. Hence, we further explored whether myricetin promoted a fast-to-slow switch via regulating the expression of miR-499. High-dose (150 mg/kg) treatments of myricetin for short term (2 weeks) and long term (4 weeks) significantly up-regulated the expression of Myh7b both in soleus and gastrocnemius muscles (P < 0.001, Ex-2-MYR150 vs. Ex-2, Ex-4-MYR150 vs. Ex-4, Fig. 5a and b). Four-week treatment of myricetin elevated expression of miR-499 in gastrocnemius muscle (P < 0.001, Ex-4-MYR50 vs. Ex-4, Ex-4-MYR150 vs. Ex-4, Fig. 5d). In soleus muscle, high-dose (150 mg/kg) treatment of myricetin for 2 weeks evidently increased the expression of miR-499 (P < 0.001, Ex-2-MYR150 vs. Ex-2, Fig. 5c). However, as for long-term treatment, low-dose (50 mg/kg) myricetin dramatically boosted miR-499 expression (P < 0.001, Ex-4-MYR50 vs. Ex-4, Fig. 5c). Notably, we found that the mRNA and protein expression of Sox6, a transcriptional repressor of slow-twitch contractile protein genes, was markedly decreased both in soleus and gastrocnemius muscles after myricetin treatment (Fig. 5e-g).
Fig. 5.
The effects of myricetin on regulating miR-499/Sox6 axis in vivo and in vitro. The mRNA expressions of Myh7b (a: soleus; b: gastrocnemius), miR-499 (c: soleus; d: gastrocnemius) and Sox6 (e: soleus; f: gastrocnemius) in rats were determined by Real-time PCR. Protein levels of Sox6 (g) in soleus and gastrocnemius muscles were measured by immunoblotting. The mRNA expressions of miR-499 (h), Myh7b (i), Sox6 (j), and PGC-1α (l) in L6 myotubes were determined by Real-time PCR. Protein levels of Sox6 (k) and PGC-1α (m) in L6 myotubes were measured by immunoblotting. Data are presented as mean ± SD. *P<0.05, **P<0.01, ***P<0.001
To confirm the findings in vivo, we next examined the effects of myricetin in L6 myotubes. Myricetin dose-dependently elevated the mRNA expression of miR-499 and Myh7b (Fig. 5h and i). Meanwhile, mRNA and protein expression of Sox6 were markedly decreased (Fig. 5j and k). Additionally, myricetin markedly up-regulated the expression of both mRNA and protein of PGC-1α (Fig. 5l and m). Taken together, these data suggest that the effects of myricetin on regulating miR-499/Sox6 signaling pathway may contribute, at least in part, to the reprogramming of fast-to-slow fiber type switch.
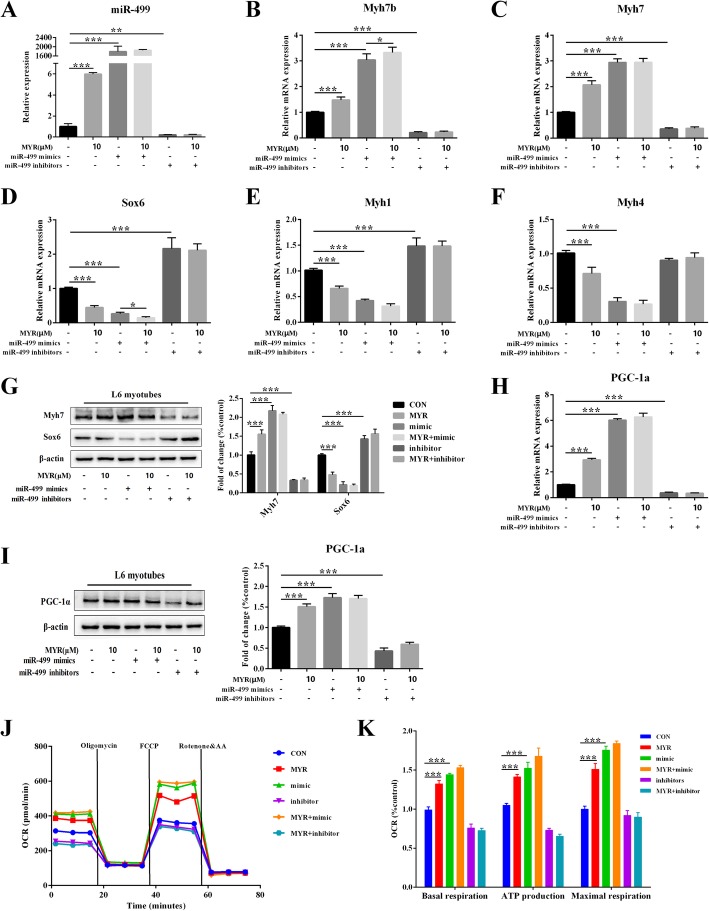
miR-499 is required for the myricetin effects on myofiber type conversion
To verify the role of miR-499 in the effects of myricetin to promote myofiber type conversion, we transfected miR-499 inhibitor and mimic into L6 myotubes. The transfection of miR-499 inhibitor declined mRNA expression of miR-499 by 78.4% (P < 0.01, Fig. 6a). Overexpression of miR-499 resulted in markedly increases of mRNA expression of Myh7b and Myh7, and protein expression of Myh7 in L6 myotubes (P < 0.001, Fig. 6b, c and g). Significant decreases of mRNA expression of Sox6, Myh1, and Myh4 were spotted in L6 myotubes (P < 0.001, Fig. 6d, e and