Abstract
Background
Increased neural error-signals have been observed in obsessive-compulsive disorder (OCD), anxiety disorders, and inconsistently in depression. Reduced neural error-signals have been observed in substance use disorders (SUD). Thus, alterations in error-monitoring are proposed as a transdiagnostic endophenotype. To strengthen this notion, data from unaffected individuals with a family history for the respective disorders are needed.
Methods
The error-related negativity (ERN) as a neural indicator of error-monitoring was measured during a flanker task from 117 OCD patients, 50 unaffected first-degree relatives of OCD patients, and 130 healthy comparison participants. Family history information indicated, that 76 healthy controls were free of a family history for psychopathology, whereas the remaining had first-degree relatives with depression (n = 28), anxiety (n = 27), and/or SUD (n = 27).
Results
Increased ERN amplitudes were found in OCD patients and unaffected first-degree relatives of OCD patients. In addition, unaffected first-degree relatives of individuals with anxiety disorders were also characterized by increased ERN amplitudes, whereas relatives of individuals with SUD showed reduced amplitudes.
Conclusions
Alterations in neural error-signals in unaffected first-degree relatives with a family history of OCD, anxiety, or SUD support the utility of the ERN as a transdiagnostic endophenotype. Reduced neural error-signals may indicate vulnerability for under-controlled behavior and risk for substance use, whereas a harm- or error-avoidant response style and vulnerability for OCD and anxiety appears to be associated with increased ERN. This adds to findings suggesting a common neurobiological substrate across psychiatric disorders involving the anterior cingulate cortex and deficits in cognitive control.
Key words: Anxiety disorders, depression, endophenotype, error-related negativity, family study, obsessive-compulsive disorder, substance use disorder, transdiagnostic risk marker, unaffected first-degree relatives
Introduction
Obsessive-compulsive disorder (OCD) is characterized by intrusive obsessions and repetitive compulsions. The lifetime prevalence is estimated to be 1–3% (Ruscio et al., 2010). Despite its prevalence and its impact on life quality, the pathophysiology of OCD is not well understood. Further, despite accumulating evidence for heritability of OCD and associated traits (Pauls et al., 2014), reliable evidence for the involvement of specific genes has not emerged (Pauls et al., 2014; International Obsessive Compulsive Disorder Foundation Genetics and Studies, 2017). OCD is etiologically complex, with multiple genetic, epigenetic, and environmental factors as well as their interactions contributing to its development (Pauls et al., 2014). Phenotypic heterogeneity, overlap in symptoms, and high comorbidity between psychiatric disorders (Insel et al., 2010; Ruscio et al., 2010) further complicate research and it remains a major scientific challenge to unravel the etiology of complex disorders such as OCD. The Research Domain Criteria (RDoC) initiative and the endophenotype concept are hoped to help overcoming these problems (Gottesman and Gould, 2003; Insel et al., 2010; Miller and Rockstroh, 2013). RDoC takes a transdiagnostic perspective and conceptualizes psychopathology with a stronger grounding in neuroscience and in relation to dysfunctions in fundamental dimensions of behavior and neurobiology (Insel et al., 2010). Endophenotypes are heritable biological or psychological traits that represent simpler signs to etiological underpinnings and are assumed to be closer to genes and disease mechanisms (Gottesman and Gould, 2003; Insel et al., 2010; Miller and Rockstroh, 2013). Both concepts consistently emphasize a grounding in neuroscience, a focus on disease mechanisms, and transdiagnostic processes (Miller and Rockstroh, 2013).
