Abstract
Background/Objective:
India is the world's most biodiverse region and is undergoing a period of dramatic social and economic change. Due to population's explosion, climate change and lax implementation of environmental policies, the incidence of breast cancer is increasing. From population-based cancer registry data, breast cancer is the most common cancer in women in urban registries where it constitutes more than 30% of all cancers in females. We conducted a meta-analysis of all breast cancer case–control studies conducted in India during 1991–2018 to find pooled estimates of odds ratio (OR).
Materials and Methods:
Eligible studies were identified through a comprehensive literature search of PubMed, EMBASE, and HINARI databases from 1991 to January 2018. This analysis included 24 observational studies out of 34 that reported the case–control distribution of reproductive factors, body mass index (BMI) and type of residence. The analysis was performed using RevMan 5.3 (Review Manager, 2017) applying the random-effects model.
Results:
A total of 21,511 patients (9889 cases and 11,622 controls) were analyzed, resulting in statistically significant association between breast cancer and the following reproductive factors: never breastfeed (OR: 3.69; 95% confidence interval [CI]: 1.70, 8.01), menopausal age >50 years (OR: 2.88; 95% CI: 1.85, 3.85), menarche age <13 years (OR: 1.83; 95% CI: 1.34, 2.51), null parity (OR: 1.58; 95% CI: 1.21, 2.06), postmenopause (OR: 1.35; 95% CI: 1.13, 1.62), and age at the 1st pregnancy >25 years (OR: 1.57; 95% CI: 1.37, 1.80). Family history (FH) of breast cancer (OR: 5.33; 95% CI: 2.89, 9.82), obesity (OR: 1.19; 95% CI: 1.00, 1.42), and urban residence (OR: 1.22; 95% CI: 1.03, 1.44) were also found to be significant risk factors.
Conclusion:
The results of this meta-analysis are indicative of significant associations between reproductive factors and breast cancer risk, profoundly so among women experiencing menopause after the age of 50, women who never breastfeed and FH of breast cancer.
Keywords: Breast cancer, India, meta-analysis, reproductive risk factors
Introduction
Breast cancer is the most common female cancer worldwide, including India (Global cancer statistics, 2012). About 12% of women in the general population will develop breast cancer sometime during their lives.[1] Worldwide, breast cancer is the fifth most common cause of cancer death (after colon cancer, lung cancer, liver cancer, and stomach cancer).[2,3] India is the world's most bio-diverse region and is undergoing a period of dramatic social and economic change. Due to population's explosion, climate change, and lax implementation of environmental policies, the incidence of breast cancer is increasing. From population-based cancer registry data, breast cancer is the most common cancer among women in urban registries where it constitutes more than 30% of all cancers in females. The death rate connected with breast cancer varies in different regions, depending on the stage of diagnosis, treatment quality, prevalence of assorted subtypes, and therapy effectiveness. Breast cancer treatments include surgery, radiation therapy, chemotherapy, hormone therapy, and targeted therapy.[4,5]
By 2020, an estimated 26% increase in breast cancer is projected, predominantly in developing countries, with a rise from 1,00,000 to 1,31,000 new cases annually in the Indian population.[6] It is unfortunate that by 2030, the global incidence of breast cancer will be burgeoning to more than 2 million new cases per year; in India, it will reach up to 2 Lakh/year.[7,8,9] The American Cancer Society estimates that 1,78,480 new cases of invasive breast cancer were diagnosed in 2007 in the USA and about 2% by 50 years of age, 4% by age 60, 7% by age 70, and 10% by age 80.[10] Previous study among Indian women elucidated that breast cancer incidence is increasing although age-adjusted rates vary with the different regions and cancer registries. Factors potentially associated with breast cancer risk include age, early menarche, childbearing, breastfeeding, use of oral contraceptive, late menopause, hormone replacement therapy, exogenous hormones, previous benign breast disease, breast density, diet, alcohol, smoking, and family history (FH).[11,12,13,14,15]
