Abstract
The relatively modest spatial resolution of positron emission tomography (PET) increases the likelihood of partial volume effects such that binding potential (BPND) may be underestimated. Given structural grey matter losses across adulthood, partial volume effects may be even more problematic in older age leading to overestimation of adult age differences. Here we examined the effects of partial volume correction (PVC) in two studies from different sites using different high-affinity D2-like radioligands (18 F-Fallypride, 11C-FLB457) and different PET camera resolutions (∼5 mm, 2.5 mm). Results across both data sets revealed that PVC increased estimated BPND and reduced, though did not eliminate, age effects on BPND. As expected, the effects of PVC were smaller in higher compared to lower resolution data. Analyses using uncorrected data that controlled for grey matter volume in each region of interest approximated PVC corrected data for some but not all regions. Overall, the findings suggest that PVC increases estimated BPND in general and reduces adult age differences especially when using lower resolution cameras. The findings suggest that the past 30 years of research on dopamine receptor availability, for which very few studies use PVC, may overestimate effects of aging on dopamine receptor availability.
Keywords: Aging, D2 receptors, dopamine, partial volume correction, positron emission tomography imaging
Introduction
Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) have been used for over three decades to obtain in vivo measures of individual differences in the human dopamine (DA) system.1–3 Nearly all of the studies examining adult age differences have reported significantly lower levels of available DA receptors in older compared to younger adults. Age-related DA receptor decline has been one of the most well-documented effects in the aging brain. A recent meta-analysis of 95 cross-sectional PET/SPECT studies revealed strong, negative correlations between age and D1-like receptors, r = −0.77 (frontal cortical r = −0.76, striatal r = −0.77), and D2-like receptors, r = −0.56 (frontal cortical r = −0.66, striatal r = −0.54).4 To the extent that cross-sectional estimates can be used as an approximation of longitudinal age-related change, together these studies suggest that about 8–12% of DA receptors are lost per decade across adulthood.4 Importantly, another very well-documented age-related neurobiological effect in humans is the structural loss of grey matter which shows similarly strong effects of age. Across several studies on adult age differences in brain volume, average correlations between age and brain volume vary between regions with large effects in frontal cortex (r = −0.56), medium effects in striatum (r = −0.40) and temporal cortex (r = −0.37), and small effects in thalamus (r = −0.28), medial temporal lobe (MTL) structures (r = −0.25), parietal cortex (r = −0.20), occipital cortex (r = −0.19), and cingulate gyrus (r = −0.17).5,6 Given that grey matter loss may result in potentially large partial volume effects in aging studies using PET data, partial volume correction (PVC) could affect estimates of age differences in DA receptor availability.
While PVC approaches have been included as additional analyses in some 11C-raclopride PET studies of adults, showing an increase in D2-like receptor availability of ∼40–60% in striatum after PVC,7,8 studies have not systematically investigated how differences in grey matter volume in older age affect PVC estimates of receptor availability in humans. It is somewhat surprising that almost none of the published PET studies of human age differences in DA receptor availability take into account structural changes with age. Admittedly, PVC approaches rely on adequate MR-PET registration and include assumptions about radiotracer binding (uniform uptake across an anatomical region of interest) that may not always be valid.9 Compared to MRI, PET has relatively modest spatial resolution, increasing the likelihood of partial-volume effects in general, but especially in old age. Only one study of which we are aware provides direct comparisons of adult age effects on DA receptor availability (binding potential, BPND) with and without controlling for brain volume.10 Using 11C-raclopride in non-human primates and employing a variation of the Müller-Gärtner approach for PVC,11 Morris et al.10 show that the effects of age on striatal binding potential are larger in uncorrected data (r = −0.82) compared to PVC data (r = −0.57). The finding suggests that most published human PET neuroimaging studies may have overestimated the effects of age on striatal DA receptors since the majority of studies used lower resolution cameras and did not correct for partial volume averaging. A similar inference may be expected for cortical regions, but this has not been directly tested. Given that loss of frontal grey matter volume is particularly steep,5,6 assessment of the effects of aging and partial volume averaging on DA D2-like receptor availability (BPND) using high affinity tracers (including 11C-FLB457 and 18F-Fallypride) capable of measuring D2-like BPND in cortex is particularly needed.
In the present study, we employed the geometric transfer matrix (GTM) method12,13 to perform PVC. GTM has been demonstrated to outperform other commonly used PVC methods (Muller-Gartner et al.11, Meltzer et al.14) on simulated (including errors in segmentation, registration, and point spread function specification) data15 and to have better recovery coefficient performance on 11C-raclopride data.16 Furthermore, GTM PVC is implemented in the commonly used PET kinetic modeling and analysis software package, PMOD (https://www.pmod.com/; Zurich, Switzerland). Here, we examined the effects of GTM PVC (hereafter referred to as PVC) on estimated non-displaceable binding potential (BPND) in general and on the estimated age effects on BPND. To examine the generalizability of the PVC effects, we compared uncorrected and PVC data in two cross-sectional, adult life-span data sets collected at two different sites (Yale, Vanderbilt) using two different high affinity, D2/D3 radioligands (11C-FLB457, 18F-Fallypride) that allow measurement of extrastriatal D2-like receptors and cameras with different resolutions (Siemens HRRT: 2.5 mm FWHM resolution,17 GE Discovery STE: ∼5 mm FWHM resolution18). We expected PVC to increase BPND in general and reduce the estimated adult age effects. We expected the effects of PVC to be larger in lower compared to higher resolution data.
Methods
Participants
Healthy participants were recruited from the community for 11C-FLB457 (hereafter referred to as FLB) PET scans performed at Yale University or 18F-Fallypride (hereafter referred to as Fallypride) scans performed at Vanderbilt University.
The FLB dataset consisted of 37 adults ranging in age from 26 to 79 years (mean = 47.81 ± 16.93; 17 males); written informed consent was obtained for all subjects. FLB subjects were medically and mentally healthy and were assessed by a physical examination, comprehensive metabolic panel and complete blood count, and medical and psychiatric histories. Inclusion criteria were the following: no prescription or illicit drug use, no history of tobacco or nicotine use, no current uncontrolled medical conditions such as cardiovascular, endocrine, renal, liver, or thyroid pathologies, no history of neurological or psychiatric disorders, alcohol consumption no greater than 21 drinks/week for women and 35 drinks/week for men, and no claustrophobia or other MRI contraindications. Females had negative pregnancy tests at intake and on the day of the scan.