f). Intriguingly, compared with miR-499 mimic alone, myricetin together with miR-499 mimic acted robustly curtailed mRNA expression of Sox6 (P < 0.05, Fig. 6d). Furthermore, we investigated PGC-1α and mitochondrial respiration capacity in myotubes, and found that myricetin significantly amplified basal mitochondrial respiration oxygen consumption rate (OCR), ATP production, maximal respiration, and the expression of PGC-1α (Fig. 6h-k). Notably, inhibition of miR-499 overturned the effects of myricetin in L6 myotubes, indicating that miR-499 played a crucial role in the effects of myricetin on promoting the reprogramming of fast-to-slow fiber type switch.
Fig. 6.
miR-499/Sox6 axis is involved in inducing fast-to-slow fiber type transition by myricetin. After transfected with inhibitor or mimic of miR-499, the mRNA expressions of miR-499 (a), Myh7b (b), Myh7 (c), Sox6 (d), Myh1 (e), and Myh4 (f) in L6 myotubes were determined. Protein levels (g) of Myh7 and Sox6 were measured by immunoblotting. The mRNA (h) and protein (i) levels of PGC-1α were monitored in the transfected L6 myotubes. Oxygen consumption rates (OCR) of myotubes (j) were determined by Seahorse XFp metabolic flux analysis. Basal respiration, ATP production and maximal respiration (k) were analyzed. Data are presented as mean ± SD. *P<0.05, **P<0.01, ***P<0.001
Discussion
In the present study, we found that natural flavonoid myricetin notably augmented endurance capacity of SD rats and increased the TTE treadmill running. Meanwhile, myricetin enhanced proportions of slow-twitch fibers in rats’ muscles. Interestingly, myricetin stimulated the expression of miR-499 both in vivo and in vitro, while inhibiting miR-499 could block the effects of myricetin on myotubes. Our results uncovered a novel mechanism that myricetin improved endurance capacity through promoting fast-to-slow myofiber transition via miR-499 pathway.
Skeletal muscle force can be distinctly modulated by exercise, as clearly shown in athletes. These adaptive changes, known as muscle plasticity, can be achieved by modifying the structural and functional properties of individual fibers or the proportion of distinct fiber populations in a muscle or a muscle group [5]. The present study showed that exercise indeed remarkably improved TTE treadmill running in SD rats. Interestingly, our in vivo data also showed that myricetin treatment potently amplified endurance capacity by remarkably increasing TTE. Previous studies have demonstrated that natural compounds, such as quercetin and resveratrol, could enhance endurance capacity [22, 23]. Meanwhile, we found that myricetin remarkably reduced the levels of lactate after exhaustion treadmill running. It is well accepted that muscle metabolic changes, such as lactate accumulation, are involved in the development of muscle fatigue [24]. Hence, myricetin enhanced anti-fatigue ability of rats, which might contribute, at least in part, to the increase of exercise endurance.