The preceding study has reported the highest increase in breast cancer incidence to be among women in Asian countries. Breast cancer has been found to be the most common cancer type among urban Indian women and the second most common among the rural Indian women.[11] In India, breast cancer incidence rates are approximately 100% higher among urban women than among rural women.[16] Physical activities have been strappingly suggested to reduce breast cancer risk among premenopausal and postmenopausal women independent of its effect on obesity, possibly by reducing estrogens production, and influencing age at menarche and menopause. The high occurrence of breast cancer among urban women can be concurrent with numerous factors, including having late sex, having fewer children, and breastfeeding them for a shorter period compared with rural women. This ultimately increases their exposure to estrogens and thus subsequently, upsurge the risk of developing breast cancer.[17] In addition, urban Indian women also tend to consume a western diet, which leads to obesity and high alcohol intake. All these factors contribute enormously to enhancing the risk of breast cancer. An increase in the number of cases and the net mortality due to breast cancer could have resulted from the large population, inadequate screening programs, and lack of proper education.[18]
Except Das et al. 2012,[19] all other studies reported that women residence of the urban area are at higher risk of breast cancer.[20,21,22,23] Dietary pattern and use of oral contraceptive pills (OCP) were the controversial risk factor of breast cancer.[9,21,24,25,26,27] Similarly, Meshram and Kulkarni 2009 and Wirth et al. 2014 reported that obese women have lower risk of breast cancer.[28,29] Considering the aforementioned issues, we conducted a meta-analysis of all case–control studies of breast cancer risk conducted in India during 1991–2018 to find pooled estimates of odds ratio (OR) for the corresponding reproductive and other risk factors in Indian women as compared to controls.
Materials and Methods
This study is a quantitative research meta-analysis on Indian women to find reproductive factors as risk factors for breast cancer. Eligible studies were identified through a comprehensive literature search of PubMed, EMBASE, and HINARI databases from 1991 to January 2018. The inclusion criteria were recognized to minimize bias or heterogeneity in the selection of studies [Figure 1]: (i) Full-text articles published between January 1991, and March 2018; (ii) Indian studies with Indian population; (iii) Case–control studies on breast cancer; (iv) original articles containing results related to reproductive factors, body mass index (BMI), and type of residence. Clinical trials, cohort studies, and case–control studies based on genetic polymorphism as the outcome were excluded from the meta-analysis. Search was bounded only for English articles published in journals.
Figure 1.
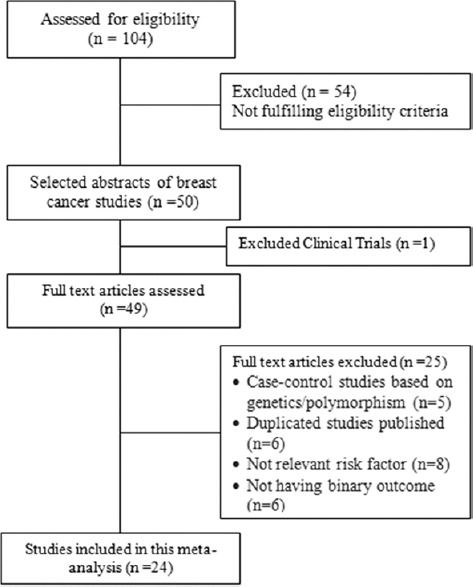
CONSORT diagram
Data synthesis and statistical analysis
The following information was extracted from eligible articles: pre–post menopausal status, age at menarche (<13 years and ≥13 years), age at first pregnancy (<25 years and ≥25 years), breastfeeding (yes/no), parity (multi-parity vs. nulliparity), marital status (unmarried and ever married), age of menopause (<50 years and ≥50 years), BMI (<25 and ≥25), FH of breast cancer (Yes/No), use of OCP (ever used, never used), place of residence (urban/rural), and dietary pattern (vegetarian/nonvegetarian).