Approval for the FLB study protocol was obtained from the Yale University Human Investigation Committee and the Yale-New Haven Hospital Radiation Safety Committee and all participants completed written informed consent. All study procedures were approved in accordance with the Declaration of Helsinki’s guidelines for the ethical treatment of human participants.
The Fallypride subjects analyzed here were selected from a larger set of data19 to match the FLB subjects in age range (Fallypride n = 64; aged 26 to 83, mean = 51.7 ± 17.21; 30 males). Specifically, a similar mean age and sex distribution was chosen across four age ranges: 20–35 (m = 29.9, FLB; 30.4, Fallypride), 36–50 (mean = 43.8, FLB; 42.9, Fallypride), 51–65 (mean = 58.3, FLB; 58.9, Fallypride), 66+ (mean = 72.4, FLB; 74.4, Fallypride). The oldest age grouping (66+ individuals) contained all available subjects in both datasets and consisted of more females (75% in FLB; 62.5% in Fallypride) than males. Fallypride subjects were medically and mentally healthy and were assessed by a physical examination, comprehensive metabolic panel and complete blood count, EKG and interviews of medical and psychiatric history. Inclusion criteria were the following: no illicit drug use in last two months, no use of any psychotropic medication in last six months, no current uncontrolled medical condition such as neurological, cardiovascular, endocrine, renal, liver, or thyroid pathology, no history of neurological or psychiatric disorders, no current tobacco or nicotine use, alcohol consumption no greater than eight ounces of whiskey (∼5 standard alcoholic drinks)/week, and no claustrophobia or other MRI contraindications. Females had negative pregnancy tests at intake and on the day of the scan. Approval for the Fallypride study protocol was obtained from the Vanderbilt University Human Research Protection Program and the Radioactive Drug Research Committee and all participants completed written informed consent.
See Supplementary Table 1 for demographic measures for subjects from the two study sites. All study procedures were approved in accordance with the Declaration of Helsinki s guidelines for the ethical treatment of human participants.
PET imaging
FLB data acquisition and preprocessing
[11C]FLB 457, 5-bromo-N-[[(2S)-1- ethyl-2-pyrrolidinyl]methyl]-3-methoxy-2-(methoxy-11C) benzamide was synthesized as previously described by Sandiego et al.20 PET scans were acquired on the high resolution research tomograph (HRRT; Siemens Medical Solutions, Knoxville, TN, USA). [11C]FLB 457 (median specific activity: 7.80 mCi/nmol) was injected intravenously as a bolus (315 MBq; average = 8.62 mCi, SD = 2.03) over 1 min by an automated infusion pump (Harvard Apparatus, Holliston, MA, USA). Prior to each scan, a six-minute transmission scan was performed for attenuation correction. Dynamic scan data were acquired in list mode for 90 min following the administration of [11C]FLB 457 and reconstructed into 27 frames (6 × 0.5 mins, 3 × 1 min, 2 × 2 mins, 16 × 5 mins) with corrections for attenuation, normalization, scatter, randoms, and dead time using the MOLAR (Motion-compensation OSEM List-mode Algorithm for Resolution-Recovery Reconstruction) algorithm.21 Event-by-event, motion correction22 was applied using a Polaris Vicra optical tracking system (NDI Systems, Waterloo, Canada) that detects motion using reflectors mounted on a cap worn by the subject throughout the duration of the scan. Prior to the PET scan, T1-weighted magnetic resonance (MR) images (MPRAGE protocol; TR = 2.4 s, TE = 1.9 ms, FOV = 256 × 256 mm, voxel size = 1 × 1 × 1 mm) were acquired on a 3T Trio whole-body scanner (Siemens Medical Systems, Erlangen, Germany).
Fallypride data acquisition and preprocessing
[18F]-Fallypride, (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3[18F]fluoropropyl)-2,3-dimethoxybenzamide was produced in the radiochemistry laboratory attached to the PET unit at Vanderbilt University Medical Center, following synthesis and quality control procedures described in US Food and Drug Administration IND 47,245. Prior to the PET scan, T1-weighted magnetic resonance (MR) images (TFE SENSE protocol; Act. TR = 8.9 ms, TE = 4.6 ms, 192 TFE shots, TFE duration = 1201.9 s, FOV = 256 × 256 mm, voxel size =1 × 1 × 1 mm) were acquired on a 3T Philips Intera Achieva whole-body scanner (Philips Healthcare, Best, The Netherlands). PET data were collected on a GE Discovery STE (DSTE) PET scanner (General Electric Healthcare, Chicago, IL, USA). Serial scan acquisition was started simultaneously with a 5.0 mCi (185 MBq; average = 5.06 mCi, SD = 0.23) slow bolus injection of DA D2/3 tracer [18F]-Fallypride (median specific activity: 9.24 mCi/nmol). CT scans were collected for attenuation correction prior to each of the three emission scans, which together lasted approximately 3.5 h with two breaks for subject comfort. Acquisition times for the dynamic PET scans have been reported previously.23 After decay correction and attenuation correction, PET scan frames were corrected for motion using SPM824 with the 20th dynamic image frame of the first series serving as the reference image. The realigned PET frames were then merged and re-associated with their acquisition timing info in PMOD’s PVIEW module to create a single 4D file for use in PMOD’s PNEURO tool for further analysis (see below).
