Abstract
Prostate cancer (PCa) is the most common cause of malignancy in males and the third leading cause of cancer mortality in the United States. The standard care for primary PCa with local invasive disease mainly is surgery and radiation. For patients with distant metastases, androgen deprivation therapy (ADT) is a gold standard. Regardless of a favorable outcome of ADT, patients inevitably relapse to an end-stage castration-resistant prostate cancer (CRPC) leading to mortality. Therefore, revealing the mechanism and identifying cellular components driving aggressive PCa is critical for prognosis and therapeutic intervention. Cancer stem cell (CSC) phenotypes characterized as poor differentiation, cancer initiation with self-renewal capabilities, and therapeutic resistance are proposed to contribute to the onset of CRPC. In this review, we discuss the role of CSC in CRPC with the evidence of CSC phenotypes and the possible underlying mechanisms.
Keywords: cancer stem cell, castration-resistant prostate cancer, neuroendocrine differentiation, transdifferentiation
INTRODUCTION
Prostate cancer (PCa) remains the most commonly diagnosed cancer, from the 2017 cancer statistic report, there were 180 890 estimated new cases in 2017, and PCa is also the third leading cause of death, with 26 120 estimated deaths in the United States.
Clinical treatment for primary PCa includes radical prostatectomy and radiotherapy. Androgen deprivation therapy (ADT) is commonly used to decrease the androgen-dependent tumor burden of metastatic PCa (mPCa). Castration-resistant PCa (CRPC) is defined as the reappearance of cancer lesion from metastatic site(s) often with rising prostate-specific antigen (PSA) in patient's serum. CRPC is recognized as the end-stage disease since patients do not respond to chemotherapeutics very well with average 6-month to 1-year survival. Although recent introduction of second-line anti-androgen agents has prolonged patients’ survival, PCa eventually develops therapy-resistant phenotypes. Clinically, therapy-resistant tumors can be divided into several different phenotypes such as neuroendocrine, androgen receptor (AR) variants, and AR hyperactivation due to gene amplification and/or mutation, which raise a critical question for the cell origin of these subtypes. Many studies have demonstrated the presence of stem-like population in normal prostate and abnormal prostate,1,2,3 which raises the potential role of cancer stem cell (CSC) in cancer progression. In this article, we have summarized the potential pathways associated with CSC leading to CRPC and the possible therapeutic strategies to improve the clinical outcomes of PCa patients.
CANCER STEM CELL IN CRPC
Cell markers for CSC
The CSC theory, as a potential mechanism for CRPC, has raised significant attention in recent years. In general, embryonic stem cell is pluripotent with the ability of developing into different tissue types. Somatic stem cell in each organ is considered to be quiescent most of time with limited number and capable of self-renewing and differentiation maintained in a homeostatic balance. Isaacs and Coffey4 first proposed that prostate stem cell resides in basal cell population; its expansion underlies the development of benign prostatic hyperplasia (BPH). Further study suggested that enrich stem cell is in the proximal duct of prostate.5 During normal prostate development, androgen binding to the AR in surrounding stromal cells plays a key role of basal stem cell different into luminal cell population.6 Recent study using gene-tracing technology indicated that luminal cell population has stem cells as well with less potency than basal stem cell.7 For CSC, its self-renewal ability allows a single cell remained after therapies to repopulate entire tumor population as therapeutic resistance. Further, the pluripotency of CSC is capable of differentiating into different cell types such as neuroendocrine. It has been reported that prostate CSC is likely derived from basal cell population.8 Normal prostate gland can be divided into epithelial, stromal, and neuroendocrine cells; the epithelial cells include luminal and basal cells. Among all these heterogeneous cell types in the normal prostate, the gene expression profile of basal cell is highly correlated with that of stem cell. Noticeably, the basal cell gene profiles are enriched in advanced, anaplastic, castration-resistant, and metastatic PCa in the human PCa sample set.9 However, it is still unclear whether clonal expansion and/or adaptation through epithelial-to-mesenchymal transition (EMT)10,11,12 or transdifferentiation13 during therapies results in the expansion of CSC.
Several markers have been commonly used to identify CSC population including CD44, stem cell antigen (Sca-1 or Ly6A), prominin-1 (CD133), and ATP-binding cassette subfamily G member 2 (Junior blood group) (ABCG2). CD44 is often associated with CSC, which is a cell membrane receptor that is involved in cell–cell interactions, adhesion, and migration. Especially, cells expressing CD44, but lacking CD24 (CD44+ CD24−) PCa cells, were identified as the CSC population with in vivo and in vitro models.14 These CD44+ CD24− PCa cells have the ability of forming spheres and producing tumor from a single cell, which is known as stem cell self-renewal ability. Sca-1 is a mouse glycosyl phostidylinositol-anchored surface protein that expressed by stem cells or progenitor cells. However, the human homolog of Sca-1 has not been identified yet. Studies in mouse model demonstrated that cells with Sca-1 expression have tumor-initiating ability, and tumor cells expressing higher Sca-1 were correlated with their aggressive phenotype.15,16 CD133 is a transmembrane glycoprotein, and is known as a marker for basal stem cell as well as PCa-initiating cell. Richardson et al.17 reported that a subset of CD133+ population exhibited higher clonogenic potential than CD133− population. Furthermore, these CD133+ populating can fully differentiate to prostatic acini from in vivo animal model. In addition, studies demonstrated that CD133 involved in cell growth, cell development, and tumor progression, in which the expression of CD133 was significantly increased in cancer-initiating cells using patient-derived primary cell model.18 ABCG2 is the ATP-binding cassette membrane transporter. Patient-derived cells with high ABCG2 expression correlated with cell that expresses stem cell markers, and these subsets of cells have shown to gain multidrug resistance and be responsible for the recurrence of PCa.19 Although these CSC markers20 listed in this review are indeed correlated with CSC population associated with cancer progression, recurrence, and therapy resistance, there is still lacking a specific PCa CSC marker.
Molecular signaling pathways lead to CSC in CPRC
Three signaling pathways have been suggested to be critical for CSC development including Wnt, Sonic Hedgehog, and Notch signaling pathways. Several reports have demonstrated that targeting these signaling pathways along with conventional treatment can prevent the emergence of CRPC.21,22
Wnt
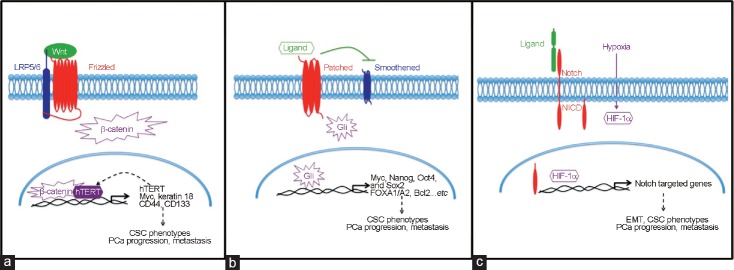
In the canonical of Wnt pathway, Wnt ligands bind to Frizzled and low-density lipoprotein receptor–related protein (LRP) 5/6, which activate downstream molecular targets, leading to the accumulation and nuclear translocation of β-catenin, subsequently affecting cell survival; while, in the noncanonical pathways, Wnt activates downstream effectors and activates targeted gene expression and cytoskeleton rearrangement, resulting in altered cell survival. Abnormal Wnt signaling has been found in several cancer types, including brain, breast, and colorectal cancer.23 In PCa, elevated β-catenin expression was often found in the nucleus of cancer cells.24 Importantly, Wnt signal regulates self-renewal ability of several cell models including LNCaP, C42B, and PC3 cell in an AR-independent manner,25,26 while downregulated Wnt/β-catenin pathway significantly suppresses stem cell-like properties.27 Furthermore, Wnt3 has been shown to increase the expression of its downstream effectors, as well as CSC markers including CD133 and CD44, which subsequently lead to sphere formation.25 In addition, Zhang et al.23 demonstrated that human telomerase reverse transcriptase (hTERT)-expressing PCa cells have higher Wnt/β-catenin activity and can thereby regulate the self-renewal and differentiation activity of PCa cells. Collectively, Wnt plays a key role in CSC development in CRPC (Figure 1a).