The analysis included 24 observational studies out of 49 that reported the case–control distribution of reproductive factors, BMI, FH, and type of residence. Pooled ORs with 95% confidence interval (CIs) were reported with forest plots separately for each factor. The analysis was performed using RevMan 5.3 (Review Manager, 2011) applying Mental Haenszel method by the random-effects model.
Results
A total of 24 case–control studies were analyzed out of 34 with a sample size of 21,511 (9889 cases and 11,622 controls). A total of 104 articles were identified from database searches. Clinical trials and case–control studies based on genetics/polymorphism were excluded from the meta-analysis. Characteristics of the studies included in the analysis are described in Table 1. Figure 2 depicts the regional distribution of breast cancer cases reported by case–control studies performed in India. Most of the reported studies were done in the Indian states of Kerala, Tamil Nadu, Haryana, Maharashtra, and Northeast, whereas few studies were done in New Delhi, Punjab, and West Bengal.
Table 1.
Characteristics of selected studies for meta-analysis
| Author | Year | City/place | Cases | Controls | Ratio | Type of control | Center |
|---|---|---|---|---|---|---|---|
| Asegaonkar et al. | 2012 | Aurangabad (Maharashtra) | 50 | 50 | 1:1 | CC | SC |
| Babita et al. | 2014 | Rohtak (Haryana) | 128 | 128 | 1:1 | HC | SC |
| Bhadoria et al. | 2013 | AIIMS (New Delhi) | 320 | 320 | 1:1 | HC | SC |
| Das et al. | 2012 | Kolkata (West Bengal) | 105 | 105 | 1:1 | HC | SC |
| Datta et al. | 2009 | Kolkata (West Bengal) | 267 | 267 | 1:1 | HC | SC |
| Devi et al. | 2015 | Assam, Meghalaya, Tripura, Mizoram, (North East) | 462 | 770 | 1:2 | CC | MC |
| Dey et al. | 2009 | Trivandrum (Kerala) | 900 | 1208 | 1:1 | HC | SC |
| Gajalakshmi et al. | 1991 | Chennai (Tamil Nadu) | 238 | 238 | 1:1 | HC | SC |
| Gajalakshmi et al. | 2009 | Chennai (Tamil Nadu), Trivandrum (Kerala) | 1866 | 1873 | 1:1 | HC | MC |
| Gathani et al. | 2017 | Jaipur (Rajasthan), Ahmedabad (Gujarat), Vellor and Bangalore (Karnataka), Coimbatore (TamilNadu), Trivandrum (Kerala), Silchar (Assam) | 2101 | 2255 | 1:1 | HC | MC |
| Jayalekshmi et al. | 2009 | Trivandrum (Kerala) | 264 | 792 | 1:3 | CC | PBCR |
| Kapahi et al. | 2014 | Amritsar (Punjab) | 102 | 102 | 1:1 | HC | SC |
| Lodha et al. | 2011 | Bhopal (Madhya Pradesh) | 215 | 215 | 1:1 | CC | PBCR |
| Mathew et al. | 2009 | Chennai (Tamil Nadu), Trivandrum (Kerala) | 1866 | 1873 | 1:1 | HC | MC |
| Meshram et al. | 2009 | Nagpur (Maharashtra) | 105 | 210 | 1:2 | CC | SC |
| Mohite et al. | 2015 | Satara (Maharashtra) | 217 | 217 | 1:1 | HC | SC |
| Parmeshwari et al. | 2013 | Kottayam (Kerala) | 20 | 80 | 1:4 | CC | PBCR |
| Rao et al. | 1994 | Mumbai (Maharashtra) | 689 | 711 | 1:1 | HC | SC |
| Sambyal et al. | 2015 | Amritsar (Punjab) | 200 | 200 | 1:1 | HC | SC |
| Samson et al. | 2007 | Chennai (Tamil Nadu) | 250 | 500 | 1:2 | HC | SC |
| Saxena et al. | 2009 | AIIMS, New Delhi | 413 | 410 | 1:1 | HC | SC |
| Singh et al. | 2011 | AIIMS, New Delhi, Ballabhgarh (Haryana) | 320 | 320 | 1:1 | HC | MC |
| Singh et al. | 2013 | Rohtak (Haryana) | 128 | 128 | 1:1 | HC | SC |
| Wirth et al. | 2014 | Mumbai (Maharashtra) | 255 | 249 | 1:1 | HC | SC |
SC=Single centric, MC=Multi-centric, CC=Community control, HC=Hospital control, PBCR=Population-based cancer registry
Figure 2.