Partial volume correction procedure for FLB and fallypride data
We implemented the volume of interest-(VOI) based partial volume correction (PVC) procedure available through PMOD’s PNEURO module. This procedure utilizes the GTM method12,13 which restricts PVC to PET signal of structurally defined VOIs. For the VOI-based PVC, we entered the point spread function representing the resolution of the PET data to allow for proper correction. For the Fallypride data, the point spread function was set at 5 × 5 × 5 mm, while it was 2.5 × 2.5 × 2.5 mm for the FLB data. We used the 1 mm Hammers atlas25 available internally in PNEURO and PNEURO’s deep nuclei option to parcellate each subject’s grey matter as determined from a segmentation of their T1 MRI image into 30 bilateral cortical areas (including amygdala and hippocampus), 5 bilateral deep nuclei (caudate, putamen, ventral striatum, thalamus, and pallidum), plus bilateral cerebellum, and brainstem. This was accomplished in PNEURO using a database of 26 normal (age: 34 ± 12) T1 MRI scans that were manually segmented by neuroanatomically trained operators, of which the most comparable brain hemispheres to a specific subject’s T1 were selected using anatomical landmarks (anterior/posterior commissure, inter-hemispheric point, and inter-caudate point) and a calculation of the average thickness of the frontal horn of the left and right ventricles. The selected knowledge base hemispheres were then elastically matched to the subject hemisphere using a hierarchical approach (1. global affine transformation, 2. individual structure adjustment with free form deformation algorithm) to create a set of structure definitions that was combined with the grey and white matter segmentation to produce a maximum probability atlas of base structures (deep nuclei, grey matter, cerebellum) with the grey matter (at probability > 0.3) being further parcellated via intersection with the specified cortical atlas (here, Hammers). After parcellation, the MRI and PET data were matched (registered via a rigid matching procedure based on the normalized mutual information criterion), PET data resampled to the MRI space (1 × 1 × 1 mm), and GTM PVC performed within each VOI. The PNEURO parcellation VOIs were then used to extract time activity curves (TACs) from the PET data before and after PVC. These TACs were then fit using the simplified reference tissue model26 to obtain BPND values using PMOD’s PKIN module with a merged, bilateral cerebellum VOI serving as the reference region.
Regions of interest for analysis
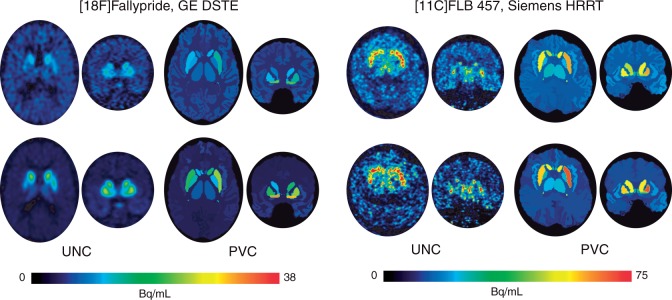
To limit the number of comparisons, we created volume-averaged BPND summary measures for large cortical and subcortical areas of interest (Supplementary Table 2). We limited our analyses to these seven areas where averaged levels of uncorrected BPND exceeded group-averaged BPND obtained from white matter TACs that we also fit in PKIN. Since no specific binding is expected in white matter, we assumed white matter BPND values present in the uncorrected data reflected non-physiological noise. For FLB, white matter BPND was 0.63 ± 0.19, while Fallypride white matter BPND was 0.47 ± 0.13. Regions with BPND values at or below the mean white matter-based thresholds were not included in analyses. Using this uncorrected white matter-based BPND cutoff, anterior cingulate cortex (ACC; group average BPND: 0.57 ± 0.17), MTL (MTL; amygdala + hippocampus + parahippocampal gyrus; BPND: 1.10 ± 0.19), and (remainder of) temporal cortex (BPND: 0.70 ± 0.22) were the only cortical areas analyzed in the Fallypride dataset, with MTL and temporal cortex BPND greater than one standard deviation from white matter BPND. Signal was high enough in the FLB data to report BPND from the remainder of frontal cortex (not part of ACC; BPND: 0.77 ± 0.27) and parietal cortex (BPND: 0.83 ± 0.32), with parietal cortex BPND greater than one standard deviation from white matter BPND. The thalamus also possessed high enough signal to analyze in both datasets (Fallypride BPND: 1.95 ± 0.33; FLB BPND: 3.15 ± 0.54). Striatal BPND was only estimable in the Fallypride dataset. This resulted in four large anatomical areas where age differences in BPND could be assessed in both datasets (ACC, MTL, temporal cortex, and thalamus). Table S1 displays demographic data and grey matter volumes from the seven regions of interest we examined from the two PET datasets. Figure 1 displays PET images at two different frames (time points) from one example subject before and after PVC for each data set. Examples of the anatomical regions are delineated in the PVC images. The uncorrected images depict voxelwise PET data with BPND estimates later obtained by fitting an averaged TAC within each region. The PVC approach (see Partial volume correction procedure, above) resulted in a single corrected value for the data in each region for each frame, which was extracted and fit to estimate PVC BPND.
Figure 1.
Example PET images (axial/transverse and coronal) of tracer uptake at frame 18 (top row) and frame 25 (bottom row) for a 60-year-old male subject within the Fallypride data collected with a GE DSTE data (left) and a 60-year-old male subject within the FLB data collected with a Siemens HRRT (right).
Statistical analyses
Statistical analyses were performed in STATA. To examine effects of PVC on regional BPND values, we compared average BPND between uncorrected and PVC data using paired t-tests across all regions of interest in each dataset (five for Fallypride: ACC, MTL, temporal cortex, thalamus, and striatum; six for FLB: ACC, MTL, temporal cortex, thalamus, frontal cortex, and parietal cortex). To assess age differences in BPND, we ran simple linear regressions of mean-centered age as a predictor of uncorrected and PVC BPND for each region in each study. We also calculated effect sizes from standardized coefficients (Pearson correlations) of simple age effects on uncorrected and PVC BPND in these regions using standardized variables in linear regressions. To assess the impact of PVC on age effect sizes, we statistically compared the differences in the age effects estimated using PVC versus uncorrected data using Wald statistics (in the form of Student’s t). Finally, we ran additional analyses to examine whether the age effects on uncorrected BPND after regressing out volume within each region would approximate the age effects on the PVC BPND data. Here, too, we statistically compared the differences in the age effects (estimated using PVC versus uncorrected data with volume regressed out) using Wald statistics (in the form of Student’s t). All point estimates are reported with 95% confidence intervals (CIs) in tables and figures. Graphs depicting age effects with 95% CIs were produced in R software and assembled in Adobe Illustrator.
All data and code are publicly available on OSF: https://osf.io/wfk6j/.