Figure 1.
Schematic representation of signal contributes to CSC phenotype. (a) Wnt proteins bind to both frizzled receptor proteins and the co-receptor LRP5/6. This binding further facilitates the activation of β-catenin. Activated β-catenin translocates into nucleus and promotes Wnt downstream gene transcription. Furthermore, Wnt/β-catenin induced hTERT also acts as a transcriptional factor, resulting a positive feedback in the enhanced expression of Wnt target genes that leads to cancer-promoting functions and CSC phenotypes. (b) Sonic Hedgehog pathway is initiated by binding one of the three secreted Hedgehog ligands to its receptor. This binding releases Smoothened that modulates the expression of three Gli zinc-finger transcription factors. (c) The Notch receptor is activated by ligand binding, which is presented by a neighboring cell. Notch activation releases an active fragment, NICD. NICD then translocates into the nucleus and promotes the expression of targeted genes. Notch-dependent signaling induces several genes associated with differentiation, survival, stemness, and EMT, which all relate to PCa progression and metastasis. Further, Notch signaling is often activated by hypoxia through HIF-1α. CSC: cancer stem cell; PCa: prostate cancer; hTERT: human telomerase reverse transcriptase; LRP: low-density lipoprotein receptor-related protein; Gli: glioma-associated oncogene homolog; Sox 2: sex determining region Y-box 2; FOXA1/A2: forkhead box A1/A2; NICD: notch intracellular domain; HIF-1α: hypoxia-inducible factor 1 subunit alpha; EMT: epithelial-to-mesenchymal transition; Bcl2: B-cell lymphoma 2; Oct4: octamer-binding transcription factor 4.
Sonic Hedgehog
Sonic Hedgehog signaling pathway is a conserved process that controls cell renewal and cell survival. Hedgehog signaling is initiated by hedgehog family ligands (Sonic, Desert, and Indian). These ligands bind to membrane receptors Patched (Ptch1 and 2) and Smoothened on the primary cilium, leading to the activation and nuclear translocation of glioma-associated oncogene homolog (Gli) (Gli 1, 2, and 3), which trigger the expression of targeted genes that regulate cell survival. Abnormal Hedgehog signaling pathway has been found in several cancer types, including brain, gastrointestinal, lung, breast, and prostate cancers.28,29 Some studies also demonstrated that Hedgehog involved in tumor progression and CSC proliferation.30,31,32 Importantly, using several PCa cell line models,29 i.e., LNCaP, Du145, PC3, 22Rv1, and LAPC4, as well as xenograft model (CWR22),30 several Gli-related genes have been shown to correlate with the progression of CPRC. These Gli-related genes, including CDKN2A/p16/INK4A, Myc, and CDK2, promote AR-independent tumor cell growth33,34 and lead to tumor recurrence.35 Data from PCa patients-derived tissue microarray and PCa cell line models (PC3, LNCaP, and Du145) indicated that forkhead box A1 (FOXA1) was found to promote the progression of CRPC.36,37 Cell model (LNCaP, PC3, Du145, and LAPC4) also showed that the expression of B-cell lymphoma 2 (Bcl2) correlates with therapy resistance ability in CRPC.38 Importantly, increased Hedgehog signaling was found in PCa CSC population (CD44+ CD24−) in vivo and in vitro.14 Taken together, Hedgehog downstream signaling pathway contributes to CRPC progression and therapy-resistant phenotypes of CSC (Figure 1b).
Notch
Notch signaling pathway has been extensively studied and has been correlated to promote CSC phenotype in several cancers. Notch signaling is mediated by four Notch receptors, including Notch1–4, and five ligands including delta-like (DLL) 1, DLL 3, DLL4, Jagged 1, and Jagged 2, were involved. Various types of cancers exhibit dysfunction of the Notch pathway in their cells.39 Importantly, Notch interacts with AR pathway and the phosphoinositide 3-kinase (PI3k)/Akt pathway, which are the two main signaling pathways in controlling prostate development and carcinogenesis.40,41,42 Studies from cell culture models and human PCa specimens have demonstrated that higher Notch ligand, Jagged 1-Notch1 signaling contributes to PCa progression and metastasis, and promotes EMT and CSC phenotype.43,44,45,46 Higher Notch3 expression was found in CRPC, which was induced by hypoxia condition.47 Recently, Notch4-targeted silencing, leading to the inhibition of nuclear factor kappa B (NF-κB) activity, also showed a promising anti-PCa growth and anti-EMT effect.44,48,49 These reports all point out the importance of Notch signal in PCa progression and the initiation of CSC phenotype (Figure 1c).
Caveolin-1 (Cav-1)
The Caveolin protein family including caveolin-1, -2, and -3 is the major component of caveolae; Cav-1 is the first member identified and has been extensively characterized. It is known to regulate multiple cellular functions, including cell cycle, signal transduction, endocytosis, and cholesterol trafficking and efflux. Cav-1 levels are correlated with PCa progression and metastasis.50,51,52 Extensive studies using PCa cell line (Du145, LNCaP, and 22Rv1), and human BPH sample model, also indicated that Cav-1 signaling contributes to therapy resistance and the reoccurrence of PCa.52,53,54 Using xenograft model, Cav-1 can be secreted into circulation to enhance tumor growth in a paracrine manner, implying that Cav-1 can be a secretory protein.55,56 Interestingly, other study has demonstrated that Cav-1 can elevate the Notch-1-Akt-NF-kappaB pathway, leading to chemoresistance in ovarian cancer.57 Studies from PCa cell models (LNCaP, PC3, and Du145) and immunostaining of PCa specimens have indicated that Cav-1 can interact with LRP6 to regulate Wnt-β-catenin signaling.58,59 Moreover, Cav-1 contributes to the expression of CSC-related genes including Oct4, Nanog, CD44, CD133, and ABCG2 in lung cancer cell and breast cancer models,59,60 which all strongly suggest that Cav-1 signaling involved in the induction of CSC phenotypes. However, additional data are required to link Cav-1 directly to CSC generation in CRPC.
MicroRNAs contribute to CSC properties of PCa cell
Emerging evidence has implied that microRNA (miRNA) regulation is crucial in promoting or repressing cancer metastasis via regulating the characteristics of CSCs. In particular, dysregulation of miRNAs is associated with tumor initiation and progression of PCa. A coordinated downregulation of miR-34a, let-7b, miR-106a, and miR-200 family has been observed in the progenitor stem cell population of PCa (Table 1).61
Table 1.
