Abstract
Although decreased calcium absorption, decreased bone formation, alcohol drinking, and smoking have been considered as causes of osteopenia in men, the cause is unknown in half of the cases. Many reports highlighted the association between Helicobacter pylori infection and osteoporosis, mainly in East Asia and Japan. To identify relevant factors of osteoporosis in men, we examined estrogen and calcium intakes and other lifestyle factors together with gastric mucosal atrophy caused by Helicobacter pylori infection. This study is a cross-sectional study design of 268 healthy men who underwent general medical examinations. Multivariate analysis was performed, with age, body mass index, smoking habit, drinking habit, exercise habit, estradiol level, calcium intake, and Helicobacter pylori infection and its associated gastric mucosal atrophy as the independent variables and the presence of osteopenia as the dependent variable. The adjusted odds ratio was 0.74 (95% Confidence Interval [0.29, 1.90], p = .531) and 1.31 (95% Confidence Interval [0.54, 3.21], p = .552), when Helicobacter pylori infection was positive without and with gastric mucosal atrophy, respectively. Helicobacter pylori infection and gastric mucosal atrophy were not significant factors. Low body mass index, smoking habit, and low calcium intake were significantly associated with decreased bone density. In conclusion, Helicobacter pylori infection was not a significant risk, whereas low body mass index, current smoking, and lower calcium intake had a significant influence on the development of osteopenia in men.
Keywords: osteopenia, Helicobacter pylori infection, lifestyle factors, calcium intake, estradiol level
Osteoporosis is typically considered as a disease of women; however, its incidence is increasing in men (Amin et al., 2006; Khosla, 2010). In Japan, many women without symptoms are found to have reduced bone mass during medical examinations and the start of treatment. However, no such medical examinations are performed for men, who are often diagnosed as having osteoporosis only after a fracture (Orimo et al., 2012).
It is clear that the primary cause of osteoporosis in women is increased bone resorption and decreased bone density as a result of decreased estrogen secretion (Chinda et al., 2017; Khosla, Atkinson, Melton, & Riggs, 1997; Zebaze et al., 2010). Meanwhile, testosterone aromatizes to estradiol in men as well. In adults, testosterone continues to stimulate periosteal growth, but estrogen is important in the structural maintenance of cancellous bone mass. Therefore, decreased estradiol levels in middle-aged and older men are reported to be associated with the risk of fracture as serum testosterone and estradiol levels decrease with age in men (Amin et al., 2006; Vandenput & Ohlsson, 2009).
By contrast, osteoporosis and osteopenia are multifactorial diseases, and various lifestyle habits are involved in their development. The causes of osteoporosis in men include secondary factors such as decreased calcium absorption, decreased bone formation, alcohol drinking, and smoking, while the cause is unknown in half of the cases (De Laet et al., 2005; Kanis et al., 2007; Karaguzel & Holick, 2010; Kaushal, Vohora, Jalali, & Jha, 2018).
Helicobacter pylori is highly diverse genetically. H. pylori strains possessing the cytotoxin-associated gene A (cagA) are known to be the most virulent phenotype. Figura et al. (2005) reported that the prevalence of CagA-positive H. pylori infection was higher in men with osteoporosis than in healthy individuals in a case–control study involving 80 Italian men with and 160 men without osteoporosis (Figura et al., 2005). In this report, increased levels of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6 in the patients with CagA-positive H. pylori infection may have affected bone resorption, and increases in urinary cross-laps, a bone resorption marker, may have contributed to the onset of osteoporosis. Moreover, as the genotype of H. pylori in Japan is almost solely the cagA-positive strain (Shimoyama et al., 1997), H. pylori infection may even have a closer association with decreased bone density in Japan than in the West. Furthermore, many reports highlighted the association between H. pylori infection and osteoporosis, mainly in East Asia and Japan (Asaoka et al., 2015; Kim et al., 2014; Lin, Koo, & Tsai, 2014; Mizuno et al., 2015). The East Asian type of H. pylori infection is considered to cause decreased gastric acid secretion as a result of gastric mucosal atrophy, thereby inhibiting the liberation of ionized calcium levels and causing decreased absorption. However, to date, no studies have simultaneously examined H. pylori infection and other important factors of osteoporosis, including estradiol level and calcium intake, in men.