Several lines of evidence have demonstrated that long-term endurance training induced an increased content of capillaries and mitochondria in skeletal muscle, promoted muscle fiber phenotype fast-to-slow (type II to typeI) transition, and thereby improved endurance capacity [25]. Recently, Mizunoya et al. reported that apple polyphenols improved endurance by inducing fast-to-slow shift of muscle fiber type composition [26, 27]. We expect that myricetin, also a flavonoid, might promote muscle fiber type transition, which led to endurance enhancement. And, in the present study we detected that myricetin dose-dependently increased the proportion of slow-twitch myofibers in both soleus and gastrocnemius muscles. Moreover, our in vitro data showed that myricetin up-regulated the expression of Myh7 gene, predominantly expressed in slow-twitch myofibers, and down-regulated the expression of Myh1, and Myh4 genes, predominantly expressed in fast-twitch myofibers, which further demonstrate this point. Therefore, we propose that myricetin improves exercise endurance through promoting reprogramming of fast-to-slow fiber type switch.
MyomiRs regulate myosin switching and myofiber identity. Eva van Rooij et al. demonstrated that the miR-499 transgenic mice ran more than 50% longer than wild-type littermates, indicating improved endurance due to the fast to slow myofibers reprogramming [1]. Notably, although miR-499 was found encoded by mouse Myh7b gene, it is relevant in human. Animal and human researches revealed that miR-499 expression were higher in muscles of trained “active” individuals compared with those of sedentary individuals. Moreover, a strong positive correlation was observed between miR-499’s expression and type I myofiber percentage, ATPmax and VO2max [28]. In accordance with these findings, our study found that myricetin remarkably augmented the expression of miR-499 and Myh7b both in vivo and in vitro, which further expounded how myricetin regulate the reprogramming of fast-to-slow fiber type switch.
The function of miR-499 on regulating gene expression is exerted by down-regulation of transcriptional repressors, such as Sox6 that suppresses slow-twitch contractile protein gene expressions. Researches found that miR-499 directly bound Sox6 and suppressed its expression, and eventually promoted type I fiber formation [29, 30]. In the present study, we investigated the effects of myricetin on regulating miR-499/Sox6 axis and found that myricetin significantly reduced Sox6 expression along with increase of miR-499 expression. Intriguingly, inhibition of miR-499 overturned the effects of myricetin on the suppression of Sox6, indicating that myricetin exerts its beneficial effects on muscle fiber type switch through the miR-499/Sox6 pathway. As to the precise mechanism for the regulation of miR-499 by myricetin, there are some researches might shed some light on the matter. Taken together, there are two possibilities of the underlying mechanism. First, myricetin might directly bind to miR-499 to exert its effects. Eshan Khan and colleagues found that myricetin was a potent and selective binder of 5’CAG/3’GAC motif containing RNA, and interacted with this RNA via base stacking at AA mismatch [31]. The stem-loop structure of pre-miR-499 also has a 5’CAG/3’GAC motif. Hence, myricetin might directly bind at this site of pre-miR-499. Second, myricetin might regulate the up-stream nuclear receptors, such as peroxisome proliferator-activated receptors (PPARs), to affect the expression of miR-499. The ligand-binding activation of PPARβ/δ induced fast-to-slow muscle fiber type switch and increased the muscle oxidative capacity [32]. Many natural phytochemicals, such as green tee and bitter melon extracts, are found to activate PPARβ/δ to improve insulin sensitivity [33, 34]. Myricetin, as a natural flavonoid, might activate PPARβ/δ to regulate slow-twitch myofiber related gene expressions. However, efforts are needed to elucidate the underlying mechanism.