MicroRNAs involved in prostate cancer progression to castration-resistant prostate cancer
| MicroRNAs | Target gene | Impacts on PCa progression | Reference |
|---|---|---|---|
| miR-34 | CD44 | Inhibits PCa metastasis, regeneration, and carcinogenesis | 62,63 |
| miR-383 | CD44 | Inhibits tumor-initiating potential and metastasis of CD44-positive PCa cells | 64 |
| miR-320 | CD133, CD117, CXCXR4, ABCG2 | Suppress tumor-sphere formation, chemoresistance, and tumorigenic abilities of prostatic CSCs | 27 |
| miR-7 | Klf4 | Inhibits stemness properties and impaires tumorigenesis of PCa stem-like cells | 65 |
| let-7 | EZH2 | Diminishes colonogenic ability and sphere-forming capacity of PCa cells | 61,66 |
| miR-100 | AGO2 | Regulates spheroid and colony formation of PCa cells | 68,69 |
| miR-200b | Bmi-1 | Suppresses proliferation and migration, as well as enhances chemosensitivity of PCa cells to docetaxel | 70 |
| miR-141 | Klf9 | Facilitates spheroid formation and proliferation of PCa cell | 73 |
| miR-143 miR-145 |
Oct4, Sox2, Klf4, FNDC3B | Inhibits cell viability and colony formation of bone metastatic PC3 cells. Suppresses tumor sphere formation and CSC marker expression in PC-3 cells | 74,76,77,78 |
| miR-128 | BMI-1, NANOG, TGFBR1 | Reduces sphere formation and colonogenic potential of PCa cells | 79 |
| miR-663 | Enhances cell proliferation, invasion and neuroendocrine differentiation characteristics in PCa cells | 108 |
PCa: prostate cancer; CRPC: castration-resistant PCa; CSC: cancer stem cell; Sox2: sex determining region Y-box 2; Bmi-1: B-cell-specific Moloney murine leukemia virus insertion site 1; TGFBR1: transforming growth factor beta receptor 1; Klf4: Kruppel-like factor 4; FNDC3B: fibronectin type III domain containing 3B; EZH2: enhancer of Zeste homolog 2; Klf9: Kruppel like factor 9; CXCXR4: C-X-C chemokine receptor type 4; ABCG2: ATP-binding cassette subfamily G member 2 (Junior blood group); AGO2: argonaute 2, RISC catalytic component
miR-34, a p53 downstream target gene, is known as a tumor-suppressor miRNA. Frequent hypermethylation of miR-34 has been observed in many malignancies with p53 mutation. Cheng et al.62 using conditional knockout/transgenic mouse model demonstrated that inactivation of both p53 and miR-34a in mouse prostate epithelium leads to the expansion of the prostate stem cell compartment, as well as development of early invasive adenocarcinoma and high-grade prostatic intraepithelial neoplasia. Consistent with their in vivo observations, combined deficiency of both miR-34 and p53 leads to accelerated EMT-dependent growth, enhanced self-renewal capacity, and increased cell motility in prostate stem/progenitor cells derived from the proximal region of prostatic ducts.62 In addition, miR-34a is known to be a key negative regulator of CD44, an adhesion molecule that is a key player in metastasis. CSCs derived from multiple malignant tumors have shown high expression of CD44. These CD44-positive CSC populations have colonogenic, tumor-initiating, and metastatic capacities. Liu et al.63 demonstrated that systemic delivery of miR-34a can inhibit PCa metastasis and regeneration by targeting CD44. A recent study done by Bucay et al.64 also revealed that another CD44-targeting miRNA, miR-383, is frequently downregulated due to loss of the chromosome 8p22 locus in the progression of PCa. Functionally, miR-383 is shown to inhibit tumor-initiating potential and metastasis of CD44-positive PCa cells by direct targeting of CD44.64
miR-320 is found significantly downregulated in the progression of PCa; reduction of miR-320 associated with increased β-catenin expression has been observed in a CD44-high subpopulation of PCa cells and clinical prostatic tumor specimens. By global gene expression profiling, we reported that ectopic expression of miR-320 in PCa cells leads to suppression of CSC markers such as CD133, CD117, CXCXR4, and ABCG2, as well as downstream target genes of Wnt/β-catenin pathway.27 Functionally, miR-320 deficiency facilitates the CSC properties including tumor-sphere formation, chemo-resistance, and tumorigenic abilities. Overall, this study strongly suggested that miR-320 is a potent regulator of tumor-initiating cells in prostate.
Similar to miR-320, expression level of miR-7 is also significantly reduced in a subpopulation of CD133-positive/CD44-positive PCa cells, which possess CSC-like features and are sufficient for tumorigenesis based on a limited dilution analysis. On the other hand, restoration of miR-7 in PCa cell lines results in sustained inhibition of CSC characteristics and impaired tumorigenesis via targeting Kruppel-like factor 4 (Klf4). Overall, this study implies the critical role of miR-7 in regulating the properties of PCa stem cell.65
Meanwhile, loss of the let-7 family has been observed in PCa tissue specimens, particularly in high-grade tumor. Kong et al.66 demonstrated an inverse correlation between let-7 and enhancer of Zeste homolog 2 (EZH2), a putative let-7 family target that is highly expressed in CSCs of many malignancies and is known to regulate expansion and maintenance of CSC.67 Functionally, let-7 is shown to diminish both colonogenic ability and sphere-forming capacity via targeting EZH2 in PCa cells.66
Similar to let-7, expression level of miR-100 is also significantly decreased particularly in bone metastatic PCa specimens. Wang et al.68 suggested that miR-100 regulates spheroid and colony formation of PCa cells by targeting argonaute 2, RISC catalytic component (Ago2), leading to suppression of stemness markers such as c-Myc, CD44, Klf4, and Oct4. This indicates that loss of miR-100 may promote the stemness properties of PCa. On the contrary, by screening miRNA expression in PCa patient-derived xenograft tumor lineages, a recent study done by Nabavi et al.69 demonstrated that several miRNAs (miR-100-5p, miR-411-5p, and miR-185-5p) are associated with the regression to dormancy status after ADT. Particularly, miR-100 has been recognized as a key component contributing to initiation and evolution of CRPC; it is believed that miR-100 is critical for the cell survival upon AR deprivation in AR-positive PCa cell lines.69
The miR-200 family (miR-200a/b/c and miR-141) is known for targeting mesenchymal transcription factors leading to inhibition of EMT. In particular, Yu et al.70 reported that miR-200b is significantly downregulated in PCa in vivo and in advanced PCa cell lines (LNCaP, PC3, and DU145), as well as patient samples (BPH) in vitro. Ectopic expression of miR-200b sensitizes PCa cells to chemotherapeutic reagent, docetaxel, by targeting the gene, B-cell-specific Moloney murine leukemia virus insertion site 1 (Bmi-1),70 which is a critical regulator of CSC properties in several malignancies such as breast and gastric cancers.71,72 In contrast, miR-141-3p has an opposite regulatory role. Li et al.73 utilized miR-141-3p mimics to demonstrate its effect on facilitating spheroid formation and proliferation of PCa cell line (PC3). The impact of miR-141 on promoting PC3 stemness is associated with the upregulation of Oct4, Bmi-1, sex-determining region Y-box 9 (Sox9), and CD44, suggesting that miR-141 can target a common repressor of these genes.73 Certainly, more detailed studies are needed to unveil the mechanism of action of miR-141.