Neural error-signals are a promising research target within both concepts, and alterations in error-processing are suggested to play a role in several mental disorders. The error-related negativity (ERN) is a well-validated and established electrophysiological marker of error-processing. The ERN is a negative deflection in the event-related brain potential peaking over fronto-central electrodes following an error and has been associated with activity in the midcingulate cortex predominantly the anterior cingulate cortex (ACC; Debener et al., 2005; Manoach and Agam, 2013; Grutzmann et al., 2016).†,1 The ERN has been repeatedly found to be increased in OCD patients (Endrass and Ullsperger, 2014; Riesel, 2019) and fulfills key criteria for an endophenotype (Gottesman and Gould, 2003; Miller and Rockstroh, 2013), including heritability (Anokhin et al., 2008), and a robust association with the disorder (Endrass and Ullsperger, 2014; Riesel, 2019). Furthermore, increased ERN amplitudes persist after symptom reduction over the course of cognitive behavioral therapy (CBT, Hajcak et al., 2008; Huyser et al., 2011; Riesel et al., 2015) and are also observed in unaffected first-degree relatives of OCD patients in the absence of symptoms (Riesel et al., 2011; Carrasco et al., 2013). Altogether, these results highlight that increased ERN amplitudes seem not to be the consequence or a correlate of OCD symptoms but to rather represent a promising endophenotype reflecting vulnerability for the disorder.
However, increased ERN amplitudes are not specific to OCD. Hyperactive neural error-signals in the brain have been linked to worry (Moser et al., 2013), repetitive behavior (Manoach and Agam, 2013), checking (Weinberg et al., 2015b), and anxiety proneness (Cavanagh and Shackman, 2014), symptoms that represent core features of OCD but are shared with other disorders. In the same vein, increased ERN amplitudes are not specific to OCD but have also been observed in generalized anxiety disorder (GAD), social anxiety, health anxiety, and less consistently in depression (Manoach and Agam, 2013; Endrass et al., 2014; Weinberg et al., 2015a; Gillan et al., 2017; Riesel et al., 2017). Moreover, increased amplitudes prospectively predict the development of anxiety symptoms (Lahat et al., 2014; Lamm et al., 2014; Meyer et al., 2015; Meyer et al., 2018). In contrast, reduced ERN amplitudes have been found in schizophrenia, bipolar disorder (Minzenberg et al., 2014), substance use disorder (SUD) and, inconsistently, in autism and attention-deficit/hyperactivity disorder (Manoach and Agam, 2013; Luijten et al., 2014; Gillan et al., 2017). In schizophrenia and SUD, results from a treatment study and high-risk populations indicate that error-processing deficits precede illness onset (Simmonite et al., 2012; Euser et al., 2013; Manoach and Agam, 2013; Gillan et al., 2017). Against this background, error-related brain activity qualifies as a promising transdiagnostic endophenotype with both reduction and enhancement in neural error-signals each indicating vulnerability for different types of psychopathology. To further strengthen this notion, studies examining unaffected first-degree relatives are of crucial importance given that family history is a major risk-factor for the development of psychopathology. The current study aims to replicate the finding that OCD patients and their unaffected first-degree relatives show increased ERN amplitudes. Moreover, we investigate whether alterations in neural error-signals reflect vulnerability across diagnoses by examining unaffected first-degree relatives of individuals with anxiety disorders, depression, and SUD. We hypothesize that individuals with familial risk for anxiety show increased ERN amplitudes, whereas those at risk for SUD show reduced amplitudes.
Methods
Participants
One hundred and seventeen patients with OCD, 50 unaffected first-degree relatives of patients with OCD (parents: n = 34, siblings: n = 13, offspring: n = 3), and 130 healthy comparison participants without a family history of OCD participated in the study (see Table 1). All participants were examined by trained clinical psychologists using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV (SCID, First et al., 1996) to assess past and present psychiatric disorders. For all participants family history information of psychopathology for their first-degree relatives was collected using the Family History Screen (Weissman et al., 2000), a structured interview that assesses all major DSM-IV diagnoses. Of the 130 healthy comparison participants, 76 were free of any family history for psychopathology (i.e. none of their first-degree relatives fulfilled criteria for a major DSM diagnosis), whereas the remaining reported a family history of depression (unaffected first-degree relatives of individuals with depression, n = 28), anxiety disorders (unaffected first-degree relatives of individuals with anxiety disorders, n = 27), and/or SUD (unaffected first-degree relatives of individuals with SUD, n = 27)2. All participants were between 18 and 65 years of age, had normal or corrected-to-normal vision, and reported no history of neurological disease or head trauma. Details about the subject flow and dropout reasons are presented in the supplementary information (online Supplementary Fig. 1).