Results
First, uncorrected and PVC BPND values within each region in each dataset were highly correlated (Supplementary Table 3), as expected. Fallypride correlations ran from r = 0.84 in the ACC to 0.99 in the striatum, while all correlations were r = 0.93 or greater for the FLB dataset. However, paired t-tests showed significantly higher BPND in the PVC compared to uncorrected data in the Fallypride DSTE study for all regions (thalamus, t63 = 46.74, p < 0.001, ACC, t63 = 8.70, p < 0.001, MTL, t63 = 29.37, p < 0.001, temporal cortex, t63 = 31.68, p < 0.001, striatum, t63 = 52.80, p < 0.001) and the FLB HRRT study for all regions (thalamus, t36 = 30.68, p < 0.001, ACC, t36 = 11.43, p < 0.001, MTL, t36 = 8.51, p < 0.001, temporal cortex, t36 = 14.29, p < 0.001, frontal, t36 = 18.26, p < 0.001, parietal, t36 = 6.57, p < 0.001). While PVC resulted in higher BPND for all areas of interest (data shifted upward in Figures 2 and 3), the effect was qualitatively larger for the Fallypride data collected on the DSTE, a lower resolution PET system.
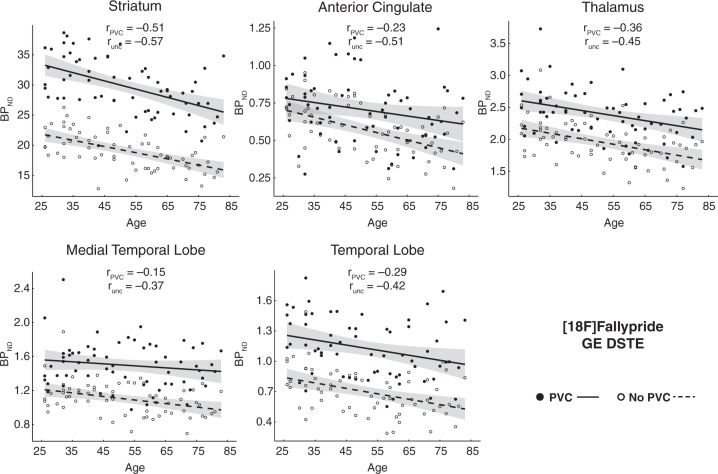
Figure 2.
Age effects on partial-volume corrected (PVC) binding potential and uncorrected (unc) binding potential from Fallypride data collected with a GE DSTE. Shaded error indicates 95% CI of age effect.
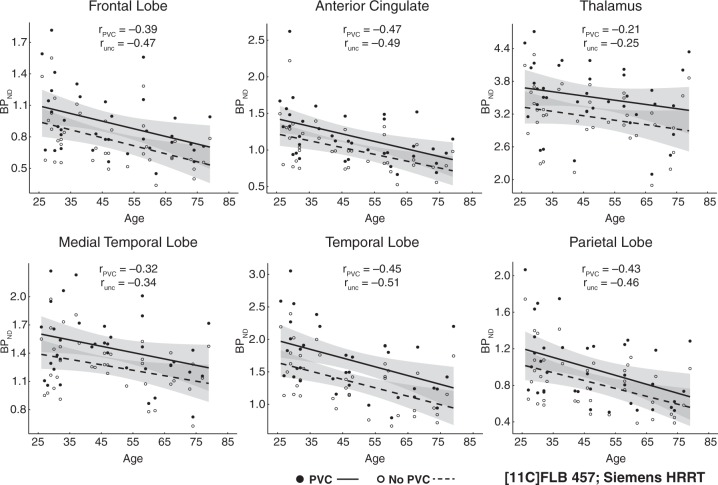
Figure 3.
Age effects on partial-volume corrected (PVC) binding potential and uncorrected (unc) binding potential from FLB457 data collected with a Siemens HRRT. Shaded error indicates 95% CI of age effect.
Mean BPND and standard errors of the BPND estimates for each region before and after PVC are reported in Supplementary Table 4. BPND estimation error increased after PVC in almost all regions across studies, but the combination of an increase in estimated BPND with a small increase in error produced higher signal relative to error ratios for most regions (see Supplementary Methods and Results for details). Within the FLB HRRT data, BPND signal relative to error (BPND/SE) increased after PVC in thalamus, parietal cortex, and frontal cortex but decreased in temporal cortex, MTL, and ACC (due to the PVC-related increase in estimation error combined with very small differences in estimated BPND). Within the Fallypride DSTE data, BPND/SE increased after PVC in thalamus, striatum, and temporal cortex, did not differ in MTL, and only decreased in ACC (see Supplementary Methods and Results for statistics).
We performed regressions to examine differences between uncorrected and PVC BPND data for both intercepts (similar to t-tests of mean BPND above) and slopes (simple linear effects of age) within each region of each dataset. Regression results are displayed in Table 1. As expected age was significantly, negatively related to D2 BPND in almost all regions in both datasets whether using uncorrected or PVC data. Consistent with the paired t-tests reported above, there were differences between PVC and uncorrected data in the intercept terms (i.e. average BPND). PVC increased BPND a minimum of ∼23% in the Fallypride DSTE study and 10–20% in the FLB HRRT study (Table 1). PVC affected age slopes differentially across the two studies, with the age slopes tending to be lower (less negative) after PVC in the Fallypride DSTE study while not significantly differing in the FLB HRRT study (Table 1). When comparing the age slopes for PVC data versus uncorrected data in the Fallypride DSTE study, we found significant differences in striatum, t = −3.05, p = 0.003, MTL, t = 2.36, p = 0.021, and ACC, t = 2.42, p = 0.019, but not the thalamus, t = 1.13, p = 0.263, or temporal cortex, t = 0.43, p = 0.668. Notably, PVC reduced age slopes in ACC and MTL in the Fallypride DSTE data to approximately half of the uncorrected values (Table 1). No regions of interest demonstrated significant differences in the age slopes on PVC versus uncorrected BPND in the FLB HRRT data, all t < 1.07, p > 0.294. Taking both datasets into account, athough some of the differences in age slopes between uncorrected and PVC data were significant, we observed relatively small numerical differences between uncorrected and PVC data in unstandardized regression slopes (Table 1) suggesting that PVC does not shift the estimated age-related rates of change very much (reported in Table 3).
Table 1.