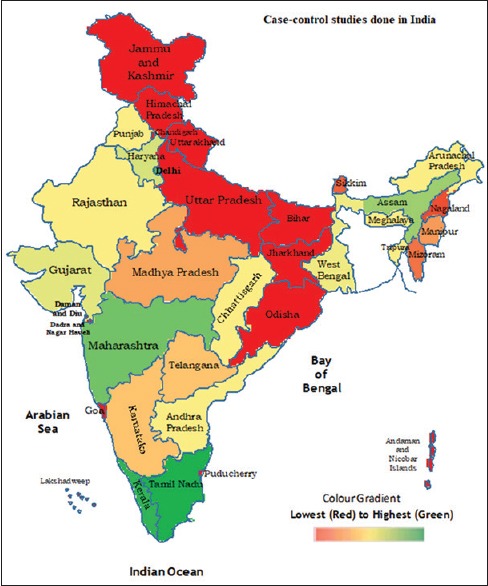
Distribution of breast cancer cases through case–control studies done in India
Modifiable risk factors
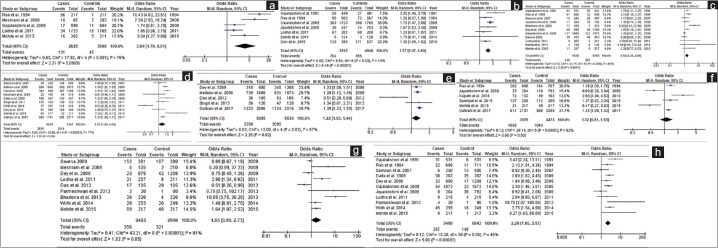
Stated modifiable risk factors of breast cancer in selected studies were BMI, age at first pregnancy, breastfeeding, OCP, parity, dietary pattern, residence, and marital status. The highest OR was observed for never breastfeed (pooled OR = 3.69, 95% CI = 1.70–8.01; P < 0.001) followed by never married (pooled OR = 2.29, 95% CI = 1.65–3.17; P < 0.001), nulli-parity (pooled OR = 1.58, 95% CI = 1.21–2.06; P < 0.001), age at first pregnancy (>25 years) (pooled OR = 1.57, 95% CI = 1.37–1.81; P < 0.001), urban residence (pooled OR = 1.12, 95% CI = 1.03–1.44; P = 0.02), and obese (BMI ≥25) (pooled OR = 1.19, 95% CI = 1.00–1.42; P = 0.04) [Figure 3]. Dietary pattern (pooled OR = 1.12, 95% CI = 0.81–1.53; P = 0.50) and use of oral contraceptive (pooled OR = 1.65, 95% CI = 0.99–2.73; P = 0.05) were not associated with breast cancer risk as pooled ORs are not statistically significant [Figure 3].
Figure 3.
