Abstract
Purpose: This study aimed to investigate whether the extracellular-to-intracellular fluid volume (E/I) ratio can predict survival in patients with metastatic cancer. Methods: Clinical data were collected from April 2016 to March 2018. Patients aged ≥19 years with metastatic solid tumor were eligible. Bioimpedance analysis was used to assess body fluid distribution and the E/I ratio. Clinical characteristics, including laboratory test results and nutrition status according to the E/I ratio, were analyzed. Cox proportional hazards models and Kaplan-Meier analysis were used to identify risk factors for mortality. Results: In total, 87 patients were included in the study. The 87 patients were divided into 2 groups according to the median E/I ratio: a high E/I group (E/I ratio ≥1.0, n = 43) and a low E/I group (E/I ratio <1.0, n = 44). Poor performance status, fluid retention, malnutrition, elevation of C-reactive protein levels, and decreases in hemoglobin, albumin, and protein levels were significantly associated with the high E/I group. The median overall survival time was 1.6 and 12.5 months in the high E/I and low E/I groups, respectively (P < .001). In the multivariate analysis, poor performance status, leukocytosis, fluid retention, and E/I ratio were independent prognostic factors, and the E/I ratio was the strongest prognostic factor predicting overall survival (hazard ratio = 3.49, 95% confidence interval = 1.75-6.96, P < .001). Conclusions: The E/I ratio can predict survival time in patients with metastatic cancer. More rigorous research is required to confirm this result.
Keywords: extracellular-to-intracellular fluid volume ratio, body composition, bioimpedance analysis, cancer, survival
Introduction
Only 20.9% of patients with advanced or metastatic tumor survive for 5 years according to national statistics in Korea, and 4% to 30% survive in the United States.1,2 Predicting survival time is important for deciding between aggressive anticancer therapies to prolong survival time and supportive care to manage patient symptoms and quality of life as the appropriate treatment plan for patients with metastatic cancer.3,4
Several prognostic methods have been developed for clinicians to treat patients with an optimal treatment plan. Clinical symptoms including anorexia, dyspnea, and delirium and laboratory data including leukocytosis, lymphocytopenia, and elevated C-reactive protein (CRP) levels have been suggested to be prognostic factors of survival time in patients with cancer.5 Additionally, several combinations of clinical symptoms and laboratory data, such as palliative prognostic score and palliative prognostic index, have been proposed.6 Comprehensive and objective predicting tools are still necessary to refine and complement prognostic models.7
The extracellular-to-intracellular fluid volume ratio (E/I ratio), which varies from 0.59 to 0.75 in healthy adults,8,9 represents the internal fluid distribution in human body. In particular, it shows a significant association with disease states such as inflammation, immune activation, and fluid shift due to poor tissue perfusion.8 Bioimpedance analysis (BIA) provides a noninvasive, reliable, and simple bedside technology for diagnosing subclinical fluid accumulation, including extracellular and intracellular fluid volume, utilizing the electrical properties of body tissues.10
The E/I ratio is positively associated with aging11 and malnutritional status.9 E/I ratio was also a strong independent predictor of mortality and survival in patients of end-stage chronic diseases.12,13 The E/I ratio was also reported to have a potential relationship with survival time in patients with cancer receiving palliative care.14 An elevated metabolic rate and accumulation of extracellular body fluid have been related to poor survival in patients with cancer, who often suffer from malnutrition, inflammation, and fluid retention such as pleural effusion, ascites, and edema of peripheral extremities.15,16
This study aimed to evaluate clinical characteristics including laboratory test results and nutrition status according to the E/I ratio and confirm the value of the E/I ratio as an independent prognostic factor in patients with metastatic cancer.
Study Purpose
The purpose of the study was to investigate whether the E/I ratio is related to survival time in patients with metastatic cancer.