Regression results for effects of age (mean centered) on D2-like receptor availability (BPND) from studies using [18F]Fallypride on the DSTE and [11C]FLB457 on the HRRT.
| Fallypride |
FLB |
||||
|---|---|---|---|---|---|
| Region | Intercept | Age slope | Intercept | Age slope | |
| Thalamus | unc | 1.95* (1.88, 2.03) | −0.009* (−0.013, −0.004) | 3.15* (2.97, 3.33) | −0.008 (−0.019, 0.003) |
| PVC | 2.40* (2.31, 2.49) | −0.008* (−0.013, −0.003) | 3.51* (3.30, 3.71) | −0.008 (−0.020, 0.004) | |
| ACC | unc | 0.57* (0.53, 0.61) | −0.005* (−0.007, −0.003) | 1.01* (0.91, 1.10) | −0.009* (−0.015, −0.004) |
| PVC | 0.70* (0.65, 0.76) | −0.003* (−0.006, 0.000) | 1.19* (1.08, 1.30) | −0.010* (−0.017, −0.004) | |
| MTL | unc | 1.10* (1.06, 1.15) | −0.004* (−0.007, −0.002) | 1.26* (1.17, 1.35) | −0.006* (−0.011, 0.000) |
| PVC | 1.50* (1.43, 1.56) | −0.002 (−0.006, 0.002) | 1.45* (1.33, 1.57) | −0.007 (−0.014, 0.000) | |
| Temporal Cortex | unc | 0.70* (0.65, 0.75) | −0.005* (−0.008, −0.002) | 1.35* (1.22, 1.47) | −0.013* (−0.020, −0.006) |
| PVC | 1.13* (1.06, 1.20) | −0.005* (−0.009, −0.001) | 1.68* (1.52, 1.83) | −0.014* (−0.023, −0.004) | |
| Striatum | unc | 19.11* (18.47, 19.74) | −0.102* (−0.140, −0.065) | ||
| PVC | 29.77* (28.77, 30.76) | −0.136* (−0.194, −0.078) | |||
| Frontal Cortex | unc | 0.77* (0.69, 0.85) | −0.008* (−0.013, −0.003) | ||
| PVC | 0.93* (0.83, 1.03) | −0.007* (−0.013, −0.001) | |||
| Parietal cortex | unc | 0.83* (0.73, 0.92) | −0.009* (−0.014, −0.003) | ||
| PVC | 0.98* (0.86, 1.10) | −0.010* (−0.017, −0.003) | |||
Note: Unstandardized effects reported with 95% CIs.
unc: uncorrected; PVC: partial volume corrected; HRRT: high resolution research tomograph; DSTE: discovery STE; ACC: anterior cingulate cortex; MTL: medial temporal lobe.
Significant at p < .05, two-tailed.
Table 3.
Age-related differences in D2/3 BPND per decade.
| % Difference in BPND per
decade |
|||
|---|---|---|---|
| Region | Fallypride DSTE (%) | FLB HRRT (%) | |
| Thalamus | unc | −4.62 | −2.54 |
| PVC | −3.33 | −2.28 | |
| ACC | unc | −8.77 | −8.91 |
| PVC | −4.29 | −8.40 | |
| MTL | unc | −3.64 | −4.76 |
| PVC | −1.33 | −4.83 | |
| Temporal cortex | unc | −7.14 | −9.63 |
| PVC | −4.42 | −8.33 | |
| Striatum | unc | −5.34 | |
| PVC | −4.57 | ||
| Frontal cortex | unc | −10.39 | |
| PVC | −7.53 | ||
| Parietal Cortex | unc | −10.84 | |
| PVC | −10.20 | ||
Note: Age-related differences in D2/3 BPND estimated using the intercept and age slope (beta) values from Table 1 to compute a percent yearly age-related difference in BPND, which was converted to percent per decade.
ACC: anterior cingulate cortex; PVC: partial volume correction; MTL: medial temporal lobe; unc: uncorrected; HRRT: high resolution research tomograph; DSTE: discovery STE.
Age slopes and intercepts from regressions have been frequently used in the PET literature to quantify the rate of decline per decade of adulthood. However, standardized estimates of the age effects (i.e. correlation coefficients) are also reported in the literature and can provide more general information about the age effect size. That is, age slopes communicate the estimated rate of age-related change, whereas standardized effect sizes indicate how reliably and accurately BPND can be predicted from age (i.e. scatter of points around that regression line). Thus, we also examined standardized age effects and compared these effects sizes for uncorrected and PVC data (Table 2). There were moderate differences between uncorrected and PVC data in the standardized correlation coefficients (i.e. age effect sizes) (Table 2). For the Fallypride DSTE data, all age correlations with BPND were significantly reduced using PVC compared to uncorrected data (thalamus, t = −4.96, p < 0.001, ACC, t = −4.51, p < 0.001, MTL, t = −6.22, p < 0.001, temporal cortex, t = −3.30, p = 0.002, striatum, t = −3.51, p = 0.001). Fewer PVC versus uncorrected differences in age correlations were observed in FLB HRRT data. Differences between PVC versus uncorrected FLB BPND correlations with age were observed in the thalamus, t = −4.73, p < 0.001, and frontal cortex, t = −4.66, p < 0.001, but not in the ACC, t = −0.56, p = 0.580, MTL, t = −0.33, p = 0.745, temporal cortex, t = −1.51, p = 0.140, or parietal cortex, t = −0.48, p = 0.635.
Table 2.
Standardized correlation coefficients (with 95% CIs) for the age effect sizes on D2-like receptor availability (BPND) from both studies.
| Fallypride |
FLB |
||
|---|---|---|---|
| Region | Age effect size | Age effect size | |
| Thalamus | unc | −0.448* (−0.675, −0.221) | −0.250 (−0.582, 0.082) |
| PVC | −0.361* (−0.597, −0.124) | −0.215 (−0.550, 0.120) | |
| ACC | unc | −0.509* (−0.727, −0.290) | −0.494* (−0.792, −0.196) |
| PVC | −0.230 (−0.476, 0.018) | −0.472* (−0.774, −0.169) | |
| MTL | unc | −0.370* (−0.605, −0.134) | −0.338* (−0.660, −0.015) |
| PVC | −0.152 (−0.403, 0.098) | −0.317 (−0.643, −0.008) | |
| Temporal Cortex | unc | −0.416* (−0.647, −0.185) | −0.513* (−0.808, −0.219) |
| PVC | −0.293* (−0.536, −0.051) | −0.452* (−0.758, −0.146) | |
| Striatum | unc | −0.572* (−0.780, −0.364) | |
| PVC | −0.511* (−0.729, −0.292) | ||
| Frontal cortex | unc | −0.471* (−0.773, −0.168) | |
| PVC | −0.394* (−0.709, −0.078) | ||
| Parietal cortex | unc | −0.455* (−0.761, −0.149) | |
| PVC | −0.427* (−0.737, −0.117) |
Significant at p < .05, two-tailed.