Forest plots of modifiable risk factors of breast cancer. (a) Breastfeeding (never). (b) Age at 1st pregnancy (>25 years). (c) Parity (Nulli). (d) BMI (≥25). (e) Residence (Urban). (f) Dietary Pattern (Veg). (g) OCP (Yes). (h) Marital status (never)
Nonmodifiable risk factors
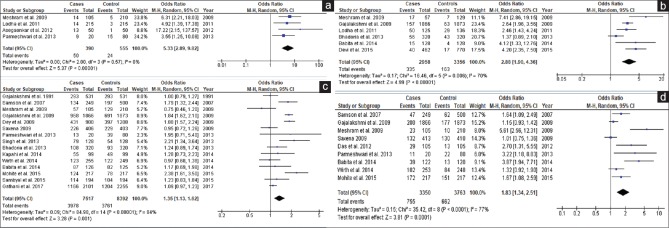
Figure 4 represents forest plots for nonmodifiable risk factors of breast cancer in selected studies, i.e. FH of breast cancer, menopausal status, age at menarche, and age of menopause. The highest OR was recorded in FH (pooled OR = 5.33, 95% CI = 2.89–9.82; P < 0.001) followed by menopausal age >50 years (pooled OR = 2.99, 95% CI = 1.90–4.36; P < 0.001), menarche onset age <13 years (pooled OR = 1.83, 95% CI = 1.34–2.51; P < 0.001), and menopausal status (pooled OR = 1.35, 95% CI = 1.13–1.62; P < 0.001).
Figure 4.
Forest plots of non-modifiable risk factors of breast cancer. (a) Family history (Yes). (b) Menopausal status (post). (c) Age at menopause (>50 years). (d) Age at menarche (<13 years)
Publication bias
Funnel plot assumes that observed effect sizes with similar precision (i.e., with similar standard error) should be more or less symmetrically distributed around the combined effect size. Publication biased is assessed by funnel plot [Figure 5]. Although there was no clear asymmetry, however, the plot demonstrates no significant publication bias.
Figure 5.
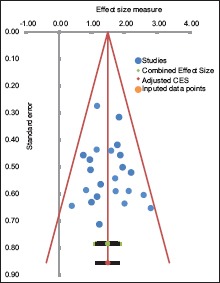
Funnel plot of studies included in the analysis
Limitations of the study
This meta-analysis incorporated only published studies and we are conscious of the so-called “file drawer effect problem” whereby statistically non significant studies are hardly published. As such there is the legitimate reason the results presented here may overestimate the true ORs. A comment about the relative quality of the included studies is warranted too. Furthermore, the extensiveness of the search guarantees that important studies were not omitted.
Discussion
The result of the meta-analysis done in this study reveals that modifiable factors such as BMI, age at first pregnancy, breastfeeding, OCP use, parity, and marital status are risk factors of breast cancer in addition to nonmodifiable risk factors. Similar findings are given in Datta et al., Devi et al., Dey et al., Gathani et al., Mathew et al. (2008), Meshram and Kulkarni, Mohite and Mohite, Samson et al., Singh and Jangra, Wirth et al., Bhadoria et al., Parameshwari et al., Saxena et al., Jayalekshmi et al., Rao et al., and Babita et al.[9,10,12,17,20,21,22,23,24,26,28,29,30,31,32,33] and others. Marital status and urban residence are not independent factors as they are associated with sedentary lifestyle and dietary pattern. The statement “use of oral contraceptive is a risk factor of breast cancer” is always contradictory in literature.
Women with nulli-parity and women with first pregnancy after 25 years have 58% and 57% breast cancer risks, respectively, as compared to women with parity and pregnancy before 25 years. In this study, the investigating team also confirms the findings in Babita et al., Dey et al. Lodha et al., Meshram and Kulkarni Gajalakshmi et al. Gajalakshmi et al., Mohite and Mohite, Rao et al., Saxena et al., Samson et al., and Jayalekshmi et al.[9,20,24,26,28,30,32,33,34,35,36] and others in this regard. Women with menopause after 50 years are almost three times more likely to have breast cancer as compared to women with menopause before 50 years.[12,17,28,30,35,36]
There is 35% higher risk of breast cancer to women with post-menopausal status as compared to post-menopausal status. However, it is directly linked with age at menopause. For more information, the reader is referred to Gajalakshmi et al. Gajalakshmi et al. Samson et al., Meshram and Kulkarni Dey et al. (2009), Saxena et al., Parameshwari et al., Singh and Jangra, Bhadoriya et al., Kapahi et al., Wirth et al., Babita et al. Mohite and Mohite, Sambyal et al. and Gathani et al.[9,17,20,21,23,25,27,28,29,30,31,32,33,34,35] and others. Women with <13 years onset age of menarche are at 85% higher risk of breast cancer.[9,19,28,29,30,31,32,33,35]
From the findings of this study, FH of breast cancer is a risk factor of breast cancer confirming the findings of Asegaonkar et al., Lodha et al., Parameshwari et al. and Meshram and Kulkarni[11,28,31,36] and others. Our results show that women with FH are 5.3 times more likely to have breast cancer risk as compared to women without any history of breast cancer.