Table 1.
Demographic and clinical characteristics and performance data of patients with OCD, unaffected first-degree relatives, and unaffected comparison subjects
| Patients with OCD | Unaffected first-degree relatives of OCD patients | Healthy comparison participants | Statistic | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | |||
| Demographical data | ||||||||
| N | 117 | 50 | 130 | |||||
| Mean age, years | 31.9 | 9.4 | 45 | 14.8 | 32.1 | 9.9 | F(2, 294) = 30.22 | 0.00 |
| Gender (% female) | 53 | 64 | 58 | χ2(2) = 1.79 | 0.41 | |||
| Verbal IQ | 103.2 | 10 | 106.5 | 9.6 | 104.8 | 9.4 | F(2, 294) = 2.17 | 0.12 |
| Family history | ||||||||
| Depressive disorders n in % | 45.3 | 54 | 21.5 | χ2(2) = 30.44 | <0.001 | |||
| SUD n in % | 19.7 | 14 | 20.8 | χ2(2) = 17.70 | <0.001 | |||
| Anxiety disorders n in % | 43.6 | 42 | 20.8 | χ2(2) = 17.70 | <0.001 | |||
| Clinical data | ||||||||
| Obsessive-compulsive inventory-revised | 27.32 | 12.17 | 5.78 | 6.12 | 3.85 | 3.87 | F(2,294) = 261.82 | <0.001 |
| Beck depression inventory-II | 18.81 | 10.70 | 3.46 | 4.38 | 2.53 | 3.32 | F(2,294) = 169.30 | <0.001 |
| State-trait-anxiety-inventory traita | 53.12 | 10.34 | 33.58 | 7.52 | 32.19 | 7.54 | F(2,288) = 191.21 | <0.001 |
| Harm avoidant motivation (OC-TCDQ)a | 9.92 | 3.52 | 2.82 | 2.18 | 2.24 | 1.89 | F(2,288) = 271.80 | <0.001 |
| Sense of incompleteness (OC-TCDQ)a | 15.20 | 5.40 | 5.08 | 3.79 | 4.39 | 3.27 | F(2,288) = 211.28 | <0.001 |
| Yale-brown obsessive-compulsive scaleb | 23.27 | 9.49 | ||||||
| Montgomery-Asberg depression rating scaleb | 13.23 | 9.24 | ||||||
| Age of onsetb | 23.27 | 9.49 | ||||||
| Performance datac | ||||||||
| Error rate in % | 4.41 | 3.42 | 3.44 | 2.97 | 4.33 | 3.26 | F(2287) = 0.04 | 0.97 |
| Reaction time for correct trials | 436 | 48 | 462 | 54 | 438 | 48 | F(2287) = 0.18 | 0.84 |
| Reaction time for errors | 371 | 76 | 385 | 66 | 373 | 56 | ||
| Robust post-error slowing | 40 | 45 | 52 | 60 | 39 | 47 | F(2287) = 0.28 | 0.76 |
s.d., standard deviation; OCD, obsessive-compulsive disorder; IQ, intelligence quotient; SUD, substance use disorders; OC-TCDQ, Obsessive-Compulsive Trait Core Dimensions Questionnaire.
State-Trait-Anxiety-Inventory and OC-TCDQ scores were missing for six OCD patients.
Yale-Brown Obsessive-Compulsive Scale, Montgomery-Asberg Depression Rating Scale and age of onset were only applicable in patients.
For performance data, age was included as a covariate to account for group differences. Further, performance data of six participants were missing due to technical problems (n = 2 OCD patients, n = 2 relatives, and n = 2 healthy comparison participants).