ACC: anterior cingulate cortex; PVC: partial volume correction; MTL: medial temporal lobe; unc: uncorrected.
Finally, we conducted additional analyses to examine whether simply controlling for brain volume within a region in the uncorrected data (instead of using PVC during estimation of BPND) would approximate the age effect size estimates obtained with PVC. If accurate, this would offer a computationally simpler alternative to PVC. Although many of the age effect sizes were numerically different between uncorrected data with volume regressed out and PVC data, statistical comparison revealed only two regions, the MTL (−0.35 vs. −0.15, t = 5.52, p < 0.001) and ACC (−0.45 vs. −0.23, t = 3.31, p = 0.002), where the age effect sizes significantly differed in the Fallypride DSTE data. The age effect sizes using the uncorrected data with volume regressed out did not significantly differ from the age effect sizes using PVC data within the thalamus (−0.39 vs. −0.36, t = 1.76, p = 0.084), temporal cortex (−0.23 vs. −0.29, t = −1.42, p = 0.162), or striatum (−0.51 vs. −0.51, t = −0.06, p = 0.955). In the FLB HRRT data, statistical comparison revealed two regions, the thalamus (−0.36 vs −0.21, t = 3.36, p = 0.002) and frontal cortex (−0.49 vs. −0.39, t = 5.03, p < 0.001), where the age effect sizes significantly differed. The age effect sizes using the uncorrected data with volume regressed out did not significantly differ from the age effect sizes using PVC data within the ACC (−0.44 vs. −0.47, t = −0.68, p = 0.498), MTL (−0.34 vs. −0.32, t = 0.37, p = 0.715), temporal cortex (−0.49 vs. −0.45, t = 0.90, p = 0.375), or parietal cortex (−0.40 vs. −0.43, t = −0.39, p = 0.697) for the FLB data. We conducted similar analyses regressing out both volume and sex and the pattern of results was nearly identical. See Supplementary Results and Supplementary Tables 5 to 7 for complete reporting of regression analyses controlling for volume and sex.
Discussion
Application of GTM PVC12,13 consistently increased BPND, with the increase in striatal Fallypride BPND ( ∼ 56%) consistent with the 40–60% increase observed in raclopride studies using PVC.7,8 Our findings are not surprising given that the purpose of PVC is to isolate the relevant tissue for analysis and minimize contributions from surrounding unrelated tissue (e.g. white matter) and ventricles. This increase in BPND after PVC could make a difference in whether areas with lower BPND are viewed as having sufficient DA signal to measure minor differences in BPND across subjects or conditions especially in older subjects where lower binding is expected. PVC effects were smaller from data collected on a higher resolution camera (BPND intercepts increased ∼10–25% in the HRRT FLB data versus ∼20–60% after PVC in the DSTE Fallypride data), as expected. Given that the resolution of the PET camera (HRRT) used for FLB was much higher than that used to collect the Fallypride PET data (Discovery STE), it is not surprising that PVC effects were smaller in the FLB data as partial volume effects are limited by the amount of different types of tissue contained in each data point. Higher resolution cameras, then, may minimize the need for PVC.
Estimates of age-related differences per decade in the uncorrected data (−2.54% to −10.84% across regions and studies, Table 3) were similar to previous studies27–29 with striatal D2 decline estimated at ∼5% per decade in the Fallypride data and frontal cortical D2 decline ∼8–11% in the FLB data. Our observed striatal D2 decline (∼5% before and after PVC) was somewhat lower than the 8% reported in a recent meta-analysis.4 This could be due to the extremely healthy sample of older Fallypride subjects. The Fallypride sample’s frontal and parietal grey matter volume were significantly greater than the healthy, age-matched FLB subjects (Table S1), suggesting less atrophy than in a well-matched age group. Greater general health could lead to less D2-related decline than would be observed in the general population. In a recent study, we showed that age-related decline in striatal D2 BPND is less steep in healthier samples (e.g. more physically active adults).30 The frontal cortical decline of 10.4% in the uncorrected data was closer to the meta-analysis’s reported 9.3%4 than the PVC value of 7.5%. Thus, estimates of frontal cortical D2 decline with age may be particularly over-estimated if PVC is not performed, which was the case for all D2 studies in the recent meta-analysis.4
Significant differences emerged between uncorrected and PVC data in the estimated age-related rates of decline (unstandardized regression slopes) in the striatum, MTL, and ACC in the Fallypride DSTE study. The striatum was the only region to show a significant increase in age slope after PVC (Table 1). However, the combination of an increased intercept and slope after PVC (β = −0.136 on intercept of 29.77 BPND compared to uncorrected: β = −0.102 on intercept of 19.11 BPND; Table 1) resulted in similar estimates of age-related decline in both the uncorrected (5.3%) and PVC data (4.6%). Thus, although the slope difference was significant, the estimated age-related change per decade was almost identical. In contrast, PVC had a larger effect on reducing age slopes on cortical (MTL, ACC) D2-like BPND (MTL 3.64% to 1.33%; ACC 8.77% to 4.29%) in the Fallypride DSTE study. None of the age-related rates of decline differed significantly between uncorrected and PVC data in the higher resolution FLB HRRT data. Overall, though, PVC had a numerically small effect on the age slopes in both data sets.
Interestingly, while the differences in unstandardized age effects on BPND (slopes of the regression lines; Table 1 and Figures 2 and 3) between PVC and uncorrected data were numerically small and often non-significant (especially in the FLB data), the standardized effects of age on BPND (correlation coefficients, i.e. spread of data around the regression line; Table 2) were significantly and moderately less negative after PVC, especially in the Fallypride DSTE data (Table 2).