Conclusion
This meta-analysis comprises studies from almost all the Indian regions. It confirms the results that the FH of breast cancer, never breastfeeding, nulli-parity, age at menarche (<13 years), age at menopause >50 years, first pregnancy age >25 years, BMI more than 25 years, post-menopausal status and never married are risk factors of breast cancer for women in India.
Financial support and sponsorship
Gayatri Vishwakarma had been awarded the grant of “Indian Council for Medical Research International Fellow” from Indian Council for Medical Research for providing funding to visit UTHSCT as visiting scientist.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to appreciate the help provided by the Director of the Library at the University of Texas Health Science Center at Tyler (UTHSCT). The authors thank Dr. Lipi B. Mahanta, IASST, Guwahati for providing the access to the data from her published research. Dr. Gayatri Vishwakarma acknowledges the Indian Council for Medical Research (ICMR) for providing funding to visit UTHSCT as visiting scientist. Dr. Vishwakarma is grateful to the Rajiv Gandhi Cancer Institute and Research Centre for permitting her visit to UTHSCT.
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al., editors. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute, Bethesda, MD. 2017. [Last assessed on 2018 Feb 13]. Available from: https://seer.cancer.gov/csr/1975_2014 .
- 2.Cardoso F, Spence D, Mertz S, Corneliussen-James D, Sabelko K, Gralow J, et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005-2015) Breast. 2018;39:131–8. doi: 10.1016/j.breast.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Kurian AW, Clarke CA, Carlson RW. The decline in breast cancer incidence: Real or imaginary? Curr Oncol Rep. 2009;11:21–8. doi: 10.1007/s11912-009-0005-7. [DOI] [PubMed] [Google Scholar]
- 4.Emens LA, Davidson NE. Adjuvant hormonal therapy for premenopausal women with breast cancer. Clin Cancer Res. 2003;9:486S–94S. [PubMed] [Google Scholar]
- 5.Jiwa M, Long A, Shaw T, Pagey G, Halkett G, Pillai V, et al. The management of acute adverse effects of breast cancer treatment in general practice: A video-vignette study. J Med Internet Res. 2014;16:e204. doi: 10.2196/jmir.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breast Cancer in Developing Countries. Lancet. 2009;374:1567. doi: 10.1016/S0140-6736(09)61930-9. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 8.Mohite AP, Mohite RV. Dietary factors and breast cancer: A case control study from rural India. Asian J Med Sci. 2014;6:55–60. [Google Scholar]
- 9.Mohite AP, Mohite RV. Reproductive risk factors and breast cancer: A case control study from rural India. Bangladesh J Med Sci. 2015;14:258–64. [Google Scholar]
- 10.Datta K, Biswas J. Influence of dietary habits, physical activity and affluence factors on breast cancer in East India: A case-control study. Asian Pac J Cancer Prev. 2009;10:219–22. [PubMed] [Google Scholar]
- 11.Asegaonkar S, Chaudhari SC, Bardapurkar JS. Lipid profile in breast cancer patients from rural India. J Indian Med Assoc. 2012;110:831–2, 837. [PubMed] [Google Scholar]