The main character of slow-twitch fibers is their high contents of mitochondria and oxidative enzymes. Intriguingly, we found that myricetin elevated oxygen consumption rates in L6 myotubes, indicating an increase of ATP production and max respiration. Inhibition of miR-499 attenuated the beneficial effects of myricetin on oxidative metabolism. Moreover, the nuclear receptor coactivator PGC-1α (PPARγ coactivator-1α) was up regulated after myricetin treatment and down regulated when miR-499 was curbed. As we know, PGC-1α is a downstream transcriptional regulator of AMPK (AMP-activated protein kinase), key sensor of cellular metabolic and energy homeostasis, which plays a crucial role in skeletal muscle oxidative metabolism and fiber-type specification [35–37]. Recently, Liu Jing et al. demonstrated that miR-499 repressed expression of Fnip1 (folliculin-interacting protein-1) by directly binding, and therefore activated downstream AMPK/PGC-1α signaling pathway [38]. Taken together, our data showed that myricetin improved muscle oxidative metabolism by elevating expression of miR-499 and subsequently activated PGC-1α signaling pathway.
In summary, our current study has further elucidated the mechanism underlying the effect of myricetin. To the best of our knowledge, this study provides the first evidence that myricetin improves endurance capacity by inducing muscle fiber type transition, and microRNA miR-499 plays an important role in the effect of myricetin. Although more work is needed to elucidate the intermolecular interactions, our findings suggest that myricetin is a potential candidate to improve endurance capacity.
Conclusions
Skeletal muscle is essential both to exert physical performance and maintain energy homeostasis. Certain stimuli, like endurance exercise, can induce fast-to-slow muscle fiber type switch, and subsequently improve aerobic capacity and metabolic status. In the current study, we showed that myricetin improved endurance capacity by regulating fast-to-slow muscle fiber type conversion. In addition, the in vivo and in vitro experiments showed that this function of myricetin was associated with miR-499. Our findings have important implications for improving the health of sedentary people and people with muscle diseases.
Acknowledgements
Not applicable.
Funding
This study was supported by research grants from the Chongqing Natural Science Foundation (project No. cstc2018jcyjAX0040), the Key Projects for Scientific Research (project No. AWS17J014) and the Pre-research Foundation (project No. 2016XYY03).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMPK
Adenosine 5′-monophosphate activated protein kinase
- ATP
Adenosine triphosphate
- BSA
Bovine serum albumin
- CCK-8
Cell Counting Kit-8
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s Modified Eagle’s medium
- DMSO
Dimethyl sulfoxide
- DNA
Deoxyribonucleic acid
- EDTA
Ethylenediaminetetraacetic acid
- FCCP
Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GAS
Gastrocnemius
- GLUT4
Glucose transporter type 4
- HRP
Horseradish Peroxidase
- MHC
myosin heavy chain
- Myh
Myosin heavy chain
- Myh1
Myosin heavy chain1
- Myh4
Myosin heavy chain4
- Myh7
Myosin heavy chain7
- Myh7b
Myosin heavy chain7b
- NRF-1
Nuclear respiratory factor 1
- OCR
Oxygen consumption rate
- PBS
Phosphate buffered saline
- PCR
Polymerase chain reaction
- PGC-1α
Peroxlsome proliferator-activated receptor-γ coactlvator-1α
- PPARβ
Peroxisome Proliferator-Activated Receptor β
- PVDF
Polyvinylidene fluoride
- qRT-PCR
Quantitative real time polymerase chain reaction
- RNA
Ribonucleic acid
- SD
Sprague Dawley
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SOL
Soleus
- Sox6
Sex-determining region Y-box 6
- TNNI1
Troponin I type 1
- TTE
Time to exhaustion
Authors’ contributions
LL, MM and JZ participated in study design. Luting Wu carried out the study and wrote the manuscript that was revised and approved by LL, MM, and LY. LR, HL, MZ, and LY assisted with the animal’s exercise training and run-to-fatigue experiment. All authors read and approved the final manuscript.
Ethics approval
This animal study was performed according to institutional guidelines with the approval of the Institutional Animal Care and Use Committee of the Army Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Luting Wu, Email: 13629712827m@sina.cn.
Li Ran, Email: 475336539@qq.com.
Hedong Lang, Email: lang0401@163.com.