Methods
Study Design and Eligibility
This was a retrospective chart review study. Patients who visited the Korean Medicine Cancer Center, Kyung Hee University Hospital at Gangdong, from April 2016 to March 2018, were enrolled. Metastatic stage solid tumor was diagnostically confirmed in every patient according to the American Joint Committee on Cancer, seventh edition. Patients were eligible for inclusion if they were 19 years or older and had undergone a BIA test. If a patient received BIA test repeatedly, we used the baseline measurement for analysis in this study. However, patients who did not undergo laboratory tests 3 days before and after their BIA test and those who had clinically unstable vital signs at the time of their BIA test were excluded. The presence of at least one of following vital signs was regarded as clinically unstable: systolic blood pressure below 90 mm Hg, pulse rate above 120 beats per minute, or constant need of oxygen supplement for dyspnea. Patients who had undergone surgery within 1 month previously and patients with a medical history of end-stage renal disease (ESRD) requiring regular hemodialysis or peritoneal dialysis were also excluded. This study was approved by the institutional review board at Kyung Hee University Hospital at Gangdong (Institutional Review Board No. KHNMC-OH 2018-05-004).
Data Collection
Clinical and medical data, including age, sex, medical history and medication, primary cancer lesion, TNM stage, major cancer treatment history, and concurrent treatment status, were collected from medical records. Major cancer treatment history was defined as surgery, chemotherapy, and radiotherapy. Body mass index, Eastern Cooperative Oncology Group performance status (ECOG-PS; 0, fully active; 1, restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; 2, ambulatory and capable of all self-care but unable to carry out any work activities; 3, capable of only limited self-care, confined to bed or chair more than 50% of waking hours; 4, completely disabled; 5, dead), and BIA results were assessed by clinical physicians. Laboratory test results 3 days before and after BIA assessment were recorded. White blood cell and platelet counts and hemoglobin, CRP, blood urea nitrogen, serum creatinine, sodium, potassium, chloride, protein, albumin, total bilirubin, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels were assessed.
Inflammation was defined as abnormal CRP elevation (CRP level >1.0 mg/dL). Anemia, hypoalbuminemia, and hyponatremia were graded according to the Common Terminology Criteria for Adverse Events version 4.0. Fluid retention was defined as abnormal fluid collection in the pericardial, pleural, or abdominal cavity or the peripheral extremities and assessed through an imaging examination confirmed by a radiologist.
Nutritional status was independently assessed by 2 professional clinical dieticians according to the Kyung Hee Neo Nutrition Risk Screening (KNNRS) score, which was developed to identify hospitalized patients with malnutrition risk based on the Subjective Global Assessment (SGA) and the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines.17-19
According to the KNNRS, nutritional status was divided into 3 categories: 0 to 3, well nourished (absence of malnutrition or normal nutrition); 4 to 10, mild to moderate malnutrition; and 11 to 20, severe malnutrition.
Bioimpedance Measurement
Bioimpedance was measured using the BIA 450 bioimpedance analyzer (Medinex, Seoul, Republic of Korea) and the unilateral wrist-ankle method. Immediately preceding the BIA, the height and weight of the patient were measured. We avoided the measurement both less than 30 minutes and more than 6 hours after meal time to avoid the patient from undergoing excessive satiety or starvation. In the absence of excessive satiety or starvation, the patient was stabilized in supine position for 15 minutes and toilet visit was recommended before the test. After 15 minutes, the patient underwent the test in the supine position. Four bipolar electrodes were placed on the wrist, dorsum of the right hand, anterior ankle, and dorsum of the right foot. A 60-kHz frequency was used to measure the intracellular and extracellular fluid of the patients, which took approximately 2 minutes. The E/I ratio was calculated as the extracellular fluid amount divided by intracellular water amount. Toilet visit was recommended before the test.
All measurements were performed by the same examiner throughout the study. The patients were divided into 2 groups according to the median values of the E/I ratio distribution: a low E/I ratio (E/I <1.0) and a high E/I ratio (E/I ≥1.0) group.
