Abstract
Pyoderma gangrenosum is an ulcerating disease associated with a high degree of morbidity and mortality. Currently, little is known about the pathophysiology of pyoderma gangrenosum, though it has been linked to increased levels of inflammatory cytokines including interleukin-23. As pyoderma gangrenosum is a rare disease, evidence for pyoderma gangrenosum treatment is dependent on reporting of cases with successful therapies. Here, we describe a case of pyoderma gangrenosum developing on the lateral leg of a medically complex 47-year-old male already on chronic immunosuppressive therapy, who achieved successful wound healing with the use of ustekinumab, a monoclonal antibody targeting inhibition of interleukin-12 and interleukin-23. This case lends further evidence for the role of interleukin-23 in the pathogenesis of recalcitrant pyoderma gangrenosum and also suggests that healthcare providers may consider a trial of ustekinumab in pyoderma gangrenosum that has failed previous topical treatments or systemic immunosuppression.
Keywords: Pyoderma gangrenosum, ustekinumab, biologics
Introduction
Pyoderma gangrenosum (PG) is an immune-mediated disease that classically presents with ulceration. It has been associated with significant morbidity, as it is linked with inflammatory bowel disease (IBD), rheumatoid arthritis, hematologic malignancies, and monoclonal gammopathy.1 Moreover, PG is associated with a threefold increased risk of mortality compared with the general population.1 Since the underlying pathophysiology remains poorly understood, a variety of therapeutic strategies have been proposed, but unfortunately, none are supported by high-quality evidence.2 This is largely related to the fact that controlled research studies on PG are difficult to perform given the condition only affects about 3–10 patients/million/year.3 Consequently, documentation of treatment successes in isolated patients through case studies is of relatively high importance for PG evidence-based medicine.
Recently, it has been proposed that recalcitrant PG lesions may demonstrate increased interleukin (IL)-23 expression.4 Correspondingly, cases have emerged suggesting ustekinumab, a humanized monoclonal antibody targeting the shared p40 subunit of IL-12 and IL-23, may hold promise for inducing remission of refractory PG.5–7 Here, we, adding to this body of literature, demonstrate the use of ustekinumab to treat PG that developed in a medically complex patient already receiving systemic immunosuppressive therapy.
Case report
A 47-year-old medically complex male with myasthenia gravis on long-term systemic corticosteroids, diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, gout, and obstructive sleep apnea presented to the dermatology clinic with painful undermined ulcers on the right lower leg. About 1 year prior to these ulcers developing, he had traumatized this area on his leg after bumping into a metal rod. Although this wound healed, within about 4 months, he subsequently developed several painful, rapidly expanding violaceous ulcers with undermined borders to the lower leg. PG was suspected based on morphology and history and was supported by results of a 4 mm punch biopsy, which revealed mixed dermal infiltrate of predominantly neutrophils, with overlying dermal fibrosis at the deep edge of the dermal biopsy. Workup revealed no evidence of inflammatory bowel disease, no features or serology consistent with autoimmune arthritis, and no other known associations with PG. However, he was found to have a monoclonal gammopathy of unknown significance (with a normal bone marrow biopsy), hepatic steatosis, and was anti-HBC positive, hepatitis B core antibody reactive, though HBsAg was negative and hepatitis B surface antibody was protective. It should be noted that the PG developed while he was on high-dose prednisone for his myasthenia gravis. His PG was initially treated with topical clobetasol 0.05% ointment and intralesional corticosteroid injections with limited success. For his medical comorbidities, intravenous immunoglobulin (IVIg; 100 g/day for two consecutive days per month) was initiated in July 2017 and mycophenolate mofetil (1 g twice daily) was started in October 2017, and both treatments were maintained to the end of the case report period. Ustekinumab was initiated in December 2017 according to the IBD dosing protocol with a 520-mg IV infusion at week 0, and 90-mg subcutaneous injections at week 8 and then every 8 weeks thereafter. On treatment with ustekinumab over a 6-month period, the patient experienced a dramatic improvement in the size of the ulcer, noting a decrease in size by approximately half. Before and after treatment with ustekinumab response is depicted in Figure 1.
Figure 1.