To determine the relevant factors for the development of osteopenia in men, the present study investigated the association of H. pylori infection and gastric mucosal atrophy with decreased bone density in men together with estrogen level, calcium intake, and several lifestyle factors.
Methods
Subjects
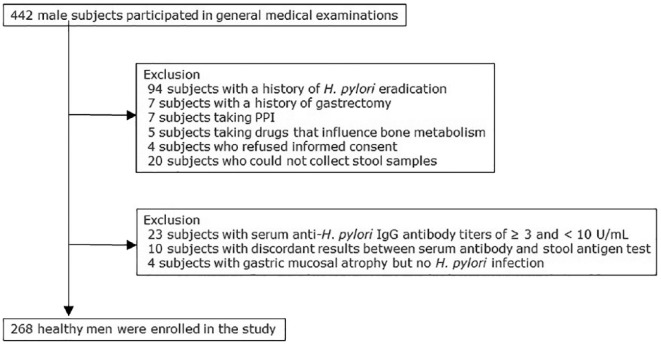
The present study had a cross-sectional study design and included 268 healthy men (age range, 19–90 years; mean age: 49.1 ± 15.1 years) who underwent general medical examinations in the Iwaki area in Hirosaki City, Aomori Prefecture, in 2014. Details of the study flow of the subjects are outlined in Figure 1. All the study subjects participated in response to a public announcement. The incidences of H. pylori infection and atrophic gastritis are particularly high in this region of Japan (Shimoyama et al., 2012). Patients with a history of H. pylori eradication, gastrectomy, proton pump inhibitor (PPI) use, receiving treatment for osteoporosis, or taking drugs that influence bone metabolism (e.g., steroids) were excluded from the study. We measured the bone density of the subjects and examined its association with age, body mass index (BMI), smoking habits, drinking habits, exercise habits, estradiol levels, daily calcium intake, and H. pylori infection and its associated gastric mucosal atrophy.
Figure 1.
Study flow of the subjects. A total of 268 subjects were enrolled from 442 healthy men who participated in general medical examinations.
The target sample size was 211 when the significance level was 5% and the statistical power was 80%. The final sample size of the study was 268, and the power of this analysis was calculated as 91.6%.
Measurement of Bone Density
The AOS-100NW Bone Densitometer, which uses quantitative ultrasonography (QUS) at the calcaneus, was used to measure bone density (Black et al., 1992). Osteo sono assessment index (OSI) scores were measured, and subjects with a T-score of ≤−1.0 SD (based on a comparison with the mean OSI score of young people [20–44 years]) were defined as having osteopenia in accordance with the World Health Organization diagnostic criteria (Karaguzel & Holick, 2010).
Serum and Stool Samples
H. pylori infection was diagnosed by testing for serum anti-H. pylori immunoglobulin G (IgG) antibodies and stool H. pylori antigens. The enzyme immunoassay (EIA) kit E-plate (Eiken Chemical Co., Ltd. Tokyo, Japan) was used to test for serum anti-H. pylori (IgG) antibodies with an antibody titer of ≥10 U/ml as positive and <3 U/ml as negative. Those whose test results were ≥3 and <10 U/ml were excluded, as they tended to be false negatives and infection was difficult to diagnose accurately (Chinda et al., 2018). Those who tested negative for H. pylori infection but had a suspected gastric mucosal atrophy were excluded, as they were likely to have a past infection. H. pylori antigen levels in stool samples were measured using Testmate Pylori Antigen EIA (Wakamoto Co., Ltd, Tokyo, Japan; Kyowa Medex Co., Ltd, Tokyo, Japan). The subjects whose antibodies and antigen test results matched were classified as infected or uninfected, and those for whom the results did not match were excluded from the study. Serum levels of pepsinogens (PGs) were measured simultaneously. Participants with PG I levels of <70 ng/ml and PG I/II levels of <3.0 ng/ml were excluded because of the suspicion of gastric mucosal atrophy and the likelihood of a past H. pylori infection, even if the subjects were negative for both serum anti-H. pylori IgG antibodies and stool H. pylori antigens (Chinda et al., 2018).