Data Analysis
All variables are expressed as mean ± standard deviation or median (range). For continuous variables, an independent-sample t test was performed. For variables not normally distributed, the Kruskal-Wallis test was used. For categorical variables, a χ2 test was performed. Hazard ratios of all-cause mortality in the study population were assessed with Cox proportional hazard ratio models. Univariate and multivariate analyses were performed, and mortality was plotted using a Kaplan-Meier survival curve. A log-rank test was used to assess statistical differences in survival. Differences were considered statistically significant for P < .05. Every analysis was calculated and plotted using PASW Statistics for Windows (SPSS) version 18.0 (SPSS Inc, Chicago, IL).
Results
Between April 2016 and March 2018, 133 patients who were diagnosed with cancer visited the Korean Medicine Cancer Center, Kyung Hee University Hospital at Gangdong, and underwent BIA. There were 32 cases of localized or limited-stage cancer and 14 cases with incomplete laboratory test results. Finally, 87 patients were included (Figure 1). The median follow-up period was 16.4 months (2.6-24.8 months), with 13.4 months in the high E/I ratio group and 16.7 months in the low E/I ratio group. The median overall survival time was 5.5 months in all patients (95% confidence interval [CI] = 4.3-6.7 months).
Figure 1.
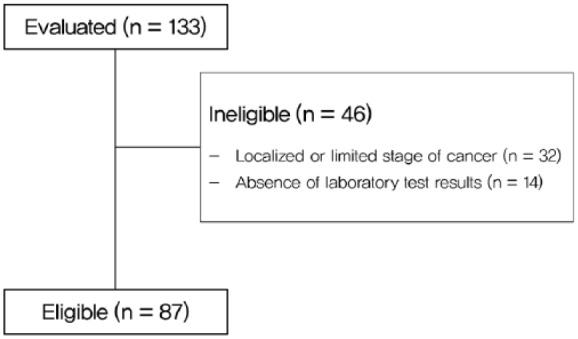
Study enrollment flowchart.
Demographic Characteristics
Of the 87 patients included in this study, 44 (50.6%) were men and 43 (49.4%) were women. The mean age was 65.1 (range = 36-95) years. The most common type of cancer was gastrointestinal tract cancer: 33 patients (37.9%) had gastrointestinal cancer, 21 (24.1%) had hepatobiliary cancer, 14 (16.1%) had urogenital cancer, and 6 (6.9%) had lung cancer. Every patient was in stage IV according to TNM staging. More than half of the patients underwent surgery and/or chemotherapy; 47 patients (54.0%) had surgery, 57 patients (65.5%) received chemotherapy, and 21 patients (24.1%) experienced radiotherapy (Table 1).
Table 1.
General Characteristics of Patients.
| Characteristic | Value |
|---|---|
| Age, years | 65.1 (36-95) |
| Male sex, n (%) | 44 (50.6%) |
| BMI, kg/m2 | 21.5 ± 3.7 |
| OS, months | 5.5 (4.3-6.7) |
| Primary lesion, n (%) | |
| Gastrointestinal | 33 (37.9%) |
| Hepatobiliary | 21 (24.1%) |
| Urogenital | 14 (16.1%) |
| Lung | 6 (6.9%) |
| Breast | 3 (3.4%) |
| Head and neck | 2 (2.3%) |
| Other | 8 (9.2%) |
| Treatment history, n (%) | |
| Surgery | 47 (54.0%) |
| Chemotherapy | 57 (65.5%) |
| Radiotherapy | 21 (24.1%) |
Abbreviations: BMI, body mass index; OS, overall survival.