Both miR-143 and miR-145 are known to regulate bone metastasis of PCa. Huang et al.74 reported that both miR-143 and miR-145 can inhibit colony formation, suppress sphere-forming capacity, and reduce expression of CSC markers (CD133, CD44, Oct4, c-Myc, and Klf4) in bone-metastasis-derived PCa cell line. The finding of this study strongly suggested that miR-143 and miR-145 may play a crucial role in regulating CSCs in bone metastatic PCa. Accumulating studies have indicated that miR-145 negatively regulates pluripotency of embryonic stem cells via targeting several stemness markers such as Oct4, Sox2, and Klf4.75,76 Ozen et al.77 demonstrated that ectopic expression of miR-145 leads to reduced cell renewal of PCa cell lines by targeting Sox2 gene expression. By screening the miRNA expression between 3D-sphere and 2D-adherent PCa cells, Fan et al.78 unveiled that progressively elevated miR-143 is found in the sphere-re-adherent culture of PCa cells, suggesting that miR-143 is involved in stem cell formation.
In contrast to those miRNAs with CSC-promoting activities, it is significantly reduced miR-128 in PCa compared to benign prostate tissue that may have an opposite function. Indeed, Jin et al.79 demonstrated that overexpression of miR-128 leads to diminished CSC properties by reducing sphere formation and clonogenic potential in PCa cells. Mechanistically, miR-128 is shown to target on several self-renewal genes such as BMI-1, NANOG, and transforming growth factor beta receptor 1 (TGFBR1).79 Overall, this study highly suggested that miR-128 regulates tumor initiation in PCa by limiting the CSC properties mediated by BMI-1 and NANOG.
NEUROENDOCRINE DIFFERENTIATION (NED) IN CRPC AND CSC
Prostate NED carcinoma is considered as a type of prostatic epithelial neoplasms that have NED feature,80 which usually identified by histopathological examination with NED markers. Therefore, NED can be found in small cell carcinoma, carcinoid, and carcinoid-like tumors, as well as prostatic adenocarcinoma (Figure 2). Importantly, NED has been found in recurrent CPRC after second line of ADT.81,82 Although small cell carcinoma in prostate only counts for less than 2% of total PCa population,80 neuroendocrine prostate cancer (NEPC) is detected in 10%–20% of CRPC population.83 Interestingly, a study with PCa cells (Du145 and PC3) and xenograft models demonstrates that CD44+ is selectively expressed in neuroendocrine cells and these cells are responsible for PCa recurrence.84 NEPC is an end stage of CRPC and most patients survive less than a year after recurrence.85 Clinically, NEPC is identified based on histology features. In general, the tumor cell morphology was similar to high-grade neuroendocrine cancers, which have high numbers of mitotic cells with nuclear molding and chromatin-like “salt and pepper” similar to small cell.81,86 In addition, there are neuroendocrine markers that can be used for validation.
Figure 2.
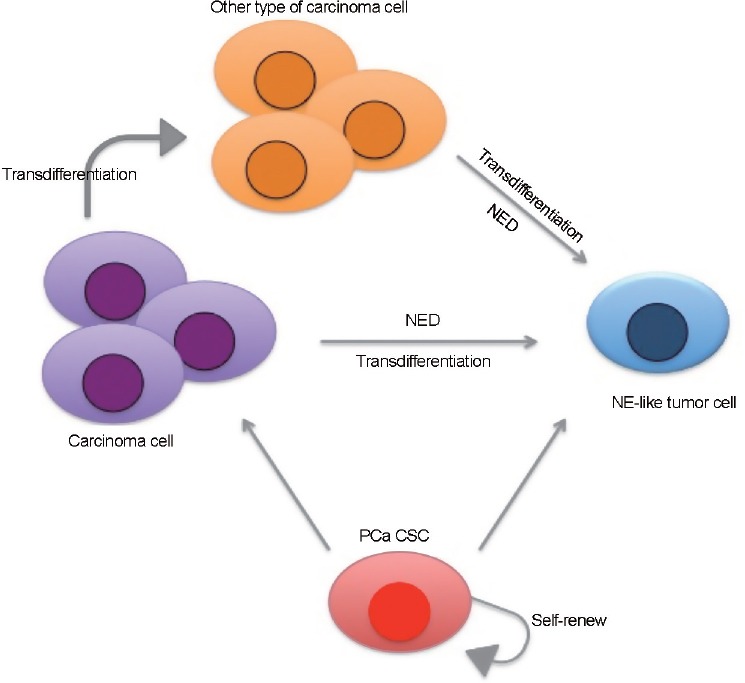
Schematic representation of the relationship of CSC, PCa cells, and NE-like cell. CSC maintains its multipotency to form different types of tumor cell and is capable of self-renewing to expand its progeny. NE-like tumor cell can originate from the same type of tumor cells that undergo NED or transdifferentiation from other type of tumor cell (i.e., adenocarcinoma to small cell adenocarcinoma with NE phenotype). CSC: cancer stem cell; PCa: prostate cancer; NE: neuroendocrine; NED: neuroendocrine differentiation.
Cell markers for NED in CRPC
There are several general NED markers that are currently used to diagnose NEPC, and the presence of at least one of these is diagnostic of the condition. These markers include (1) neuron-specific enolase (NSE), a cell-specific isoenzyme of the glycolytic enzyme enolase, and it is one of the most reliable markers for the diagnosis of small cell in lung cancer;87 (2) synaptophysin (SYP), a major synaptic vesicle protein, is usually combined with the neuroendocrine secretory protein, chormogranin A (ChgA), for diagnosis;88 (3) ChgA, a secretory protein found in the neuroendocrine cells, has commonly used in detecting neuroendocrine tumors;89 (4) CD56, also known as neural cell adhesive molecule (NCAM), which belongs to a group of cell surface glycoproteins involved in cell-cell adhesion.90 On the other hand, NEPC cells often lack the expression of luminal markers such as PSA and prostatic acid phosphatase.91 Recently, FOXA2 has also been suggested to be a reliable marker in diagnosis of NEPC.92
Molecular mechanisms leading to NED in CRPC
Several studies have indicated that Myc (N-Myc or c-Myc) plays a key role in NEPC and also Myc gene amplification or protein overexpression is often detected in clinical specimens.93 Recently, Lee et al.94 have indicated that ectopic expression of N-Myc in basal cell population of prostate potentiates NEPC through the activation of Akt signaling pathway with human patient sample model in vivo and in vitro. Other embryonic transcription factors such as FOXA1 and FOXA2 are associated with NEPC. FOXA1 is known as a pioneering factor in modulating AR activity, and is also involved in prostate epithelial differentiation by altering chromatin tertiary structure. However, the loss of FOXA1 is found to facilitate NEPC through the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathways in both cell line model (TRAMP-C and 22Rv1) and human patient sample.95 In contrast, FOXA2 expression was detected in NEPC, but not in primary and metastatic PCa samples (NCI-H660, PC3),92 and it cooperates with hypoxia-inducible factor 1 subunit alpha (HIF-1α) activity to facilitate NED in PCa cell model (PC3, NCIH660, LAPC4, and LNCaP).96 In addition, other key regulators in neuronal differentiation such as BRN2, POU-domain transcription factor, have recently shown to regulate Sox2, which contributes to NED in PCa cell model (PC3, NCI-H660, LNCaP, and LAPC4).97
Under the influence of tumor microenvironment, studies with mouse PCa in vitro and in vivo model, as well as LNCaP cell model, have found that interleukin 6/signal transducer and activator of transcription 3 (IL-6/STAT3) pathway associated with aurora kinase A (AURKA), N-Myc, and EZH2 have been shown to induce NEPC.98,99 Mechanistically, in LNCaP cell model, autophagy process is involved in IL-6-induced NED and therapy-resistant phenotype in PCa model;100 thus, targeting autophagy synthesis or related signaling pathways can block neuroendocrine cell differentiation.101,102 In addition, secretory protein secretogranin II (SgII), a neuroendocrine secretory protein-like ChgA that is widely distributed throughout the neuroendocrine system, has been shown to induce NEPC phenotype.103 G-protein coupled receptor kinase 3 (GRK3) expression correlated with PCa progression has been shown to increase neuroendocrine phenotypes associated with ADT resistance in PCa cells (NCI-H660, PC3, LNCaP, and VCaP).104 In contrast to oncogenic factors, loss of retinoblastoma 1 (RB1) and tumor protein p53 (TP53) further facilitates ADT resistance of PCa cells that exhibit several NED markers in both clinical patient sample and mouse model.105,106
MicroRNAs correlated with NED in PCa
Dang et al.107 demonstrated that the treatment of enzalutamide or casodex increases the infiltration of mast cells secreting C-C motif chemokine ligand 8 (CCL8) and IL-8 that can promote NED phenotypes by upregulating miR-32 in PCa cells. Indeed, overexpression of miR-32 leads to enhanced NED characteristics in PCa cells associated with elevated NSE. Overall, this study demonstrates that the potential impact of immune cells on the emergence of NED in PCa is mediated by microRNA.107
Meanwhile, by examining PCa tissue specimens, Jiao et al.108 revealed a significant upregulation of miR-663 in CRPC. Functionally, overexpression of miR-663 in LNCaP cell results in enhanced cell proliferation, invasion, and NED. Clinically, expression level of miR-663 is correlated with PCa grade, node, and metastasis (TNM) staging of PCa,108 suggesting miR-663 as a potent regulator in NEPC.