The standardized age effects sizes (i.e., amount of variance in BPND explained by age) were significantly reduced after PVC in all regions in the Fallypride DSTE data as well as in the thalamus and frontal cortex in the FLB HRRT data (Table 2). For studies collected on standard resolution cameras such as the DSTE and/or using Fallypride, our data suggest it may be necessary to use PVC to avoid overestimation of the effects of age on D2-like receptor decline. The changes in standardized age effects suggest that age may be a weaker predictor of DA D2 receptor availability than what is currently reported in the literature.4 That is, although rates of decline are relatively unaffected by PVC (with the exceptions of MTL and ACC), these rates of decline would produce less precise predictions of an individual subject’s BPND based on their age (given the increased spread of data around the regression line after PVC). Overall, these data suggest that the majority of the age effects reported in the literature may be overestimated since almost no studies have used PVC and the effects of PVC in our study are consistent with the conclusions of a previous PVC study in non-human primates.10 Averaging over all regions in the Fallypride DSTE data (which is similar in resolution or even higher compared to most of the published studies4), PVC reduces the age effect size by about 0.15. If the size of the PVC effect we observed here is representative of what one would expect if PVC were performed in the published PET studies (e.g., using raclopride, FLB, and Fallypride, which made up the majority of the D2 data in a recent meta-analysis),4 the meta-analytic estimates of age effect sizes on D2 receptors may be closer to r = –0.41 than r = –0.56.
We conducted additional analyses to examine whether simply controlling for regional volume in uncorrected data would approximate the effects of PVC. Based on statistical tests of differences in age effects sizes, this approach seems to work for some regions but not others. In the MTL and ACC, controlling for volume in the uncorrected data still greatly overestimated the age effect sizes. These results suggest that studies focusing on these regions using standard resolution cameras should use PVC. However, for other brain regions, controlling for volume may be a satisfactory alternative especially given the limitations of PVC (detailed below).
There are several qualifications and limitations to these studies. Although the effects of PVC were smaller in the study using a higher resolution camera, the differences in the estimated age effect sizes across datasets may be attributable to other factors beyond the differences between scanner resolution. The two datasets involved subject populations from different cities (although with similar recruitment and screening) and used different radiotracers. Although, mechanistically, it is evident why camera resolution would have an effect, it is unclear how the properties of the different radiotracers used would impact the utility of PVC. Nevertheless, the D2/3 radioligands used (FLB and Fallypride) have slightly different D2 versus D3 affinity,31,32 kinetics,33 and are differentially sensitive to endogenous DA levels.34 The acquisition and preprocessing of the two PET datasets also differed. The FLB data had motion correction applied throughout the acquisition using a Polaris Vicra optical tracking system,22 while the motion correction for the Fallypride data was applied post-reconstruction and only accounted for across (not within) frame motion. In addition, the Fallypride data were acquired over three dynamic scan sessions with short breaks for subject comfort, while the FLB data were acquired in one dynamic scan session, which would lead to a greater need to realign the Fallypride data. It is possible these differences in realignment and data acquisition could have led to more variance in the Fallypride data.
All PVC correction methods make certain assumptions that are applied equally across regions and subjects in a dataset. For the GTM method12,13 applied here, the technique assumes each VOI has homogenous uptake of the PET ligand. Thus, the current method does not account for specific subregions of structures (like subnuclei of the amygdala) that may have different distributions of D2-like receptors. Furthermore, the method we employed through PNEURO relies heavily on accurate PET to MRI registration. It is possible that minor registration errors resulted in some PET signal being assigned to the incorrect anatomical structure (caudate versus the subcallosal area of ACC, for example). In spite of this, the PET TACs used to estimate BPND before and after PVC originated from the same registered data. So, any signal mixing due to minor PET-MRI registration differences would be present in both the uncorrected and PVC data to an equal degree.
A final and important consideration when using PVC is that it has the potential to introduce additional variance in BPND estimates.12,13 As expected, we found that PVC increased BPND (by overcoming partial volume effects in our data) as discussed above, but also increased standard error (SE) of the BPND estimates (possibly due to non-exact PET-MR registration, among other factors). Despite PVC increasing BPND estimation variation for most regions in both studies, the concomitant increase in BPND after PVC led to significant increases in BPND signal relative to error (BPND/SE) in most areas supporting its application to recover signal despite introducing more variance. However, across both datasets, ACC BPND/SE significantly decreased and MTL BPND/SE was not improved, suggesting that researchers should consider the trade-off between increased signal and estimation error before performing GTM PVC, especially in these regions. In the present studies, we only used GTM. The precision of estimation after PVC will vary depending on the PVC method as well as many other factors such as the size and shape of ROIs, PET camera, image co-registration, or tracers used. Differences in both signal and estimation error should be evaluated when considering the application of PVC in future studies.
In conclusion, our analyses indicate that PVC reduces the size of age-related differences in BPND, especially on standard-resolution PET cameras. Even with the high resolution HRRT system, age effect sizes on BPND varied when using uncorrected or PVC PET data for some regions. In contrast to the age effect sizes, PVC had a smaller and less consistent effect on the estimated rates of decline with age (i.e. slope of the age effect on BPND). Based on these data, the overall recommendation of whether or not to use PVC depends on a future study’s PET resolution, the accuracy with which the PVC algorithm estimates BPND, and whether the study is focused on quantifying standardized age effect sizes or estimated rates of decline with age. If a researcher is attempting to obtain estimates of age effect sizes using a standard resolution PET system, the present data suggest that using PVC may provide more accurate estimates that are less susceptible to biases introduced by grey matter loss for most but not all brain regions (e.g. ACC). The use of PVC appears to be less critical for the estimation of age-related rates of decline or when using very high resolution systems like the HRRT (with the possible exception of estimating age effect sizes in thalamus and frontal cortex).