There were no patients with an ECOG-PS of 0, 29 patients (33.3%) had an ECOG-PS of 1, 27 (31.0%) had an ECOG-PS of 2, 17 patients (19.5%) had an ECOG-PS of 3, and 14 patients (16.1%) had an ECOG-PS of 4. Although 29 patients (29.3%) had a normal nutritional status, the majority of patients experienced malnutrition, with 55 patients (63.2%) with mild to moderate malnutrition and 3 patients (3.4%) with a severe malnutrition status. Anemia of grade 2 or higher was observed in 32.2% of patients, hypoalbuminemia in 25.3%, and hyponatremia in 9.2%.
Patient Characteristics According to 2 Quantiles of E/I Ratio
The overall characteristics of the patient groups divided according to E/I ratio are shown in Table 2.
Table 2.
Characteristics in the Low and High E/I Ratio Groups.
| Characteristics | Low E/I Ratio (E/I <1.0, n = 44) | High E/I Ratio (E/I ≥1.0, n = 43) | P |
|---|---|---|---|
| BMI, kg/m2 | 21.8 ± 3.5 | 21.0 ± 4.0 | .272 |
| ECOG-PS | .045* | ||
| 0-2 | 33 (75.0%) | 25 (53.5%) | |
| 3-4 | 11 (25.0%) | 20 (45.6%) | |
| Fluid retention (presence of pleural fluid, ascites and/or peripheral edema) | 7 (15.9%) | 28 (65.1%) | <.001* |
| Malnutrition | .007* | ||
| Absence (well nourished) | 21 (47.7%) | 8 (18.6%) | |
| Mild to moderate | 22 (50.0%) | 33 (76.7%) | |
| Severe | 1 (2.3%) | 2 (4.7%) | |
| WBC (/µL) | 6954.3 ± 3037.9 | 7443.3 ± 4151.2 | .533 |
| CRP (mg/dL) | 2.3 ± 3.4 | 4.8 ± 6.8 | .037* |
| Hb (g/dL) | 11.6 ± 2.1 | 10.2 ± 1.5 | .001* |
| Sodium (mEq/L) | 136.4 ± 3.4 | 134.9 ± 4.2 | .071 |
| Chloride (mEq/L) | 103.4 ± 3.3 | 101.8 ± 4.6 | .075 |
| Protein (mg/dL) | 6.78 ± 0.7 | 6.33 ± 0.9 | .010* |
| Albumin (mg/dL) | 3.63 ± 0.6 | 3.14 ± 0.6 | .001* |
| Bilirubin (mg/dL) | 0.70 ± 0.4 | 1.57 ± 3.7 | .137 |
Abbreviations: E/I, extracellular-to-intracellular fluid volume; BMI, body mass index; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; WBC, white blood cell count; CRP, C-reactive protein; Hb, hemoglobin.
Indicates that the P value was statistically significant (P < .05).
Patients in the high E/I ratio group had a poor performance status, indicated by an ECOG-PS of 3 or worse (46.5% vs 25.0%, P = .045), experienced more fluid retention including pleural effusion and ascites (65.1% vs 15.9%, P < .001), and experienced more malnutrition (81.4% vs 52.3%, P = .007). In the high E/I ratio group, the CRP level was significantly higher (4.8 ± 6.8 vs 2.3 ± 3.4 mg/dL, P = .037), while the hemoglobin level (10.2 ± 1.5 vs 11.6 ± 2.1 g/dL, P = .001), serum protein level (6.33 ± 0.9 vs 6.78 ± 0.7 mg/dL, P = .010), and albumin level (3.14 ± 0.6 vs 3.63 ± 0.6 mg/dL, P = .001) were significantly lower.
Univariate and Multivariate Analyses for Mortality
In the univariate analysis, low body mass index, poor ECOG-PS, leukocytosis, inflammation, anemia, hyponatremia, hypoalbuminemia, fluid retention, malnutrition, and E/I ratio were predictors of overall survival. In the adjusted multivariate analysis, poor ECOG-PS, leukocytosis, fluid retention, and E/I ratio were significant predictors of overall survival (hazard ratio [95% CI]): poor ECOG-PS 2.51 (1.16-5.44), leukocytosis 2.65 (1.19-5.92), fluid retention 2.17 (1.05-4.49), and E/I ratio 3.49 (1.75-6.96; Table 3).