OCD patients were recruited via the outpatient clinic at Humboldt-Universität zu Berlin, Germany, and fulfilled criteria for OCD as verified with the SCID. Exclusion criteria for patients were: a current or lifetime diagnosis of psychotic, SUD, or bipolar disorders as well as neuroleptic medication in the past 4 weeks and/or benzodiazepines use in the past 2 weeks. Fifty OCD patients were currently taking psychotropic medication (serotonin reuptake inhibitors: n = 38, serotonin–norepinephrine reuptake inhibitors: n = 6, tricyclic antidepressants: n = 6). The majority of patients had one to three comorbid axis-I disorders: major depression (n = 27 remitted, n = 31 current episode), dysthymia (n = 10), panic disorder (n = 3), agoraphobia (n = 2), social phobia (n = 10), specific phobia (n = 10), GAD (n = 5), unspecific somatoform disorder (n = 6), pain disorder (n = 1), hypochondria (n = 1), body dysmorphic disorder (n = 1), anorexia nervosa (n = 1), binge eating disorder (n = 2), tic disorder (n = 6), excoriation disorder (n = 1).
First-degree relatives of patients with OCD were recruited via OCD patients that were diagnosed and treated at the outpatient clinic at Humboldt-Universität zu Berlin. OCD patients gave written informed consent for contacting them. For all first-degree relatives of OCD patients the diagnosis of their affected relative was verified in a face-to-face SCID. OCD relatives were only included if they themselves were free of past or present OCD. Additional exclusion criteria applied to unaffected first-degree relatives of OCD patients were: lifetime diagnosis of psychotic, bipolar, or SUD, and psychotropic medication in the past 4 weeks.
Healthy comparison participants were recruited via public advertisements and were matched for age, gender, and education level to OCD patients. Exclusion criteria for comparison participants were as follows: psychoactive medication in the past 3 months; any current or past axis-I disorder; and a family history of OCD. The Family History Screen (Weissman et al., 2000) indicated that 28 healthy participants had first-degree relatives with depression, 27 first-degree relatives with anxiety disorders, and/or 27 reported to have first-degree relatives with SUD. Thus, the effects of a family history for depression, SUD (alcohol and substance use), and anxiety disorders (GAD, specific phobia, social phobia, agoraphobia, panic disorder) were examined. The number of cases did not allow to examine familial risk for other disorder such as schizophrenia, or attention-deficit/hyperactivity disorder or to separately analyze different anxiety disorders with sufficient power2. Note that family history information for unaffected first-degree relatives of individuals with SUD, anxiety, or depression was not verified by a direct interview with the family members but was derived via the Family History Screen (Weissman et al., 2000).
All participants gave written informed consent after receiving written and verbal information about the study, and were monetarily compensated for their time. The study was conducted in accordance with the ethical guidelines of the revised Declaration of Helsinki, as confirmed by the ethics committee of Humboldt-Universität zu Berlin.
Measures
All participants completed the Beck-Depression-Inventory II (Beck et al., 1996), the Obsessive-Compulsive Inventory-Revised (Foa et al., 2002), and the State-Trait Anxiety Inventory (STAI, Spielberger et al., 1983) to assess self-reported severity of depressive, obsessive-compulsive, and anxiety symptoms, respectively. The Obsessive-Compulsive Trait Core Dimensions Questionnaire (Ecker and Gonner, 2008) was used to assess harm avoidance and sense of incompleteness in the participants. The Wortschatztest (Schmidt and Metzler, 1992) was applied to measure verbal intelligence. In addition, for patients only, severity of obsessive-compulsive and depressive symptoms was rated by trained clinicians using the Yale-Brown Obsessive-Compulsive Scale (Goodman et al., 1989) and the Montgomery-Asberg Depression Rating Scale (Montgomery and Asberg, 1979).
Task
An arrow-version of the flanker task was administered using Presentation software (Neurobehavioral Systems, Inc., Albany, California). On each trial, five vertically aligned arrows were presented and participants were instructed to indicate the direction of the central arrow. The stimuli were presented for 100 ms, followed by a 1000 ms response interval, followed by an inter-trial interval that varied randomly between 200 and 1200 ms. Half of the trials were congruent and half were incongruent. Eight blocks with 60 trials and at the beginning 20 practice trials were presented. After each block, participants were reminded to respond both quickly and accurately. The duration of the experiment was about 25 min.