Supplementary Material
Acknowledgments
Thanks to Kruti Vekaria, Jaime Castrellon, Scott Perkins, Tahj Blow, and Audrey Odonkor for assistance with data collection, Aaron Tetreault and Miki Wilkinson for assistance with data processing, and William Jagust for advice on partial volume correction approaches.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from the US National Institute on Aging to DHZ (R01-AG044838) and GRSL (R00-AG042596), the US National Institute on Drug Abuse to CTS (F32-DA041157) and LCD (F32-DA036979), and the US National Center for Advancing Translational Sciences (UL1TR000445).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
GRSL and DHZ designed the study. JLC, DM, and RLC collected the data and assisted with data processing and analysis. MDSJ, AV, and DTK assisted with data processing and analysis. CTS and GRSL analyzed the data with input from LCD, KLS, EDM, and DHZ. CTS, JLC, DHZ, and GRSL wrote the first draft of the paper. All authors contributed to the final draft of the paper.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Elsinga PH, Hatano K, Ishiwata K. PET tracers for imaging of the dopaminergic system. Curr Med Chem 2006; 13: 2139–2153. [DOI] [PubMed] [Google Scholar]
- 2.Wagner HN, Jr., Burns HD, Dannals RF, et al. Imaging dopamine receptors in the human brain by positron tomography. Science 1983; 221: 1264–1266. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RM. Imaging methods for evaluating brain function in man. Neurobiol Aging 2003; May–June(24 Suppl 1): S21–S35; discussion S37–S29. [DOI] [PubMed]
- 4.Karrer TM, Josef AK, Mata R, et al. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging 2017; 57: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raz N. The aging brain observed in vivo: differntial changes and their modifiers. In: Cabeza R, Nyberg L, Park DC. (eds). Cognitive neuroscience of aging: Linking cognitive and cerebral aging, New York: Oxford University Press, 2004, pp. 17–55. [Google Scholar]
- 6.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 2006; 30: 730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez D, Slifstein M, Broft A, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003; 23: 285–300. [DOI] [PubMed] [Google Scholar]
- 8.Mawlawi O, Martinez D, Slifstein M, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21: 1034–1057. [DOI] [PubMed] [Google Scholar]
- 9.Erlandsson K, Buvat I, Pretorius PH, et al. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys Med Biol 2012; 57: R119–R159. [DOI] [PubMed] [Google Scholar]
- 10.Morris ED, Chefer SI, Lane MA, et al. Loss of D2 receptor binding with age in rhesus monkeys: importance of correction for differences in striatal size. J Cereb Blood Flow Metab 1999; 19: 218–229. [DOI] [PubMed] [Google Scholar]
- 11.Muller-Gartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992; 12: 571–583. [DOI] [PubMed] [Google Scholar]
- 12.Rousset OG, Collins DL, Rahmim A, et al. Design and implementation of an automated partial volume correction in PET: application to dopamine receptor quantification in the normal human striatum. J Nucl Med 2008; 49: 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med 1998; 39: 904–911. [PubMed] [Google Scholar]
- 14.Meltzer CC, Kinahan PE, Greer PJ, et al. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med 1999; 40: 2053–2065. [PubMed] [Google Scholar]
- 15.Quarantelli M, Berkouk K, Prinster A, et al. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. J Nucl Med 2004; 45: 192–201. [PubMed] [Google Scholar]
- 16.Harri M, Mika T, Jussi H, et al. Evaluation of partial volume effect correction methods for brain positron emission tomography: quantification and reproducibility. J Med Phys 2007; 32: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong HW, van Velden FH, Kloet RW, et al. Performance evaluation of the ECAT HRRT: an LSO-LYSO double layer high resolution, high sensitivity scanner. Phys Med Biol 2007; 52: 1505–1526. [DOI] [PubMed] [Google Scholar]
- 18.Teras M, Tolvanen T, Johansson JJ, et al. Performance of the new generation of whole-body PET/CT scanners: discovery STE and discovery VCT. Eur J Nucl Med Mol Imag 2007; 34: 1683–1692. [DOI] [PubMed] [Google Scholar]
- 19.Dang LC, Samanez-Larkin GR, Castrellon JJ, et al. Associations between dopamine D2 receptor availability and BMI depend on age. Neuroimage 2016; 138: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandiego CM, Gallezot JD, Lim K, et al. Reference region modeling approaches for amphetamine challenge studies with [11C]FLB 457 and PET. J Cereb Blood Flow Metab 2015; 35: 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carson RE, Barker WC, Liow JS. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. Nucl Sci Symp Conf Record IEEE 2003; 5: 3281–3285. [Google Scholar]
- 22.Jin X, Chan C, Mulnix T, et al. List-mode reconstruction for the Biograph mCT with physics modeling and event-by-event motion correction. Phys Med Biol 2013; 58: 5567–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CT, Dang LC, Cowan RL, et al. Variability in paralimbic dopamine signaling correlates with subjective responses to d-amphetamine. Neuropharmacology 2016; 108: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametic maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2: 189–210. [Google Scholar]
- 25.Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp 2003; 19: 224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4: 153–158. [DOI] [PubMed] [Google Scholar]
- 27.Kaasinen V, Vilkman H, Hietala J, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging 2000; 21: 683–688. [DOI] [PubMed] [Google Scholar]
- 28.Inoue M, Suhara T, Sudo Y, et al. Age-related reduction of extrastriatal dopamine D2 receptor measured by PET. Life Sci 2001; 69: 1079–1084. [DOI] [PubMed] [Google Scholar]
- 29.Ishibashi K, Ishii K, Oda K, et al. Regional analysis of age-related decline in dopamine transporters and dopamine D2-like receptors in human striatum. Synapse 2009; 63: 282–290. [DOI] [PubMed] [Google Scholar]
- 30.Dang LC, Castrellon JJ, Perkins SF, et al. Reduced effects of age on dopamine D2 receptor levels in physically active adults. Neuroimage 2017; 148: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halldin C, Farde L, Hogberg T, et al. Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med 1995; 36: 1275–1281. [PubMed] [Google Scholar]
- 32.Mukherjee J, Constantinescu CC, Hoang AT, et al. Dopamine D3 receptor binding of (18)F-fallypride: evaluation using in vitro and in vivo PET imaging studies. Synapse 2015; 69: 577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandehey NT, Moirano JM, Converse AK, et al. High-affinity dopamine D2/D3 PET radioligands 18F-fallypride and 11C-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab 2010; 30: 994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris ED, Yoder KK. Positron emission tomography displacement sensitivity: predicting binding potential change for positron emission tomography tracers based on their kinetic characteristics. J Cereb Blood Flow Metab 2007; 27: 606–617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.