Table 3.
Univariate and Multivariate Analyses by Cox Proportional Hazard Ratio Model for Mortality.
| Unadjusted Univariate Analysis | Adjusted Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| BMI (BMI <20.0 vs ≥20.0) | 1.96 (1.13-3.41) | .015* | ||
| ECOG-PS (3-4 vs 0-2) | 4.34 (2.30-8.18) | <.001* | 2.51 (1.16-5.44) | .020* |
| Leukocytosis (WBC >10 000 vs ≤10 000) | 5.16 (2.60-10.22) | <.001* | 2.65 (1.19-5.92) | .017* |
| Inflammation (CRP >1.0 vs ≤1.0) | 2.44 (1.42-4.20) | .001* | ||
| Anemia (Hb <10.0 vs ≥10.0) | 2.09 (1.00-4.37) | .046* | ||
| Hyponatremia (Na <130.0 vs ≥130.0) | 2.47 (1.11-5.51) | .027* | — | — |
| Hypoalbuminemia (albumin <3.0 vs ≥3.0) | 5.16 (2.88-9.25) | <.001* | ||
| Bilirubinemia (Bilirubin >1.0 vs ≤1.0) | 1.64 (0.87-3.07) | .123 | — | — |
| Fluid retention (presence vs absence) | 5.40 (3.05-9.56) | <.001* | 2.17 (1.05-4.49) | .038* |
| Malnutrition (presence vs absence) | 2.60 (1.32-5.11) | .006* | ||
| E/I ratio (<1.0 vs ≥1.0) | 3.52 (2.01-6.16) | <.001* | 3.49 (1.75-6.96) | <.001* |
Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; ECOG-PS, Eastern Cooperative Oncology Group performance status; WBC, white blood cell count; CRP, C-reactive protein; Hb, hemoglobin; E/I, extracellular-to-intracellular fluid volume.
Indicates that the P was statistically significant (P < .05).
Survival Curve
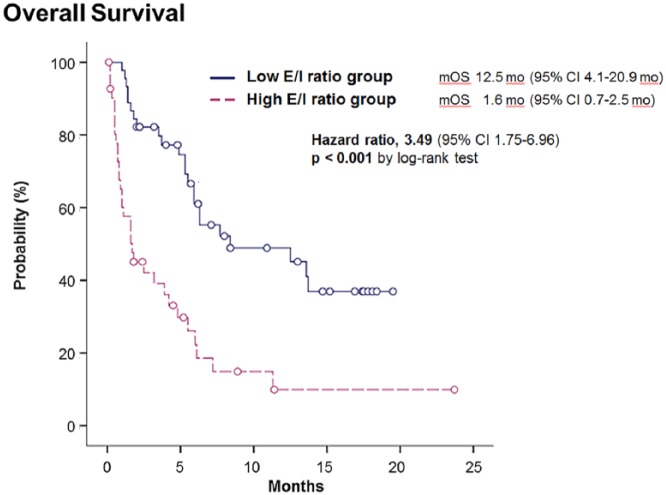
The overall survival of patients differed significantly between the high and low E/I ratio groups. The median overall survival of the high E/I ratio group according to the Kaplan-Meier survival curve was 1.6 months (95% CI = 0.7-2.5 months), whereas that of the low E/I ratio group was 12.5 months (95% CI = 4.1-20.9 months; P < .001; Figure 2).
Figure 2.
Overall survival according to E/I ratio.
Abbreviations: E/I, extracellular-to-intracellular fluid volume; mOS, median overall survival; MO, months; CI, confidence interval.
The high E/I ratio group had a significantly shorter survival time (median overall survival time 1.6 vs 12.5 months, log-rank P < .001).