In addition, Liang et al.109 reported that hypoxia can promote NED in PCa cell lines such as LNCaP and PC3 by inducing the expression of a polycistronic miRNA cluster containing miR-106b, miR-93, and miR-25, which can suppress the expression of neuron-restrictive silencer factor, also known as RE1-Silencing Transcription factor (REST) gene, but increase of pro-neuronal genes such as paired mesoderm homeobox protein 2A (PHOX2A), absent small and homeotic disks protein 1 (ASH-1), and ChgA. Clinically, an inverse correlation between miR-106b-25 and REST is observed in PCa with high Gleason grade.109 Altogether, this study suggested that loss of REST due to elevation of miR-106b-25 under hypoxia condition might promote NED in PCa.
TRANSDIFFERENTIATION IN CPRC
Transdifferentiation is the process of cell conversion from one type to another type (Figure 2). For example, pancreatic progenitor cells transdifferentiate into hepatocyte-like cells.110 Recent data clearly show that somatic cells can undergo reprograming process by exogenously introducing key transcription factors such as Sox2, Klf4, c-Myc, Oct4, Nanog, and lin-28 homolog A (LIN28).111,112 For cancer cells, particularly, high-grade poorly differentiated cancer cells exhibiting CSC phenotypes can transdifferentiate into different cell types by turning on similar genes endogenously.113 For example, loss of Rb1 and TP53 underlying cancer lineage plasticity106 is mediated by increased Sox2 expression in these cells.114 Similarly, loss of PTEN and TP53 facilitates ADT resistance and initiates transdifferentation event in adenocarcinoma, which is evidenced by elevated NED markers.115 All these data conclude that CSC is the result of cancer cell de-differentiation through genetic alteration and/or epigenetic alteration from tumor microenvironment. In CRPC, accumulating evidence supports that CSC plays a central role of therapeutic resistance, NED. Thus, developing anti-CSC strategy is expected to improve the survival of CRPC patients.
CONCLUSION
The presence of CSC has been identified in hematopoietic and testicular cancers but less known in solid tumors, particularly PCa. Accumulating data support the critical role of prostate CSC in disease progression. Despite these progresses, there is still lacking human PCa-specific CSC marker(s). Further, the underlying mechanisms of transdifferentiation as well as NED in CSC are not fully understood, which will be critical for further developing better therapeutic strategies.
AUTHOR CONTRIBUTIONS
CJL and UGL prepared manuscript. JTH outlined framework and finalized manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We thank Dr. Samarpita Sengupta for editorial assistance. This work was supported in part by grants from the United States Army (W81XWH-16-1-0474 to Jer-Tsong Hsieh).
REFERENCES
- 1.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–19. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 2.Maitland NJ, Collins A. A tumour stem cell hypothesis for the origins of prostate cancer. BJU Int. 2005;96:1219–23. doi: 10.1111/j.1464-410X.2005.05744.x. [DOI] [PubMed] [Google Scholar]
- 3.Schalken JA, van Leenders G. Cellular and molecular biology of the prostate: stem cell biology. Urology. 2003;62:11–20. doi: 10.1016/s0090-4295(03)00758-1. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 1989;2:33–50. doi: 10.1002/pros.2990150506. [DOI] [PubMed] [Google Scholar]
- 5.Goto K, Salm SN, Coetzee S, Xiong X, Burger PE, et al. Proximal prostatic stem cells are programmed to regenerate a proximal-distal ductal axis. Stem Cells. 2006;24:1859–68. doi: 10.1634/stemcells.2005-0585. [DOI] [PubMed] [Google Scholar]
- 6.Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205:181–93. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- 7.Moad M, Hannezo E, Buczacki SJ, Wilson L, El-Sherif A, et al. Multipotent basal stem cells, maintained in localized proximal niches, support directed long-ranging epithelial flows in human prostates. Cell Rep. 2017;20:1609–22. doi: 10.1016/j.celrep.2017.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schalken JA. Molecular and cellular prostate biology: origin of prostate-specific antigen expression and implications for benign prostatic hyperplasia. BJU Int. 2004;93(Suppl 1):5–9. doi: 10.1111/j.1464-410x.2003.04633.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Park D, Zhong Y, Lu Y, Rycaj K, et al. Stem cell and neurogenic gene-expression profiles link prostate basal cells to aggressive prostate cancer. Nat Commun. 2016;7:10798. doi: 10.1038/ncomms10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harner-Foreman N, Vadakekolathu J, Laversin SA, Mathieu MG, Reeder S, et al. A novel spontaneous model of epithelial-mesenchymal transition (EMT) using a primary prostate cancer derived cell line demonstrating distinct stem-like characteristics. Sci Rep. 2017;7:40633. doi: 10.1038/srep40633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong D, Sethi S, Li Y, Chen W, Sakr WA, et al. Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes. Prostate. 2015;75:161–74. doi: 10.1002/pros.22901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abell AN, Johnson GL. Implications of mesenchymal cells in cancer stem cell populations: relevance to EMT. Curr Pathobiol Rep. 2014;2:21–6. doi: 10.1007/s40139-013-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–44. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 14.Klarmann GJ, Hurt EM, Mathews LA, Zhang X, Duhagon MA, et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis. 2009;26:433–46. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:6942–7. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, et al. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci U S A. 2005;102:7180–5. doi: 10.1073/pnas.0502761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–45. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 18.Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, et al. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–11. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzel E, Karatas OF, Duz MB, Solak M, Ittmann M, et al. Differential expression of stem cell markers and ABCG2 in recurrent prostate cancer. Prostate. 2014;74:1498–505. doi: 10.1002/pros.22867. [DOI] [PubMed] [Google Scholar]
- 20.Harris KS, Kerr BA. Prostate cancer stem cell markers drive progression, therapeutic resistance, and bone metastasis. Stem Cells Int. 2017;2017:8629234. doi: 10.1155/2017/8629234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–64. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu JW, Rizzo P, Pannuti A, Golde T, Osborne B, et al. Notch signals in the endothelium and cancer “stem-like” cells: opportunities for cancer therapy. Vasc Cell. 2012;4:7. doi: 10.1186/2045-824X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K, Guo Y, Wang X, Zhao H, Ji Z, et al. WNT/β-catenin directs self-renewal symmetric cell division of hTERThigh prostate cancer stem cells. Cancer Res. 2017;77:2534–47. doi: 10.1158/0008-5472.CAN-16-1887. [DOI] [PubMed] [Google Scholar]