As for estrogen levels, estradiol is a metabolite of testosterone with the strongest effect and, thus, was measured in this study. The reference range for men is 20–60 pg/ml and <20 pg/ml was considered low.
Questionnaire and Interviews
Interviews concerning lifestyle habits were also conducted. Questionnaires were sent to all participants prior to the general medical examinations. The subjects brought the questionnaire themselves on the day of the examination and the answers were confirmed by more than a dozen investigators in one-to-one interviews. Smoking and drinking habits were categorized as present, past, or none. Exercise habits were defined as the presence of periodic exercise at least once per week over the course of a year.
The brief-type self-administered diet history questionnaire (BDHQ) was distributed to the subjects, and daily calcium intake was calculated on the basis of 1-month food menus. On the basis of the Dietary Reference Intakes for Japanese 2015 recommended by the Japanese Ministry of Health, Labour and Welfare (MHLW), low calcium intake was defined as levels below the recommended intake of 650 mg/day.
Statistical Analyses
SPSS version 24.0J (SPSS Inc., Chicago, IL, USA) was used for data management and analysis. A univariate analysis was used to compare age, BMI, smoking habit, drinking habit, exercise habit, estradiol level, daily calcium intake, and H. pylori infection and associated gastric mucosal atrophy between the two groups (osteopenia and non-osteopenia groups). A multivariate analysis was also performed, with age, BMI, smoking habit, drinking habit, exercise habit, estradiol level, calcium intake, and H. pylori infection and its associated gastric mucosal atrophy as the independent variables and the presence of osteopenia as the dependent variable. The adjusted odds ratio (OR) was calculated on the basis of the multiple logistic regression analysis.
Study Approval
The present study was a joint project conducted by the Iwaki District of Hirosaki City and Hirosaki University Graduate School of Medicine. The study was approved by the Hirosaki University institutional review board (approval No. 2014-377). All the subjects who participated in health examinations as part of the project received an explanation of the details of the examination and principal aims of the study. Written consent was obtained prior to study commencement.
Results
The results of the comparison and univariate analysis of each factor related to the presence/absence of osteopenia are shown in Table 1. Osteopenia was identified in 57 (21.3%) of the 268 subjects. Significant differences in age, BMI, smoking habit, and calcium intake were found in the univariate analysis when the groups with and without osteopenia were compared.
Table 1.
The Subject’s Background and Univariate Analysis for Each Factor Related to Presence of Osteopenia.
| Total |
Osteopenia (WHO
diagnostic criteria) |
p | |||||
|---|---|---|---|---|---|---|---|
| Negative |
Positive |
||||||
| Parameter | (n = 268) | (n = 211) | (%) | (n = 57) | (%) | ||
| Age (years old) | Mean ± SD | 49.1 ± 15.2 | 48.0 ± 14.9 | 53.3 ± 15.0 | .019 | ||
| (range) | (19–90) | (19–90) | (23–83) | ||||
| ≤50 years old | 152 | 127 | (83.6) | 25 | (16.4) | .027 | |
| >50 years old | 116 | 84 | (72.4) | 32 | (27.6) | ||
| BMI (kg/m2) | Mean ± SD | 23.7 ± 3.3 | 23.9 ± 3.3 | 23.0 ± 3.3 | .065 | ||
| (Range) | (17.2–36.3) | (17.2–36.3) | (17.6–33.0) | ||||