- 12.Devi KR, Chenkual S, Majumdar G, Ahmed J, Kaur T, Zonunmawia JC, et al. TLR2Δ22 (196174) significantly increases the risk of breast cancer in females carrying proline allele at codon 72 of TP53 gene: A casecontrol study from four ethnic groups of north eastern region of India. Tumour Biol. 2015;36:999510002. doi: 10.1007/s13277-015-3795-2. [DOI] [PubMed] [Google Scholar]
- 13.Moorman PG, Terry PD. Consumption of dairy products and the risk of breast cancer: A review of the literature. Am J Clin Nutr. 2004;80:514. doi: 10.1093/ajcn/80.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA. Family history and the risk of breast cancer: A systematic review and metaanalysis. Int J Cancer. 1997;71:8009. doi: 10.1002/(sici)1097-0215(19970529)71:5<800::aid-ijc18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Bhadoria AS, Kapil U, Sareen N, Singh P. Reproductive factors and breast cancer: A case-control study in tertiary care hospital of North India. Indian J Cancer. 2013;50:316–21. doi: 10.4103/0019-509X.123606. [DOI] [PubMed] [Google Scholar]
- 16.Mathew A, Gajalakshmi V, Rajan B, Kanimozhi VC, Brennan P, Binukumar BP, et al. Physical activity levels among urban and rural women in south India and the risk of breast cancer: A case-control study. Eur J Cancer Prev. 2009;18:368–76. doi: 10.1097/CEJ.0b013e32832e1c46. doi: 10.1097/CEJ.0b013e32832e1c46. [DOI] [PubMed] [Google Scholar]
- 17.Sloan FA, Gelband H. Cancer Control Opportunities in Low-and Middle-Income Countries1. Washington (DC): National Academies Press (US); 2007. doi: 10.17226/11797. [PubMed] [Google Scholar]
- 18.Das S, Sen S, Mukherjee A, Chakraborty D, Mondal PK. Risk factors of breast cancer among women in Eastern India: A tertiary hospital based case control study. Asian Pac J Cancer Prev. 2012;13:4979–81. doi: 10.7314/apjcp.2012.13.10.4979. [DOI] [PubMed] [Google Scholar]
- 19.Dey S, Boffetta P, Mathews A, Brennan P, Soliman A, Mathew A, et al. Risk factors according to estrogen receptor status of breast cancer patients in Trivandrum, South India. Int J Cancer. 2009;125:1663–70. doi: 10.1002/ijc.24460. [DOI] [PubMed] [Google Scholar]
- 20.Gathani T, Barnes I, Ali R, Arumugham R, Chacko R, Digumarti R, et al. Lifelong vegetarianism and breast cancer risk: A large multicentre case control study in India. BMC Womens Health. 2017;17:6. doi: 10.1186/s12905-016-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew A, Gajalakshmi V, Rajan B, Kanimozhi V, Brennan P, Mathew BS, et al. Anthropometric factors and breast cancer risk among urban and rural women in South India: A multicentric case-control study. Br J Cancer. 2008;99:207–13. doi: 10.1038/sj.bjc.6604423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh M, Jangra B. Association between body mass index and risk of breast cancer among females of north india. South Asian J Cancer. 2013;2:121–5. doi: 10.4103/2278-330X.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayalekshmi P, Varughese SC, Kalavathi, Nair MK, Jayaprakash V, Gangadharan P, et al. A nested case-control study of female breast cancer in karunagappally cohort in Kerala, India. Asian Pac J Cancer Prev. 2009;10:241–6. [PubMed] [Google Scholar]
- 24.Kapahi R, Guleria K, Sambyal V, Manjari M, Sudan M, Uppal MS, et al. Vascular endothelial growth factor (VEGF) gene polymorphisms and breast cancer risk in Punjabi population from North West India. Tumour Biol. 2014;35:11171–81. doi: 10.1007/s13277-014-2404-0. [DOI] [PubMed] [Google Scholar]