- 24.Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Detection and analysis of beta-catenin mutations in prostate cancer. Prostate. 2000;45:323–34. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19:683–97. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- 26.Wan X, Liu J, Lu JF, Tzelepi V, Yang J, et al. Activation of β-catenin signaling in androgen receptor-negative prostate cancer cells. Clin Cancer Res. 2012;18:726–36. doi: 10.1158/1078-0432.CCR-11-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ, et al. MicroRNA-320 suppresses the stem cell-like characteristics of prostate cancer cells by downregulating the Wnt/beta-catenin signaling pathway. Carcinogenesis. 2013;34:530–8. doi: 10.1093/carcin/bgs371. [DOI] [PubMed] [Google Scholar]
- 28.Tostar U, Malm CJ, Meis-Kindblom JM, Kindblom LG, Toftgard R, et al. Deregulation of the hedgehog signalling pathway: a possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J Pathol. 2006;208:17–25. doi: 10.1002/path.1882. [DOI] [PubMed] [Google Scholar]
- 29.Giakoustidis A, Giakoustidis D, Mudan S, Sklavos A, Williams R. Molecular signalling in hepatocellular carcinoma: role of and crosstalk among WNT/β-catenin, Sonic Hedgehog, Notch and Dickkopf-1. Can J Gastroenterol Hepatol. 2015;29:209–17. doi: 10.1155/2015/172356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zunich SM, Valdovinos M, Douglas T, Walterhouse D, Iannaccone P, et al. Osteoblast-secreted collagen upregulates paracrine Sonic hedgehog signaling by prostate cancer cells and enhances osteoblast differentiation. Mol Cancer. 2012;11:30. doi: 10.1186/1476-4598-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz I Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang HH, Chen BY, Wu CY, Tsao ZJ, Chen YY, et al. Hedgehog overexpression leads to the formation of prostate cancer stem cells with metastatic property irrespective of androgen receptor expression in the mouse model. J Biomed Sci. 2011;18:6. doi: 10.1186/1423-0127-18-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernard D, Pourtier-Manzanedo A, Gil J, Beach DH. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest. 2003;112:1724–31. doi: 10.1172/JCI19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, et al. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58:5718–24. [PubMed] [Google Scholar]
- 35.Lee CT, Capodieci P, Osman I, Fazzari M, Ferrara J, et al. Overexpression of the cyclin-dependent kinase inhibitor p16 is associated with tumor recurrence in human prostate cancer. Clin Cancer Res. 1999;5:977–83. [PubMed] [Google Scholar]
- 36.Gerhardt J, Montani M, Wild P, Beer M, Huber F, et al. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. Am J Pathol. 2012;180:848–61. doi: 10.1016/j.ajpath.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Sahu B, Laakso M, Ovaska K, Mirtti T, Lundin J, et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011;30:3962–76. doi: 10.1038/emboj.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCourt C, Maxwell P, Mazzucchelli R, Montironi R, Scarpelli M, et al. Elevation of c-FLIP in castrate-resistant prostate cancer antagonizes therapeutic response to androgen receptor-targeted therapy. Clin Cancer Res. 2012;18:3822–33. doi: 10.1158/1078-0432.CCR-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espinoza I, Pochampally R, Xing F, Watabe K, Miele L. Notch signaling: targeting cancer stem cells and epithelial-to-mesenchymal transition. Onco Targets Ther. 2013;6:1249–59. doi: 10.2147/OTT.S36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang L, Graham PH, Ni J, Hao J, Bucci J, et al. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol. 2015;96:507–17. doi: 10.1016/j.critrevonc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 43.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–88. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Sha J, Yang G, Huang X, Bo J, et al. Activation of Notch pathway is linked with epithelial-mesenchymal transition in prostate cancer cells. Cell Cycle. 2017;16:999–1007. doi: 10.1080/15384101.2017.1312237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su Q, Zhang B, Zhang L, Dang T, Rowley D, et al. Jagged1 upregulation in prostate epithelial cells promotes formation of reactive stroma in the Pten null mouse model for prostate cancer. Oncogene. 2017;36:618–27. doi: 10.1038/onc.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terada N, Shiraishi T, Zeng Y, Aw-Yong KM, Mooney SM, et al. Correlation of Sprouty1 and Jagged1 with aggressive prostate cancer cells with different sensitivities to androgen deprivation. J Cell Biochem. 2014;115:1505–15. doi: 10.1002/jcb.24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meunier A, Flores AN, McDermott N, Rivera-Figueroa K, Perry A, et al. Hypoxia regulates Notch-3 mRNA and receptor activation in prostate cancer cells. Heliyon. 2016;2:e00104. doi: 10.1016/j.heliyon.2016.e00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Kuang Y, Wang Y, Xu Q, Ren Q. Notch-4 silencing inhibits prostate cancer growth and EMT via the NF-kappaB pathway. Apoptosis. 2017;22:877–84. doi: 10.1007/s10495-017-1368-0. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Wang L, Mizokami A, Shi J, Zou C, et al. Down-regulation of E-cadherin enhances prostate cancer chemoresistance via Notch signaling. Chin J Cancer. 2017;36:35. doi: 10.1186/s40880-017-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, et al. Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate. 2007;67:614–22. doi: 10.1002/pros.20557. [DOI] [PubMed] [Google Scholar]
- 51.Mouraviev V, Li L, Tahir SA, Yang G, Timme TM, et al. The role of caveolin-1 in androgen insensitive prostate cancer. J Urol. 2002;168:1589–96. doi: 10.1016/S0022-5347(05)64526-0. [DOI] [PubMed] [Google Scholar]
- 52.Thompson TC, Tahir SA, Li L, Watanabe M, Naruishi K, et al. The role of caveolin-1 in prostate cancer: clinical implications. Prostate Cancer Prostatic Dis. 2010;13:6–11. doi: 10.1038/pcan.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katsogiannou M, El Boustany C, Gackiere F, Delcourt P, Athias A, et al. Caveolae contribute to the apoptosis resistance induced by the alpha (1A)-adrenoceptor in androgen-independent prostate cancer cells. PLoS One. 2009;4:e7068. doi: 10.1371/journal.pone.0007068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Ling MT, Wang Q, Lau CK, Leung SC, et al. Identification of a novel inhibitor of differentiation-1 (ID-1) binding partner, caveolin-1, and its role in epithelial-mesenchymal transition and resistance to apoptosis in prostate cancer cells. J Biol Chem. 2007;282:33284–94. doi: 10.1074/jbc.M705089200. [DOI] [PubMed] [Google Scholar]
- 55.Bartz R, Zhou J, Hsieh JT, Ying Y, Li W, et al. Caveolin-1 secreting LNCaP cells induce tumor growth of caveolin-1 negative LNCaP cells in vivo. Int J Cancer. 2008;122:520–5. doi: 10.1002/ijc.23142. [DOI] [PubMed] [Google Scholar]