| <18.5 | 8 | 3 | (37.5) | 5 | (62.5) | <.001 | |
| 18.50–24.99 | 188 | 144 | (76.6) | 44 | (23.4) | ||
| ≥25.0 | 72 | 64 | (88.9) | 8 | (11.1) | ||
| Smoking | Nonsmoker | 95 | 81 | (85.3) | 14 | (14.7) | .047 |
| Current smoker | 84 | 59 | (70.2) | 25 | (29.8) | ||
| Past smoker | 89 | 71 | (79.8) | 18 | (20.2) | ||
| Alcohol | Nondrinker | 70 | 55 | (78.6) | 15 | (21.4) | .209 |
| Current drinker | 187 | 145 | (77.5) | 42 | (22.5) | ||
| Past drinker | 11 | 11 | (100.0) | 0 | (0.0) | ||
| Exercise habit | Irregular | 191 | 150 | (78.5) | 41 | (21.5) | .901 |
| Periodical | 77 | 61 | (79.2) | 16 | (20.8) | ||
| Estradiol level | Median | 20 | 20 | 19 | |||
| (Range) | (<10–71) | (<10–71) | (<10–45) | ||||
| Normal | 167 | 130 | (77.8) | 37 | (22.2) | .648 | |
| Low | 101 | 81 | (80.2) | 20 | (19.8) | ||
| Calcium intake | Mean ± SD | 502.7 ± 245.9 | 490.9 ± 228.6 | 546.3 ± 300.0 | .132 | ||
| (Range) | (91.4–1632.3) | (91.4–1632.3) | (139.7–1593.1) | ||||
| Normal | 54 | 48 | (88.9) | 6 | (11.1) | .041 | |
| Low | 214 | 163 | (76.2) | 51 | (23.8) | ||
| H. pylori infection | Negative | 185 | 148 | (80.0) | 37 | (20.0) | .282 |
| Positive | |||||||
| Mucosal atrophy– | 44 | 36 | (81.8) | 8 | (18.2) | ||
| Mucosal atrophy+ | 39 | 27 | (69.2) | 12 | (30.8) | ||
Note. BMI = body mass index; WHO = World Health Organization.
Table 2 shows the results of the multiple logistic regression analysis with each item as an adjustment item. With regard to H. pylori infection and gastric mucosal atrophy in relation to osteopenia, the adjustment ORs were 0.74 (95% CI [0.29, 1.90]; p = .531) and 1.31 (95% CI [0.54, 3.21], p = .552) when H. pylori infection was positive without and with atrophy, respectively. H. pylori infection and gastric mucosal atrophy were not significant factors. Estradiol level had an adjustment OR of 0.87 (95% CI [0.45, 1.67], p = .680) and was also not a significant factor. Among the lifestyle factors, higher BMI was negatively associated with osteopenia, but both current smoking and low calcium intake were positively associated with osteopenia.
Table 2.
Multiple Logistic Regression Analysis for Each Factor Related to Presence of Osteopenia.
| n | Adjusted odds
ratio |
p | ||
|---|---|---|---|---|
| (95% confidence interval) | ||||
| Age | ≤50 years old | 152 | 1 | |
| >50 years old | 116 | 1.85 [0.92, 3.72] | .086 | |
| BMI | <18.5 | 8 | 1 | |
| 18.50–24.99 | 188 | 0.15 [0.03, 0.73] | .019 | |
| ≥25.0 | 72 | 0.07 [0.01, 0.38] | .002 | |
| Smoking | Nonsmoker | 95 | 1 | |
| Current smoker | 84 | 2.34 [1.04, 5.28] | .041 | |
| Past smoker | 89 | 1.40 [0.61, 3.19] | .426 | |
| Alcohol | Nondrinker | 70 | 1 | |
| Current drinker | 187 | 0.85 [0.41, 1.77] | .851 | |
| Past drinker | 11 | – | - | |
| Exercise habit | Irregular | 191 | 1 | |
| Periodical | 77 | 1.26 [0.41, 1.77] | .530 | |
| Estradiol level | Normal | 167 | 1 | |
| Low | 101 | 0.87 [0.45, 1.67] | .680 | |
| Calcium intake | Normal | 54 | 1 | |
| Low | 214 | 2.92 [1.09, 7.81] | .034 | |
| H. pylori infection | Negative | 185 | 1 | |
| Positive (mucosal atrophy–) | 44 | 0.74 [0.29, 1.90] | .531 | |
| Positive (mucosal atrophy+) | 39 | 1.31 [0.54, 3.21] | .552 |
Note. BMI = body mass index.
Discussion
The results of the present study indicated that the factors associated with osteopenia in Japanese men were low BMI, smoking, and low calcium intake. Meanwhile, no significant association was found with H. pylori infection, gastric mucosal atrophy, or estradiol level.