Electroencephalographic recording and analyses
The electroencephalogram (EEG) was recorded from 61 Ag/AgCl-electrodes using an equidistant electrode montage (EASYCAP GmbH, Herrsching-Breitbrunn, Germany). External electrodes were placed below the eyes, below T1 (ground), and on the nasion. Channels were referenced to Cz during recording and impedances were below 5 kΩ. The EEG was sampled at a rate of 1000 Hz and a resolution of 0.1 µV. EEG data were processed offline using BrainVision Analyzer 2.1 (Brain Products GmbH, Munich, Germany). The following filters were applied: a 50 Hz notch filter, a 30 Hz low-pass filter, and a 0.01 Hz high-pass filter. To correct for eye movements and eye blinks, an ocular correction independent component analysis was conducted. Data were re-referenced to average reference and response-locked segments were computed from 400 ms before and 1000 ms after response execution (baseline-correction −100 to 0 ms). Artifacts were excluded based on the following criteria: amplitude changes exceeding 50 µV between consecutive data points, voltage differences of more than 200 µV within a 200 ms interval, and voltage changes of less than 0.50 µV within a 100 ms interval. Only participants with at least six artifact-free error trials were analyzed to ensure a reliable quantification of ERN (Olvet and Hajcak, 2009). ERN and CRN were quantified as the difference between the most negative peak occurring in a 150 ms post-response epoch and the immediately preceding positive peak at electrode FCz where error-related brain activity was maximal (Riesel et al., 2011). In addition, we also calculated ΔERN (ERN minus CRN) and quantified the ERN and CRN as the mean amplitude centered around the most negative peak occurring in a 150 ms epoch following the response at electrode FCz (peak ± 20 ms). Note that the pattern of results was replicated across the different ERN quantifications and related results are reported in online Supplementary information (SI 2). Results for the CRN are presented in the online Supplementary information (SI 1).
Statistical analyses
One-way analyses of covariance were used to examine differences in ERN, symptom severity, and error rate between OCD patients, OCD relatives and healthy participants. Repeated-measurement analyses of covariance were used to analyze response times including group (OCD patients, OCD relatives, and healthy comparison participants) as between-subjects and response type (correct, error) as within-subjects factors. Age was included as a covariate in these analyses to control for age differences between groups. Additional analyses for the OCD group included medication (with n = 50, without medication n = 66) and comorbidity (with n = 86, without comorbidity n = 31) as between-subjects factors. We used hierarchical regressions to examine whether family history explained variance in ERN in unaffected comparison participants. Results for the CRN and the whole sample are presented in the online Supplementary information (SI 1 and SI 3). In a first step, the effects of age, depressive, and obsessive-compulsive symptoms were controlled. In a second step, family history information for SUD, depression, and anxiety was entered. In a third step, interactions between familial risk statuses were included. Regression and correlational analyses were performed to determine the relation between ERN and symptoms (online Supplementary information SI 4). Heritability estimates for error-related brain activity were computed using Sequential Oligogenic Linkage Analysis Routines (SOLAR) and are presented in the online Supplementary information (SI 5). All statistical tests were two-tailed with α = 0.05. Post-hoc comparisons were corrected using the Bonferroni procedure. Statistical analyses were conducted with SPSS (Version 21.0, Chicago, USA).