- 56.Tahir SA, Kurosaka S, Tanimoto R, Goltsov AA, Park S, et al. Serum caveolin-1, a biomarker of drug response and therapeutic target in prostate cancer models. Cancer Biol Ther. 2013;14:117–26. doi: 10.4161/cbt.22633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou W, Ma X, Hua W, Chen B, Cai G. Caveolin-1 mediates chemoresistance in cisplatin-resistant ovarian cancer cells by targeting apoptosis through the Notch-1/Akt/NF-kappaB pathway. Oncol Rep. 2015;34:3256–63. doi: 10.3892/or.2015.4320. [DOI] [PubMed] [Google Scholar]
- 58.Tahir SA, Yang G, Goltsov A, Song KD, Ren C, et al. Caveolin-1-LRP6 signaling module stimulates aerobic glycolysis in prostate cancer. Cancer Res. 2013;73:1900–11. doi: 10.1158/0008-5472.CAN-12-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Wang N, Li W, Liu P, Chen Q, et al. Caveolin-1 mediates chemoresistance in breast cancer stem cells via β-catenin/ABCG2 signaling pathway. Carcinogenesis. 2014;35:2346–56. doi: 10.1093/carcin/bgu155. [DOI] [PubMed] [Google Scholar]
- 60.Phiboonchaiyanan PP, Kiratipaiboon C, Chanvorachote P. Ciprofloxacin mediates cancer stem cell phenotypes in lung cancer cells through caveolin-1-dependent mechanism. Chem Biol Interact. 2016;250:1–11. doi: 10.1016/j.cbi.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Liu C, Kelnar K, Vlassov AV, Brown D, Wang J, et al. Distinct microRNA expression profiles in prostate cancer stem/progenitor cells and tumor-suppressive functions of let-7. Cancer Res. 2012;72:3393–404. doi: 10.1158/0008-5472.CAN-11-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng CY, Hwang CI, Corney DC, Flesken-Nikitin A, Jiang L, et al. miR-34 cooperates with p53 in suppression of prostate cancer by joint regulation of stem cell compartment. Cell Rep. 2014;6:1000–7. doi: 10.1016/j.celrep.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–5. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bucay N, Sekhon K, Yang T, Majid S, Shahryari V, et al. MicroRNA-383 located in frequently deleted chromosomal locus 8p22 regulates CD44 in prostate cancer. Oncogene. 2017;36:2667–79. doi: 10.1038/onc.2016.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang YL, Zhou PJ, Wei L, Li W, Ji Z, et al. MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway. Oncotarget. 2015;6:24017–31. doi: 10.18632/oncotarget.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong D, Heath E, Chen W, Cher ML, Powell I, et al. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS One. 2012;7:e33729. doi: 10.1371/journal.pone.0033729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wen Y, Cai J, Hou Y, Huang Z, Wang Z. Role of EZH2 in cancer stem cells: from biological insight to a therapeutic target. Oncotarget. 2017;8:37974–90. doi: 10.18632/oncotarget.16467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang M, Ren D, Guo W, Wang Z, Huang S, et al. Loss of miR-100 enhances migration, invasion, epithelial-mesenchymal transition and stemness properties in prostate cancer cells through targeting Argonaute 2. Int J Oncol. 2014;45:362–72. doi: 10.3892/ijo.2014.2413. [DOI] [PubMed] [Google Scholar]
- 69.Nabavi N, Saidy NR, Venalainen E, Haegert A, Parolia A, et al. miR-100-5p inhibition induces apoptosis in dormant prostate cancer cells and prevents the emergence of castration-resistant prostate cancer. Sci Rep. 2017;7:4079. doi: 10.1038/s41598-017-03731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu J, Lu Y, Cui D, Li E, Zhu Y, et al. miR-200b suppresses cell proliferation, migration and enhances chemosensitivity in prostate cancer by regulating Bmi-1. Oncol Rep. 2014;31:910–8. doi: 10.3892/or.2013.2897. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Wang C, Zhang X, Hua R, Gan L, et al. Bmi-1 regulates stem cell-like properties of gastric cancer cells via modulating miRNAs. J Hematol Oncol. 2016;9:90. doi: 10.1186/s13045-016-0323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim SH, Singh SV. The role of polycomb group protein Bmi-1 and Notch4 in breast cancer stem cell inhibition by benzyl isothiocyanate. Breast Cancer Res Treat. 2015;149:681–92. doi: 10.1007/s10549-015-3279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li JZ, Li J, Wang HQ, Li X, Wen B, et al. MiR-141-3p promotes prostate cancer cell proliferation through inhibiting kruppel-like factor-9 expression. Biochem Biophys Res Commun. 2017;482:1381–6. doi: 10.1016/j.bbrc.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 74.Huang S, Guo W, Tang Y, Ren D, Zou X, et al. miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncol Rep. 2012;28:1831–7. doi: 10.3892/or.2012.2015. [DOI] [PubMed] [Google Scholar]
- 75.Liu T, Cheng W, Huang Y, Huang Q, Jiang L, et al. Human amniotic epithelial cell feeder layers maintain human iPS cell pluripotency via inhibited endogenous microRNA-145 and increased Sox2 expression. Exp Cell Res. 2012;318:424–34. doi: 10.1016/j.yexcr.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–58. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 77.Ozen M, Karatas OF, Gulluoglu S, Bayrak OF, Sevli S, et al. Overexpression of miR-145-5p inhibits proliferation of prostate cancer cells and reduces SOX2 expression. Cancer Invest. 2015;33:251–8. doi: 10.3109/07357907.2015.1025407. [DOI] [PubMed] [Google Scholar]
- 78.Fan X, Chen X, Deng W, Zhong G, Cai Q, et al. Up-regulated microRNA-143 in cancer stem cells differentiation promotes prostate cancer cells metastasis by modulating FNDC3B expression. BMC Cancer. 2013;13:61. doi: 10.1186/1471-2407-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin M, Zhang T, Liu C, Badeaux MA, Liu B, et al. miRNA-128 suppresses prostate cancer by inhibiting BMI-1 to inhibit tumor-initiating cells. Cancer Res. 2014;74:4183–95. doi: 10.1158/0008-5472.CAN-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parimi V, Goyal R, Poropatich K, Yang XJ. Neuroendocrine differentiation of prostate cancer: a review. Am J Clin Exp Urol. 2014;2:273–85. [PMC free article] [PubMed] [Google Scholar]
- 81.Yashi M, Terauchi F, Nukui A, Ochi M, Yuzawa M, et al. Small-cell neuroendocrine carcinoma as a variant form of prostate cancer recurrence: a case report and short literature review. Urol Oncol. 2006;24:313–7. doi: 10.1016/j.urolonc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 82.Hu CD, Choo R, Huang J. Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front Oncol. 2015;5:90. doi: 10.3389/fonc.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cohen A, Richards KA, Patel S, Weiner A, Eggener SE, et al. Metastatic small cell carcinoma of the prostate: population-based analysis of patient characteristics and treatment paradigms. Urol Oncol. 2015;33:70.e1–7. doi: 10.1016/j.urolonc.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Palapattu GS, Wu C, Silvers CR, Martin HB, Williams K, et al. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate. 2009;69:787–98. doi: 10.1002/pros.20928. [DOI] [PubMed] [Google Scholar]