In a study of osteoporosis in Japanese men, H. pylori significantly increased the risk of low trabecular bone density (TBD), and gastric mucosal atrophy significantly increased the risk of low TBD and low elastic modulus of the trabecular bone in 230 men in their 50s and 60s (Mizuno et al., 2015). The possible mechanisms and etiology of osteoporosis caused by H. pylori infection include bone resorption due to a systemic reaction in addition to a local reaction caused by inflammatory cytokines (IL-1, IL-6, IL-8, TNF-α, etc.) (Asaoka et al., 2015; Kim et al., 2014; Lin et al., 2014; Mizuno et al., 2015). Decreased acid secretion due to gastric mucosal atrophy associated with H. pylori infection is assumed to inhibit the liberation of ionized calcium and to lead to decreased absorption (Asaoka et al., 2015; Kim et al., 2014; Lin et al., 2014; Mizuno et al., 2015). Many reports have been published on the association between low-acid environments with the internal use of PPIs and osteoporosis as a result of a similar mechanism (Khalili et al., 2012; Ngam-ruengphong, Leontiadis, Radhi, Dentino, & Nugent, 2011). In other words, virulent H. pylori infection, such as that with East Asian–type CagA, spreads to the gastric body at an early stage, leads to widespread gastric mucosal atrophy as a result of severe long-term active gastritis, reduces the number of parietal cells distributed in the fundic gland region, and decreases gastric acid secretion in many cases (Haruma et al., 2000). In the present study, no significant association was found between gastric mucosal atrophy caused by H. pylori infection and decreased bone density. The reason for this could be that calcium absorption levels were relatively well maintained owing to the mild decrease in gastric acid secretion as compared with that with internal use of PPIs. In addition, severe low-acid environments were avoided even if the degree of gastric mucosal atrophy may have been severe. H. pylori infection was diagnosed using only serum anti-H. pylori antibodies in studies that reported an association between H. pylori infection and osteoporosis. In many cases, diagnosis using serum anti-H. pylori antibodies may include current and past infections even at levels below the cutoff value (Chinda et al., 2018; Yanaoka et al., 2008). It has come to be known that when setting the cutoff value at 10 U/ml for the E-plates in anti-H. pylori IgG antibody assay kits, which are widely used in Japan, nearly 20% of patients with antibody titers of ≥3 and <10 U/ml will include those who have past or current infection (Chinda et al., 2018). The results of the present study may have been different from those of previous reports because H. pylori infection was strictly defined by excluding patients with anti-H. pylori antibody titers of ≥3 and <10 U/ml and by evaluating atrophy with stool antigen tests and PG.
The results of the multivariate analysis showed that decreased bone density in men was associated with low BMI, smoking habit, and daily calcium intake. The association of low BMI with the increased risk of osteoporotic fractures has also been observed (De Laet et al., 2005; Lloyd et al., 2014). reported that regardless of age, sex, and race, for each 1-kg/m2 increase in BMI, the proximal femoral bone density increased by 0.0082 g/cm3 (Lloyd et al., 2014). Low BMI was also a significant risk factor for osteopenia in the present study. The importance of weighting and muscle strength in maintaining and increasing bone density was again demonstrated in this study.
Smoking is considered a risk factor for osteoporosis, as it induces antiestrogen action, suppression of calcium absorption through the intestinal tract, blood flow disorder, vascular disorder, and arteriosclerosis. Several meta-analysis studies have been conducted on the risk of fracture due to smoking (Kanis et al., 2005; Vestergaard & Mosekilde, 2003). However, in men the risk ratio (RR) of femoral proximal fracture was 1.82 for current smokers in reference to those who have never smoked and 1.11 for former smokers, which shows no significant difference and suggests the efficacy of smoking cessation (Kanis et al., 2005). The OR of current smokers was 2.34 in relation to those who have never smoked, while that of past smokers was 1.40, with no significant difference. These results also suggest increased bone density as a result of smoking cessation.