Min Zhou, Email: zmyingyang0510@sina.com.
Li Yu, Email: gyulilove.happy@163.com.
Long Yi, Email: longgyin8341@hotmail.com.
Jundong Zhu, Email: zjdnfs@126.com.
Lei Liu, Email: liuleililian@icloud.com.
Mantian Mi, Email: mi_mt2009@hotmail.com.
References
- 1.Rooij EV, Quiat D, Johnson BA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17(5):662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberbach A, Bossenz Y, Lehmann S, et al. Altered Fiber distribution and Fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care. 2006;29(4):895–900. doi: 10.2337/diacare.29.04.06.dc05-1854. [DOI] [PubMed] [Google Scholar]
- 3.Stuart CA, McCurry MP, Marino A, et al. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab. 2013;98(5):2027–2036. doi: 10.1210/jc.2012-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albers PH, Pedersen AJ, Birk JB, et al. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes. 2015;64(2):485–497. doi: 10.2337/db14-0590. [DOI] [PubMed] [Google Scholar]
- 5.Blaauw B, Schiaffino S, Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr Physiol. 2013;3(4):1645–1687. doi: 10.1002/cphy.c130009. [DOI] [PubMed] [Google Scholar]
- 6.Rooij EV, Liu N, Olson EN. MicroRNAs flex their muscles. Trends Genet. 2008;24(4):159–166. doi: 10.1016/j.tig.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Bell ML, Buvoli M, Leinwand LA. Uncoupling of expression of an intronic microRNA and its myosin host gene by exon skipping. Mol Cell Biol. 2010;30(8):1937–1945. doi: 10.1128/MCB.01370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quiat D, Voelker KA, Pei J, et al. Concerted regulation of myofiber-specific gene expression and muscle performance by the transcriptional repressor Sox6. Proc Natl Acad Sci U S A. 2011;108(25):10196–10201. doi: 10.1073/pnas.1107413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol. 2001;90:345–357. doi: 10.1152/jappl.2001.90.1.345. [DOI] [PubMed] [Google Scholar]
- 10.Harridge SD. Plasticity of human skeletal muscle: gene expression to in vivo function. Exp Physiol. 2007;92:783–797. doi: 10.1113/expphysiol.2006.036525. [DOI] [PubMed] [Google Scholar]
- 11.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARδ agonists are exercise mimetics. Cell. 2008;134(3):405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Laher I. Exercise pills: at the starting line. Trends Pharmacol Sci. 2015;36(12):906–917. doi: 10.1016/j.tips.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Fan W, Evans RM. Exercise mimetics: impact on health and performance. Cell Metab. 2017;25(2):242–247. doi: 10.1016/j.cmet.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Conesa MT. Dietary polyphenols against metabolic disorders: how far have we progressed in the understanding of the molecular mechanisms of action of these compounds? Crit Rev Food Sci Nutr. 2017;57(9):1769–1786. doi: 10.1080/10408398.2014.980499. [DOI] [PubMed] [Google Scholar]
- 15.Somerville V, Bringans C, Braakhuis A. Polyphenols and performance: a systematic review and meta-analysis. Sports Med. 2017;47(8):1589–1599. doi: 10.1007/s40279-017-0675-5. [DOI] [PubMed] [Google Scholar]
- 16.Ross RP, Ross CA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2003;22(1):19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 17.Abdelkader B, Soulet S, Nassima C, et al. Wine polyphenols: potential agents in neuroprotection. Oxidative Med Cell Longev. 2012;2012:1–14. doi: 10.1155/2012/805762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong KC, Khoo HE. Biological effects of myricetin. Gen Pharmacol. 1997;29(2):121–126. doi: 10.1016/S0306-3623(96)00421-1. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Ding Y. Minireview: therapeutic potential of myricetin in diabetes mellitus. Food Sci Human Wellness. 2012;1(1):19–25. doi: 10.1016/j.fshw.2012.08.002. [DOI] [Google Scholar]