Discussion
This study aimed to evaluate the clinical characteristics of patients with metastatic cancer according to the E/I ratio and to investigate whether the E/I ratio was related to survival time in patients with metastatic cancer. This study showed that a high E/I ratio (≥1.0) independently predicted a poor prognosis in patients with metastatic cancer.
E/I ratio indicates the fluid distribution in the human body. Fluids in the body lubricate movement of organs, aid in the excretion of waste matter, and mediate metabolism by transporting energy and substances including cytokines. Body fluid distribution tends to maintain homeostasis through active and passive transport systems. E/I ratio has already shown a relationship with overall condition and prognostic possibility in patients with chronic disease.12,13,20
The E/I ratio in patients with cancer may be imbalanced due to several reasons. Patients with cancer experience a higher incidence of hyponatremia, which is mainly related to the syndrome of inappropriate secretion of antidiuretic hormone secretion.21,22 Cancerous tissues in the peritoneum, pleura, or pericardium induce excessive and unnecessary effusion with or without major organ failure, which can change cardiovascular permeability and induce unnecessarily excessive fluid accumulation.23 Patients with cancer often experience fluid retention such as pleural effusion, ascites, or edema in the peripheral extremities.15,16 Fluid retention in patients with cancer commonly results from dysfunction of important organs.24 Fluid imbalance is also closely associated with inflammation,25 malnutrition,26 and body composition27 in patients with cancer.
Until now, the distribution of body fluid has mainly focused on the evaluation of periodic dialysis in patients with ESRD.28,29 The higher E/I ratio in patients with renal failure was suggested to be a predictor of prognosis in the context of the malnutrition-inflammation-atherosclerosis complex,26 as many cancer patients share the characteristics of malnutrition and inflammation.30-33 The study by Crawford et al supported the suggestion that accumulation of body fluid may have a significant relationship with a poor prognosis in patients with cancer.14
Our results are consistent with the results of previous studies of patients with ESRD.28,29 Cancer patients with a high E/I ratio experienced poorer nutrition, more inflammation, and poorer survival expectancy. Notably, the median value of the E/I ratio in the present study was much higher than that in patients with ESRD. This difference can be interpreted as follows. First, terminally ill patients with cancer experienced worse fluid imbalance, which resulted in a high E/I ratio. Although ESRD studies followed patients for 60 to 150 months, more than half of those patients did not meet the median survival in the Kaplan-Meier survival curve. On the other hand, our study reported a median survival of 1.6 months in the high E/I ratio group. Second, malnutrition in patients with cancer was more related to sarcopenia and loss of muscle than to the loss of other body mass.34 Because muscle contains more water than adipocytes and other tissues, the E/I ratio can be influenced by sarcopenia in patients with malnutrition in this study.
This study has some limitations. First, because muscle contains more water than adipocytes, the E/I ratio can be influenced by the difference in muscle distribution. Second, this study was a small sample–sized retrospective study performed at a single center.
In conclusion, the E/I ratio may be used to predict survival in patients with metastatic cancer. A well-designed large-scale prospective study is required to support this result.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Traditional Korean Medicine R&D program funded by the Ministry of Health and Welfare through the Korea Health Industry Development Institute (KHIDI) (Grant No. HB16C0067).
ORCID iD: Jee Young Lee  https://orcid.org/0000-0002-1080-1915
https://orcid.org/0000-0002-1080-1915
References
- 1. National Cancer Center. Annual Report of Cancer Statistics in Korea in 2015. Sejong, Korea: Ministry of Health and Welfare; 2017. [Google Scholar]
- 2. Noone AM, Howlader N, Krapcho M, et al. SEER cancer statistics review, 1975-2015. https://seer.cancer.gov/csr/1975_2015/. Accessed April 29, 2018.