- 85.Deorah S, Rao MB, Raman R, Gaitonde K, Donovan JF. Survival of patients with small cell carcinoma of the prostate during 1973-2003: a population-based study. BJU Int. 2012;109:824–30. doi: 10.1111/j.1464-410X.2011.10523.x. [DOI] [PubMed] [Google Scholar]
- 86.Stein ME, Bernstein Z, Abacioglu U, Sengoz M, Miller RC, et al. Small cell (neuroendocrine) carcinoma of the prostate: etiology, diagnosis, prognosis, and therapeutic implications – a retrospective study of 30 patients from the rare cancer network. Am J Med Sci. 2008;336:478–88. doi: 10.1097/MAJ.0b013e3181731e58. [DOI] [PubMed] [Google Scholar]
- 87.Isgro MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125–43. doi: 10.1007/978-94-017-7215-0_9. [DOI] [PubMed] [Google Scholar]
- 88.Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE. Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci U S A. 1986;83:3500–4. doi: 10.1073/pnas.83.10.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gut P, Czarnywojtek A, Fischbach J, Baczyk M, Ziemnicka K, et al. Chromogranin A -unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci. 2016;12:1–9. doi: 10.5114/aoms.2016.57577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farinola MA, Weir EG, Ali SZ. CD56 expression of neuroendocrine neoplasms on immunophenotyping by flow cytometry: a novel diagnostic approach to fine-needle aspiration biopsy. Cancer. 2003;99:240–6. doi: 10.1002/cncr.11458. [DOI] [PubMed] [Google Scholar]
- 91.Vlachostergios PJ, Puca L, Beltran H. Emerging variants of castration-resistant prostate cancer. Curr Oncol Rep. 2017;19:32. doi: 10.1007/s11912-017-0593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park JW, Lee JK, Witte ON, Huang J. FOXA2 is a sensitive and specific marker for small cell neuroendocrine carcinoma of the prostate. Mod Pathol. 2017;30:1262–72. doi: 10.1038/modpathol.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maina PK, Shao P, Liu Q, Fazli L, Tyler S, et al. c-MYC drives histone demethylase PHF8 during neuroendocrine differentiation and in castration-resistant prostate cancer. Oncotarget. 2016;7:75585–602. doi: 10.18632/oncotarget.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee JK, Phillips JW, Smith BA, Park JW, Stoyanova T, et al. N-Myc drives neuroendocrine prostate cancer initiated from human prostate epithelial cells. Cancer Cell. 2016;29:536–47. doi: 10.1016/j.ccell.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim J, Jin H, Zhao JC, Yang YA, Li Y, et al. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene. 2017;36:4072–80. doi: 10.1038/onc.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qi J, Nakayama K, Cardiff RD, Borowsky AD, Kaul K, et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell. 2010;18:23–38. doi: 10.1016/j.ccr.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bishop JL, Thaper D, Vahid S, Davies A, Ketola K, et al. The master neural transcription factor BRN2 is an androgen receptor-suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov. 2017;7:54–71. doi: 10.1158/2159-8290.CD-15-1263. [DOI] [PubMed] [Google Scholar]
- 98.Dardenne E, Beltran H, Benelli M, Gayvert K, Berger A, et al. N-Myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell. 2016;30:563–77. doi: 10.1016/j.ccell.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun F, Zhang ZW, Tan EM, Lim ZL, Li Y, et al. Icaritin suppresses development of neuroendocrine differentiation of prostate cancer through inhibition of IL-6/STAT3 and Aurora kinase A pathways in TRAMP mice. Carcinogenesis. 2016;37:701–11. doi: 10.1093/carcin/bgw044. [DOI] [PubMed] [Google Scholar]
- 100.Chang PC, Wang TY, Chang YT, Chu CY, Lee CL, et al. Autophagy pathway is required for IL-6 induced neuroendocrine differentiation and chemoresistance of prostate cancer LNCaP cells. PLoS One. 2014;9:e88556. doi: 10.1371/journal.pone.0088556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morell C, Bort A, Vara-Ciruelos D, Ramos-Torres A, Altamirano-Dimas M, et al. Up-regulated expression of LAMP2 and autophagy activity during neuroendocrine differentiation of prostate cancer LNCaP cells. PLoS One. 2016;11:e0162977. doi: 10.1371/journal.pone.0162977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanayama M, Hayano T, Koebis M, Maeda T, Tabe Y, et al. Hyperactive mTOR induces neuroendocrine differentiation in prostate cancer cell with concurrent up-regulation of IRF1. Prostate. 2017;77:1489–98. doi: 10.1002/pros.23425. [DOI] [PubMed] [Google Scholar]
- 103.Courel M, El Yamani FZ, Alexandre D, El Fatemi H, Delestre C, et al. Secretogranin II is overexpressed in advanced prostate cancer and promotes the neuroendocrine differentiation of prostate cancer cells. Eur J Cancer. 2014;50:3039–49. doi: 10.1016/j.ejca.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 104.Sang M, Hulsurkar M, Zhang X, Song H, Zheng D, et al. GRK3 is a direct target of CREB activation and regulates neuroendocrine differentiation of prostate cancer cells. Oncotarget. 2016;7:45171–85. doi: 10.18632/oncotarget.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan HL, Sood A, Rahimi HA, Wang W, Gupta N, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res. 2014;20:890–903. doi: 10.1158/1078-0432.CCR-13-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83. doi: 10.1126/science.aah4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dang Q, Li L, Xie H, He D, Chen J, et al. Anti-androgen enzalutamide enhances prostate cancer neuroendocrine (NE) differentiation via altering the infiltrated mast cells → androgen receptor (AR) → miRNA32 signals. Mol Oncol. 2015;9:1241–51. doi: 10.1016/j.molonc.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiao L, Deng Z, Xu C, Yu Y, Li Y, et al. miR-663 induces castration-resistant prostate cancer transformation and predicts clinical recurrence. J Cell Physiol. 2014;229:834–44. doi: 10.1002/jcp.24510. [DOI] [PubMed] [Google Scholar]
- 109.Liang H, Studach L, Hullinger RL, Xie J, Andrisani OM. Down-regulation of RE-1 silencing transcription factor (REST) in advanced prostate cancer by hypoxia-induced miR-106b ~ 25. Exp Cell Res. 2014;320:188–99. doi: 10.1016/j.yexcr.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gratte FD, Pasic S, Olynyk JK, Yeoh GC, Tosh D, et al. Transdifferentiation of pancreatic progenitor cells to hepatocyte-like cells is not serum-dependent when facilitated by extracellular matrix proteins. Sci Rep. 2018;8:4385. doi: 10.1038/s41598-018-22596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 112.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–8. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prasad A, Manivannan J, Loong DT, Chua SM, Gharibani PM, et al. A review of induced pluripotent stem cell, direct conversion by trans-differentiation, direct reprogramming and oligodendrocyte differentiation. Regen Med. 2016;11:181–91. doi: 10.2217/rme.16.5. [DOI] [PubMed] [Google Scholar]
- 114.Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–8. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 2017;7:736–49. doi: 10.1158/2159-8290.CD-16-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]