Results
Demographic and behavioral data
Table 1 shows demographic, clinical, behavioral measures, and the statistics for the comparison between groups. Patients with OCD and healthy comparison participants did not differ in age (t245 = 0.14, p = 0.89). OCD relatives were significantly older compared to patients (t165 = 6.82, p < 0.001) and healthy comparison participants (t178 = 6.71, p < 0.001). Groups did not differ in gender and verbal intelligence quotient (IQ). OCD patients scored higher on symptom measures than OCD relatives (depressive symptoms: t165 = 9.78, p < 0.001; obsessive-compulsive symptoms: t165 = 11.87, p < 0.001; anxiety symptoms: t159 = 12.00, p < 0.001; harm avoidance: t159 = 13.16, p < 0.001; sense of incompleteness: t159 = 11.98, p < 0.001) and healthy comparison participants (depressive symptoms: t245 = 16.47, p < 0.001; obsessive-compulsive symptoms: t245 = 20.85, p < 0.001; anxiety symptoms: t239 = 18.12, p < 0.001; harm avoidance: t239 = 21.48, p < 0.001; sense of incompleteness: t239 = 19.10, p < 0.001). OCD relatives did not differ from healthy comparison participants in depressive (t178 = 1.52, p = 0.13), anxiety symptoms (t178 = 1.11, p = 0.27), harm avoidance (t178 = 1.74, p = 0.08) and sense of incompleteness (t178 = 1.21, p = 0.23). However, unaffected first-degree relatives reported slightly more obsessive-compulsive symptoms (t178 = 2.51, p = 0.01).
Groups did not differ in error rates. Correct responses were slower compared to errors (F1,287 = 28.31, p < 0.001, η2P = 0.09). A main effect of the covariate age (F1,287 = 52.47, p < 0.001, η2P = 0.16) reflected that increasing age was associated with slower responses (r = 0.49, p < 0.001). No main effect of group was observed after accounting for age differences. Robust post-error slowing (difference in reaction time between post-error and the associated pre-error trial) did not differ between groups.
Error-related negativity
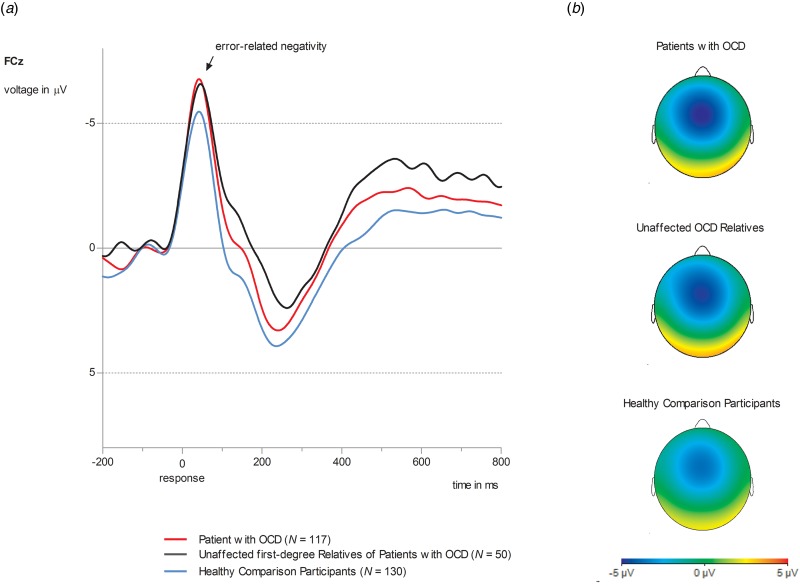
Figure 1 displays ERN for OCD patients, unaffected first-degree relatives of OCD patients and unaffected comparison participants. Results for the CRN are presented in the online Supplementary information (SI 1). A significant main effect of group (F2,293 = 4.39, p = 0.005, η2P = 0.04, d = 0.39) was observed. Both OCD patients and unaffected relatives of OCD patients showed enhanced ERN amplitudes compared to unaffected comparison participants [OCD patients v. healthy comparison participants: mean difference = 1.39, s.e. = 0.59, p = 0.05, 95% confidence interval (CI) (0.04–2.80); OCD relatives v. healthy comparison participants: mean difference = 2.49, s.e. = 0.83, p = 0.009, 95% CI (0.48–4.50)]. OCD patients and OCD relatives did not differ in ERN [mean difference = 0.19, s.e. = 0.81, p = 0.81, 95% CI (−1.40 to 1.79)]. A significant main effect of the covariate age (F1,293 = 7.85, p = 0.005, η2P = 0.03) was observed. Age and ERN showed a positive correlation (r = 0.12; p = 0.05) with increasing age being associated with more positive (i.e. smaller) amplitudes. For patients, neither a main effect for medication (F1,114 = 0.02, p = 0.89, η2P = 0.00), or comorbidity (F1,114 = 0.44, p = 0.51, η2P = 0.004) nor significant interactions involving these variables were observed (p values >0.33). When analyzing associations with symptoms across the whole sample, only harm avoidance showed a correlation with ERN (r = −0.13, p = 0.03). Higher harm avoidant motivation was associated with more negative, i.e. larger amplitudes. No other associations with symptoms or verbal IQ in the whole sample as well as symptom dimensions and clinical features in OCD patients were observed (see online Supplementary information SI 4).