- 3. Lamont EB, Christakis NA. Physician factors in the timing of cancer patient referral to hospice palliative care. Cancer. 2002;94:2733-2737. [DOI] [PubMed] [Google Scholar]
- 4. Hui D, Didwaniya N, Vidal M, et al. Quality of end-of-life care in patients with hematologic malignancies: a retrospective cohort study. Cancer. 2014;120:1572-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med. 1988;148:1586-1591. [PubMed] [Google Scholar]
- 6. Morita T, Tsunoda J, Inoue S, Chihara S. The Palliative Prognostic Index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer. 1999;7:128-133. [DOI] [PubMed] [Google Scholar]
- 7. Kao J, Gold KD, Zarrili G, et al. Clinical predictors of survival for patients with stage IV cancer referred to radiation oncology. PLoS One. 2015;10:e0124329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gligoroska JP, Todorovska L, Mancevska S, Karagjozova I, Petrovska S. Bioelectrical impedance analysis in karate athletes: BIA parameters obtained with inbody720 regarding the age. Res Phys Educ Sport Health. 2016;5:117-121. [Google Scholar]
- 9. Taniguchi M, Yamada Y, Fukumoto Y, et al. Increase in echo intensity and extracellular-to-intracellular water ratio is independently associated with muscle weakness in elderly women. Eur J Appl Physiol. 2017;117:2001-2007. [DOI] [PubMed] [Google Scholar]
- 10. Covic A, Voroneanu L, Goldsmith D. Routine bioimpedance-derived volume assessment for all hypertensives: a new paradigm. Am J Nephrol. 2014;40:434-440. [DOI] [PubMed] [Google Scholar]
- 11. Ohashi Y, Joki N, Yamazaki K, et al. Changes in the fluid volume balance between intra- and extracellular water in a sample of Japanese adults aged 15-88 yr old: a cross-sectional study. Am J Physiol Renal Physiol. 2018;314:F614-F622. [DOI] [PubMed] [Google Scholar]
- 12. Chen W, Guo LJ, Wang T. Extracellular water/intracellular water is a strong predictor of patient survival in incident peritoneal dialysis patients. Blood Purif. 2007;25:260-266. [DOI] [PubMed] [Google Scholar]
- 13. Kim EJ, Choi MJ, Lee JH, et al. Extracellular fluid/intracellular fluid volume ratio as a novel risk indicator for all-cause mortality and cardiovascular disease in hemodialysis patients. PLoS One. 2017;12:e0170272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crawford GB, Robinson JA, Hunt RW, Piller NB, Esterman A. Estimating survival in patients with cancer receiving palliative care: is analysis of body composition using bioimpedance helpful? J Palliat Med. 2009;12:1009-1014. [DOI] [PubMed] [Google Scholar]
- 15. Keraliya AR, Rosenthal MH, Krajewski KM, et al. Imaging of fluid in cancer patients treated with systemic therapy: chemotherapy, molecular targeted therapy, and hematopoietic stem cell transplantation. AJR Am J Roentgenol. 2015;205:709-719. [DOI] [PubMed] [Google Scholar]
- 16. Verbelen H, Gebruers N, Beyers T, De Monie AC, Tjalma W. Breast edema in breast cancer patients following breast-conserving surgery and radiotherapy: a systematic review. Breast Cancer Res Treat. 2014;147:463-471. [DOI] [PubMed] [Google Scholar]
- 17. Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779-785. [DOI] [PubMed] [Google Scholar]
- 18. Lacuesta-Corro L, Llido LO. The results of the validation process of a Modified SGA (Subjective Global Assessment) Nutrition Assessment and Risk Level Tool designed by the Clinical Nutrition Service of St. Luke’s Medical Center, a tertiary care hospital in the Philippines. PhilSPEN Online J Parenteral Enteral Nutr. 2013;12:1-7. [Google Scholar]