Low daily calcium intake was also a significant factor of osteopenia. Reports indicated that calcium intake is significantly associated with bone mass and bone density in men (Karaguzel & Holick, 2010; Welten, Kemper, Post, & van Staveren, 1995). The results of the 2017 National Health and Nutrition Survey report indicated that the average daily intake of calcium is 520 mg in men in Japan, which is lower than the reference amount of 650 mg. Only 10.5% of the subjects in the osteopenia group and 22.7% of the subjects in the non-osteopenia group exceeded the reference value in the present study. Absorption of calcium relies on active transport mediated by active vitamin D and concentration-dependent passive transport. It increases in proportion to calcium intake (DeLuca, 2004; Van Cromphaut et al., 2001). Rather than considering the absorption rate of calcium, including decreased acid secretion, increasing calcium intake itself may be more effective for preventing osteopenia in Japanese men, whose calcium intake is often below the reference value.
Estradiol level was not a significant factor of reduced bone density in men. Estrogen directly suppresses bone resorption by osteoclasts, but it also suppresses osteoclast differentiation by suppressing the expression of the receptor activator of nuclear factor kappa-Β ligand (Khosla et al., 1997; Zebaze et al., 2010). Serum estradiol levels are higher in elderly men than in postmenopausal women and show stronger associations with bone mineral density, bone turnover, and bone loss than testosterone levels (Amin et al., 2006; Khosla, 2010; Vandenput & Ohlsson, 2009). Evidence regarding estradiol level shows a threshold value at which male bone impairment occurs. Bone maturation is delayed in men below the threshold value as a result of increased speed of bone mass decrease and fracture rate (Vandenput & Ohlsson, 2009). In the present study, the receiver operating characteristic curve for estradiol for evaluating the optimal threshold value was examined, but the area under the curve was too small (0.573) to calculate the optimal threshold value (data not shown).
This study has several limitations. One limitation is that the present study was a cross-sectional study. Another limitation is that the association between the degree of atrophy in individuals with H. pylori infection and gastric pH was not elucidated. The relationship between the presence/absence of atrophy as detected using the PG test method and the degree of gastric pH is unclear. Although gastric pH is impossible to monitor in all subjects, good correlation has been shown between serum PG level and gastric acid secretion level (Iijima, Koike, Abe, & Shimosegawa, 2014). Furthermore, we excluded subjects with past H. pylori infection because serum PG levels are significantly modulated by the eradication of H. pylori infection. The third limitation is that bone density was measured using the calcaneal QUS, which is a more standardized and widely used tool than hip and spinal dual-energy x-ray absorptiometry (DXA). One of the reasons is that the general medical examinations in this study were performed at a public facility where DXA was not available, rather than at a medical institution. Another reason is the exposure to radiation.
In conclusion, low BMI, smoking habit, and daily calcium intake were associated with decreased bone density in the Japanese men in this study. Decreased bone density was not associated with H. pylori infection and H. pylori–associated gastric mucosal atrophy or estradiol level. The results of the present study are different from those of previous reports because H. pylori infection was defined more strictly and several original confounding factors were simultaneously evaluated.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was partly supported by JSPS KAKENHI Grant Number 17K09098 & 26460761. This study was based on the Iwaki Health Promotion Project as a project by Hirosaki University Graduate School of Medicine, in collaboration with Aomori Heath Evaluation and Promotion Center and Hirosaki City Office, Department of Health Promotion. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD: Daisuke Chinda  https://orcid.org/0000-0003-1690-5923