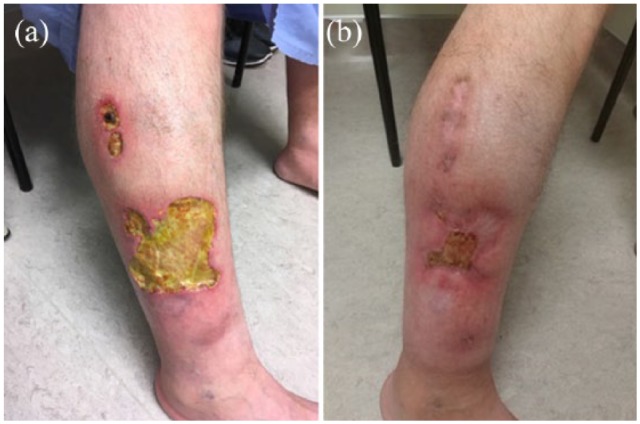
Pyoderma gangrenosum ulcers on the right lower leg (a) before treatment and (b) 6 months after treatment with ustekinumab.
Discussion
PG is a painful ulcerating condition that can lead to scarring, especially if left untreated or if treated sub-optimally over a long period of time.8 To date, no gold standard therapy exists for PG, and no treatments are supported by high-quality evidence.1 As with other autoimmune diseases, interest is increasing in the use of biologic agents, given that these drugs are designed to block specific inflammatory cytokines, thereby promoting remission of immune-mediated diseases.8 Our case has demonstrated the successful treatment of PG with the use of ustekinumab, an IL-12 and IL-23 inhibitor, in a complex patient who developed PG while already on immunosuppressive therapy. This patient had a history of a minor traumatic injury to the affected leg, which subsequently progressed to PG, indicating potential pathergy. The positive clinical response documented here is congruent with a growing number of cases also demonstrating improvements in recalcitrant PG with the use of ustekinumab.5–7 Therefore, this case further supports the use of ustekinumab in therapy for PG and also draws attention to the probable contribution of IL-23 in disease pathogenesis.
While this case does not infer causation, it suggests that future observational studies or small multicentre randomized trials would be beneficial in characterizing the effectiveness of ustekinumab in the treatment of PG. With a growing number of cases in the literature, it will also be beneficial to explore whether certain demographic or clinical features may predict responders to treatments including ustekinumab. At present, providers may consider a trial of ustekinumab in patients with PG who have not responded optimally to conventional therapy.
Acknowledgments
The authors thank the patient for his permission to document his treatment course and outcome.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: IAV was supported by the Alberta Innovates MD/PhD studentship.
Informed consent: Consent to publication occurred in the context of the photo documentation.
References
- 1. Langan SM, Groves LM, Card RW, et al. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol 2012; 132: 2166–2170. [DOI] [PubMed] [Google Scholar]
- 2. Partridge ACR, Bai J, Rosen C, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol 2018; 179(2): 290–295. [DOI] [PubMed] [Google Scholar]
- 3. Ruocco E, Sangiuliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol 2009; 23: 1008–1017. [DOI] [PubMed] [Google Scholar]
- 4. Guenova E, Teske A, Fehrenbacher B, et al. Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Arch Dermatol 2011; 147(10): 1203–1205. [DOI] [PubMed] [Google Scholar]
- 5. Greb JE, Gottlieb AB, Goldminz AM. High-dose ustekinumab for the treatment of severe, recalcitrant pyoderma gangrenosum. Dermatol Ther 2016; 29(6): 482–483. [DOI] [PubMed] [Google Scholar]
- 6. Nieto D, Sendagorta E, Rueda JM, et al. Successful treatment with ustekinumab and vacuum-assisted closure therapy in recalcitrant myelodysplastic syndrome-associated pyoderma gangrenosum: case report and literature review. Clin Exp Dermatol 2018; 44: 116–119. [DOI] [PubMed] [Google Scholar]
- 7. Goldminz AM, Botto NC, Gottlieb AB. Severely recalcitrant pyoderma gangrenosum successfully treated with ustekinumab. J Am Acad Dermatol 2012; 67(5): e237–e238. [DOI] [PubMed] [Google Scholar]
- 8. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol 2015; 95(5): 525–531. [DOI] [PubMed] [Google Scholar]