- 20.Zou D, Liu P, Chen K, et al. Protective effects of Myricetin on acute hypoxia-induced exercise intolerance and mitochondrial impairments in rats. PLoS One. 2015;10(4):e0124727. doi: 10.1371/journal.pone.0124727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung HY, Lee D, Ryu HG, et al. Myricetin improves endurance capacity and mitochondrial density by activating SIRT1 and PGC-1α. Sci Rep. 2017;7(1):6237. doi: 10.1038/s41598-017-05303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kressler J, Millard-Stafford M, Warren GL. Quercetin and endurance exercise capacity: a systematic review and meta-analysis. Med Sci Sports Exerc. 2011;43(12):2396–2404. doi: 10.1249/MSS.0b013e31822495a7. [DOI] [PubMed] [Google Scholar]
- 23.Dolinsky VW, Jones KE, Sidhu RS, et al. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol. 2012;590(11):2783–2799. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saey D, Michaud A, Couillard A, et al. Contractile fatigue, muscle morphometry, and blood lactate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(10):1109–1115. doi: 10.1164/rccm.200408-1005OC. [DOI] [PubMed] [Google Scholar]
- 25.Lindstedt SL, Hokanson JF, Wells DJ, et al. Running energetics in the pronghorn antelope. Nature. 1991;353(6346):748–750. doi: 10.1038/353748a0. [DOI] [PubMed] [Google Scholar]
- 26.Mizunoya W, Miyahara H, Okamoto S, et al. Improvement of endurance based on muscle Fiber-type composition by treatment with dietary apple polyphenols in rats. PLoS One. 2015;10(7):e0134303. doi: 10.1371/journal.pone.0134303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizunoya W, Okamoto S, Miyahara H, et al. Fast-to-slow shift of muscle fiber-type composition by dietary apple polyphenols in rats: impact of the low-dose supplementation. Anim Sci J. 2017;88(3):489–499. doi: 10.1111/asj.12655. [DOI] [PubMed] [Google Scholar]
- 28.Gan Z, Rumsey J, Hazen BC, et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J Clin Invest. 2013;123(6):2564–2575. doi: 10.1172/JCI67652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nachtigall PG, Dias MC, Carvalho RF, et al. MicroRNA-499 expression distinctively correlates to target genes sox6 and rod1 profiles to resolve the skeletal muscle phenotype in Nile tilapia. PLoS One. 2015;10(3):e0119804. doi: 10.1371/journal.pone.0119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding J, Chen J, Wang Y, et al. Trbp regulates heart function through microRNA-mediated Sox6 repression. Nat Genet. 2015;47(7):776–783. doi: 10.1038/ng.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan E, Tawani A, Mishra SK, et al. Myricetin reduces toxic level of CAG repeats RNA in Huntington’s disease (HD) and Spino cerebellar Ataxia (SCAs) ACS Chem Biol. 2017;13(1):180–188. doi: 10.1021/acschembio.7b00699. [DOI] [PubMed] [Google Scholar]
- 32.Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle Fiber type and running endurance by PPARδ. PLoS Biol. 2004;2(10):e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francesca D, Mattia DN, Elisa B, et al. Green tea extract selectively activates peroxisome proliferator-activated receptorβ/δ in cultured cardiomyocytes. Br J Nutr. 2009;101(12):1736–1739. doi: 10.1017/S0007114508145871. [DOI] [PubMed] [Google Scholar]
- 34.Sasa M, Inoue I, Shinoda Y, et al. Activating effect of Momordin, extract of bitter melon (Momordica Charantia L.), on the promoter of human PPARδ. J Atheroscler Thromb. 2009;16(6):888–892. doi: 10.5551/jat.2790. [DOI] [PubMed] [Google Scholar]
- 35.Mounier R, Théret M, Lantier L, et al. Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol Metab. 2015;26(6):275–286. doi: 10.1016/j.tem.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Röckl KS, Hirshman MF, Brandauer J, et al. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle Fiber type shift. Diabetes. 2007;56(8):2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Liang X, Zhou D, et al. Coupling of mitochondrial function and skeletal muscle fiber type by a miR-499/Fnip1/AMPK circuit. EMBO Mol Med. 2016;8(10):1212–1228. doi: 10.15252/emmm.201606372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.