https://orcid.org/0000-0003-1690-5923
Tadashi Shimoyama  https://orcid.org/0000-0001-9615-0000
https://orcid.org/0000-0001-9615-0000
References
- Amin S., Zhang Y., Felson D. T., Sawin C. T., Hannan M. T., Wilson P. W. F., Kiel D. P. (2006). Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. The American Journal of Medicine, 119, 426–433. [DOI] [PubMed] [Google Scholar]
- Asaoka D., Nagahara A., Shimada Y., Matsumoto K., Ueyama H., Matsumoto K., … Watanabe S. (2015). Risk factors for osteoporosis in Japan: Is it associated with Helicobacter pylori? Therapeutics and Clinical Risk Management, 11, 381–391. doi: 10.2147/TCRM.S80647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. M., Cummings S. R., Genant H. K., Nevitt M. C., Palermo L., Browner W. (1992). Axial and appendicular bone density predict fractures in older women. The Journal of Bone and Mineral Research, 7, 633–638. [DOI] [PubMed] [Google Scholar]
- Chinda D., Shimoyama T., Iino C., Matsuzaka M., Nakaji S., Fukuda S. (2017). Decrease of estradiol and several lifestyle factors, but not Helicobacter pylori infection, are significant risks for osteopenia in Japanese females. Digestion, 96, 103–109. doi: 10.1159/000479317 [DOI] [PubMed] [Google Scholar]
- Chinda D., Shimoyama T., Mikami T., Arai T., Chiba D., Sasaki Y., … Fukuda S. (2018). Serum pepsinogen levels indicate the requirement of upper gastrointestinal endoscopy among group A subjects of ABC classification: A multicenter study. Journal of Gastroenterology, 53, 924–931. doi: 10.1007/s00535-018-1431-9 [DOI] [PubMed] [Google Scholar]
- De Laet C., Kanis J. A., Odén A., Johanson H., Johnell O., Delmas P., … Tenenhouse A. (2005). Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporosis International, 16, 1330–1338. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. (2004). Overview of general physiologic features and functions of vitamin D. The American Journal of Clinical Nutrition, 80, 1689S–1696S. doi: 10.1093/ajcn/80.6.1689S [DOI] [PubMed] [Google Scholar]
- Figura N., Gennari L., Merlotti D., Lenzi C., Campagna S., Franci B., … Nut A. (2005). Prevalence of Helicobacter pylori infection in male patients with osteoporosis and controls. Digestive Diseases and Sciences, 50, 847–852. [DOI] [PubMed] [Google Scholar]
- Haruma K., Kamada T., Kawaguchi H., Okamoto S., Yoshihara M., Sumii K., … Miyoshi A. (2000). Effect of age and Helicobacter pylori infection on gastric acid secretion. Journal of Gastroenterology and Hepatology, 15, 277–283. [DOI] [PubMed] [Google Scholar]
- Iijima K., Koike T., Abe Y., Shimosegawa T. (2014). Cutoff serum pepsinogen values for predicting gastric acid secretion status. The Tohoku Journal of Experimental Medicine, 232, 293–300. [DOI] [PubMed] [Google Scholar]
- Kanis J. A., Johnell O., Oden A., Johansson H., De Laet C., Eisman J. A., … Tenenhouse A. (2005). Smoking and fracture risk: A meta-analysis. Osteoporosis International, 16, 155–162. [DOI] [PubMed] [Google Scholar]
- Kanis J. A., Oden A., Johnell O., Johansson H., De Laet C., Brown J., … Yoshimura N. (2007). The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporosis International, 18, 1033–1046. [DOI] [PubMed] [Google Scholar]
- Karaguzel G., Holick M. F. (2010). Diagnosis and treatment of osteopenia. Reviews in Endocrine and Metabolic Disorders, 11, 237–251. doi: 10.1007/s11154-010-9154-0 [DOI] [PubMed] [Google Scholar]
- Kaushal N., Vohora D., Jalali R. K., Jha S. (2018). Prevalence of osteoporosis and osteopenia in an apparently healthy Indian population – a cross-sectional retrospective study. Osteoporosis and Sarcopenia, 4, 53–60. doi: 10.1016/j.afos.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili H., Huang E. S., Jacobson B. C., Camargo C. A., Feskanich D., Chan A. T. (2012). Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: A prospective cohort study. British Medical Journal, 344, e372. doi: 10.1136/bmj.e372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S. (2010). Update in male osteoporosis. The Journal of Clinical Endocrinology & Metabolism, 95, 3–10. doi: 10.1210/jc.2009-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S., Atkinson E. J., Melton L. J., Riggs B. L. (1997). Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: A population-based study. The Journal of Clinical Endocrinology & Metabolism, 82, 1522–1527. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Kim Y.-H., Han K., Nam G. E., Kim G. S., Han B.-D., … Ko B. J. (2014). Atrophic gastritis: A related factor for osteoporosis in elderly women. PLoS One, 9, e101852. doi: 10.1371/journal.pone.0101852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.-C., Koo M., Tsai K.-W. (2014). Association between Helicobacter pylori infection and risk of osteoporosis in elderly Taiwanese women with upper gastrointestinal diseases: A retrospective patient record review. Gastroenterology Research and Practice, 2014, 1–5. doi: 10.1155/2014/814756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. T., Alley D. E., Hawkes W. G., Hochberg M. C., Waldstein S. R., Orwig D. L. (2014). Body mass index is positively associated with bone mineral density in US older adults. Archives of Osteoporosis, 9, 175. doi: 10.1007/s11657-014-0175-2 [DOI] [PubMed] [Google Scholar]
- Mizuno S., Matsui D., Watanabe I., Ozaki E., Kuriyama N., Watanabe Y. (2015). Serologically determined gastric mucosal condition is a predictive factor for osteoporosis in Japanese men. Digestive Diseases and Sciences, 60, 2063–2069. doi: 10.1007/s10620-015-3576-1 [DOI] [PubMed] [Google Scholar]
- Ngamruengphong S., Leontiadis G. I., Radhi S., Dentino A., Nugent K. (2011). Proton pump inhibitors and risk of fracture: A systematic review and meta-analysis of observational studies. The American Journal of Gastroenterology, 106, 1209–1218. doi: 10.1038/ajg.2011.113 [DOI] [PubMed] [Google Scholar]
- Orimo H., Nakamura T., Hosoi T., Iki M., Uenishi K., Endo N., … Fujiwara S. (2012). Japanese 2011 guidelines for prevention and treatment of osteoporosis–executive summary. Archives of Osteoporosis, 7, 3–20. doi: 10.1007/s11657-012-0109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama T., Aoki M., Sasaki Y., Matsuzaka M., Nakaji S., Fukuda S. (2012). ABC screening for gastric cancer is not applicable in a Japanese population with high prevalence of atrophic gastritis. Gastric Cancer, 15, 331–334. doi: 10.1007/s10120-012-0141-x [DOI] [PubMed] [Google Scholar]
- Shimoyama T., Fukuda S., Tanaka M., Mikami T., Saito Y., Munakata A. (1997). High prevalence of the CagA-positive Helicobacter pylori strains in Japanese asymptomatic patients and gastric cancer patients. Scandinavian Journal of Gastroenterology, 32, 465–468. [DOI] [PubMed] [Google Scholar]
- Van Cromphaut S. J., Dewerchin M., Hoenderop J. G. J., Stockmans I., Van Herck E., Kato S., … Carmeliet G. (2001). Duodenal calcium absorption in vitamin D receptor-knockout mice: Functional and molecular aspects. Proceeding of the National Academy of Sciences of the United States of America, 98, 13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenput L., Ohlsson C. (2009). Estrogens as regulators of bone health in men. Nature Reviews Endocrinology, 5, 437–443. doi: 10.1038/nrendo.2009.112 [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Mosekilde L. (2003). Fracture risk associated with smoking: A meta-analysis. Journal of Internal Medicine, 254, 572–583. [DOI] [PubMed] [Google Scholar]
- Welten D. C., Kemper H. C. G., Post G. B., van Staveren W. A. (1995). A meta-analysis of the effect of calcium intake on bone mass in young and middle aged females and males. The Journal of Nutrition, 125, 2802–2813. [DOI] [PubMed] [Google Scholar]
- Yanaoka K., Oka M., Yoshimura N., Mukoubayashi C., Enomoto S., Iguchi M., … Ichinose M. (2008). Risk of gastric cancer in asymptomatic, middle-aged Japanese subjects based on serum pepsinogen and Helicobacter pylori antibody levels. International Journal of Cancer, 123, 917–926. doi: 10.1002/ijc.23571 [DOI] [PubMed] [Google Scholar]
- Zebaze R. M. D., Ghasem-Zadeh A., Bohte A., Iuliano-Burns S., Mirams M., Price R. I., … Seeman E. (2010). Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: A cross-sectional study. The Lancet, 375, 1729–1736. doi: 10.1016/S0140-6736(10)60320-0 [DOI] [PubMed] [Google Scholar]