- 19. Lee JS, Cho MR, Lee GJ. Validation of the developed nutritional screening tool for hospital patients. Korean J Nutr. 2010;43:189-196. [Google Scholar]
- 20. Törnudd M, Hahn RG, Zdolsek JH. Fluid distribution kinetics during cardiopulmonary bypass. Clinics (Sao Paulo). 2014;69:535-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sorensen JB, Andersen MK, Hansen HH. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in malignant disease. J Intern Med. 1995;238:97-110. [DOI] [PubMed] [Google Scholar]
- 22. Grohé C, Berardi R, Burst V. Hyponatraemia—SIADH in lung cancer diagnostic and treatment algorithms. Crit Rev Oncol Hematol. 2015;96:1-8. [DOI] [PubMed] [Google Scholar]
- 23. Psallidas I, Stathopoulos GT, Maniatis NA, et al. Secreted phosphoprotein-1 directly provokes vascular leakage to foster malignant pleural effusion. Oncogene. 2013;32:528-535. [DOI] [PubMed] [Google Scholar]
- 24. Light RW. Pleural effusions. Med Clin North Am. 2011;95:1055-1070. [DOI] [PubMed] [Google Scholar]
- 25. Limthongkul S, Charoenlap P, Nuchprayoon C, Songkhla YN. Relationships between pleural fluid pH, PCO2 to pleural fluid PO2, amylase, protein, glucose and white cells in tuberculous and malignant effusions. J Med Assoc Thai. 1990;73:429-432. [PubMed] [Google Scholar]
- 26. Demirci MS, Demirci C, Ozdogan O, et al. Relations between malnutrition, inflammation, atherosclerosis and volume status. The usefulness of bioimpedance analysis in peritoneal dialysis patients. Nephrol Dial Transplant. 2011;26:1708-1716. [DOI] [PubMed] [Google Scholar]
- 27. Cao DX, Wu GH, Zhang B, et al. Resting energy expenditure and body composition in patients with newly detected cancer. Clin Nutr. 2010;29:72-77. [DOI] [PubMed] [Google Scholar]
- 28. Niebauer J, Volk HD, Kemp M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838-1842. [DOI] [PubMed] [Google Scholar]
- 29. Tai R, Ohashi Y, Mizuiri S, Aikawa A, Sakai K. Association between ratio of measured extracellular volume to expected body fluid volume and renal outcomes in patients with chronic kidney disease: a retrospective single-center cohort study. BMC Nephrol. 2014;15:189. doi: 10.1186/1471-2369-15-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian JP, Wang H, Du FH, Wang T. The standard deviation of extracellular/intracellular water is associated with all-cause mortality and technique failure in peritoneal dialysis patients. Int Urol Nephrol. 2016;48:1547-1554. [DOI] [PubMed] [Google Scholar]
- 31. Te Riele RJLM, Dronkers EAC, Wieringa MH, et al. Influence of anemia and BMI on prognosis of laryngeal squamous cell carcinoma: development of an updated prognostic model. Oral Oncol. 2018;78:25-30. [DOI] [PubMed] [Google Scholar]
- 32. Nazoe T, Matono R, Ijichi H, et al. Glasgow Prognostic Score (GPS) can be a useful indicator to determine prognosis of patients with colorectal carcinoma. Int Surg. 2014;99:512-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nde PP, Paiva BS, Hui D, Paiva CE. Validation of the Modified Glasgow Prognostic Score in advanced cancer patients receiving palliative care. J Pain Symptom Manage. 2016;51:270-277. [DOI] [PubMed] [Google Scholar]
- 34. van der Kroft G, Bours DMJL, Janssen-Heijnen DM, van Berlo DCLH, Konsten DJLM. Value of sarcopenia assessed by computed tomography for the prediction of postoperative morbidity following oncological colorectal resection: a comparison with the malnutrition screening tool. Clin Nutr ESPEN. 2018;24:114-119. [DOI] [PubMed] [Google Scholar]