- 25.Rao DN, Ganesh B, Desai PB. Role of reproductive factors in breast cancer in a low-risk area: A case-control study. Br J Cancer. 1994;70:129–32. doi: 10.1038/bjc.1994.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambyal V, Guleria K, Kapahi R, Manjari M, Sudan M, Uppal MS, et al. Association of the -2518 A/G polymorphism of MCP-1 with breast cancer in Punjab, North-West India. Asian Pac J Cancer Prev. 2015;16:7243–8. doi: 10.7314/apjcp.2015.16.16.7243. [DOI] [PubMed] [Google Scholar]
- 27.Meshram HP, Kulkarni PN. Reproductive risk factors for breast cancer: A case control study. Online J Health Allied Sci. 2009;8:1–4. [Google Scholar]
- 28.Wirth MD, Burch JB, Hébert JR, Kowtal P, Mehrotra-Kapoor A, Steck SE, et al. Case-control study of breast cancer in India: Role of PERIOD3 clock gene length polymorphism and chronotype. Cancer Invest. 2014;32:321–9. doi: 10.3109/07357907.2014.919305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babita R, Kumar N, Karwasra RK, Singh M, Malik JS, Kaur A, et al. Reproductive risk factors associated with breast carcinoma in a tertiary care hospital of North India: A case-control study. Indian J Cancer. 2014;51:251–5. doi: 10.4103/0019-509X.146759. [DOI] [PubMed] [Google Scholar]
- 30.Parameshwari P, Muthukumar K, Jennifer HG. A population based case control study on breast cancer and the associated risk factors in a rural setting in Kerala, Southern India. J Clin Diagn Res. 2013;7:1913–6. doi: 10.7860/JCDR/2013/5830.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samson M, Swaminathan R, Rama R, Sridevi V, Nancy KN, Rajkumar T, et al. Role of GSTM1 (Null/Present), GSTP1 (Ile105Val) and P53 (Arg72Pro) genetic polymorphisms and the risk of breast cancer: A case control study from South India. Asian Pac J Cancer Prev. 2007;8:253–7. [PubMed] [Google Scholar]
- 32.Saxena A, Dhillon VS, Raish M, Asim M, Rehman S, Shukla NK, et al. Detection and relevance of germline genetic polymorphisms in glutathione S-transferases (GSTs) in breast cancer patients from Northern Indian population. Breast Cancer Res Treat. 2009;115:537–43. doi: 10.1007/s10549-008-0098-y. [DOI] [PubMed] [Google Scholar]
- 33.Gajalakshmi CK, Shanta V. Risk factors for female breast cancer. A hospital-based case-control study in Madras, India. Acta Oncol. 1991;30:569–74. doi: 10.3109/02841869109092419. [DOI] [PubMed] [Google Scholar]
- 34.Gajalakshmi V, Mathew A, Brennan P, Rajan B, Kanimozhi VC, Mathews A, et al. Breastfeeding and breast cancer risk in India: A multicenter case-control study. Int J Cancer. 2009;125:662–5. doi: 10.1002/ijc.24429. [DOI] [PubMed] [Google Scholar]
- 35.Lodha R, Joshi A, Paul D, Lodha KM, Nahar N, Shrivastava A, et al. Association between reproductive factors and breast cancer in an urban set up at Central India: Acasecontrol study. Indian J Cancer. 2011;48:3037. doi: 10.4103/0019-509X.84928. [DOI] [PubMed] [Google Scholar]
- 36.Mathew A, Gajalakshmi V, Rajan B, Kanimozhi VC, Brennan P, Binukumar BP, et al. Physical activity levels among urban and rural women in south India and the risk of breast cancer: A case-control study. Eur J Cancer Prev. 2009;18:368–76. doi: 10.1097/CEJ.0b013e32832e1c46. doi: 10.1097/CEJ.0b013e32832e1c46. [DOI] [PubMed] [Google Scholar]