Fig. 1.
(a) Grand average waveforms at electrode site FCz for ERN amplitude in OCD patients (red lines, N = 117), unaffected first-degree relatives of OCD patients (black lines, N = 50) and healthy comparison participants (blue lines, N = 130). (b) Topographies of error-related brain activity for OCD patients, OCD relatives and healthy comparison participants depicting the mean activity in the time window from 0 to 100 ms after response execution.
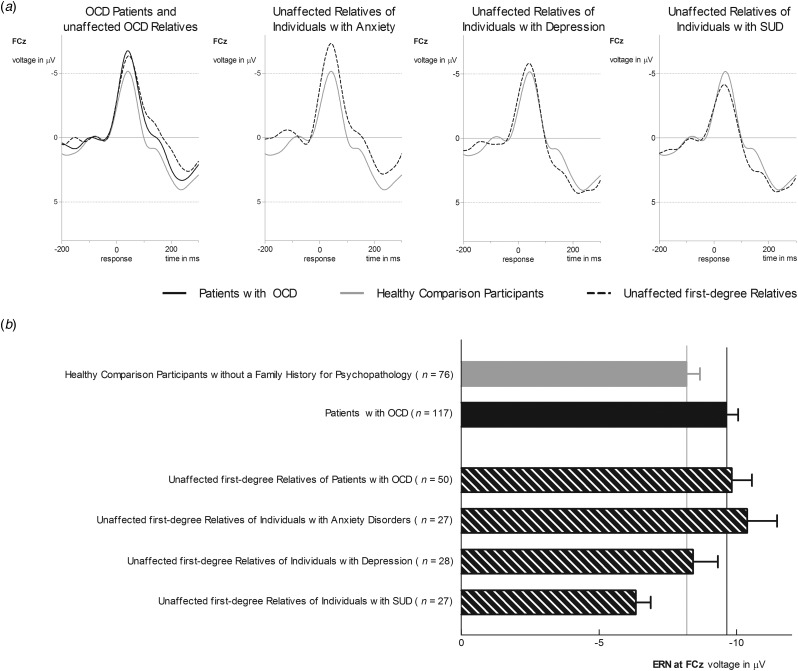
Using hierarchical regressions, we examined whether family history information explained variance in ERN in healthy comparison participants after adjusting for age, gender and symptom severity (Table 2, Fig. 2). Familial risk for SUD and anxiety (i.e. having a first-degree relative with this disorder) significantly predicted ERN magnitude in healthy participants. Unaffected first-degree relatives of individuals with SUD (i.e. healthy participants at familial risk for SUD) reported lower ERN amplitudes compared to those without familial risk for SUD [t128 = 2.49, p = 0.01, d = 0.54, mean difference = 2.40, s.e. = 0.96, 95% CI (0.49–4.30)]. Further, increased ERN amplitudes were observed in unaffected first-degree relatives of individuals with anxiety [i.e. healthy participants with a family history for anxiety; t128 = 2.83, p = 0.005, d = 0.61, mean difference = 2.70, s.e. = 0.96, 95% CI (0.81–4.59)]. Unaffected first-degree relatives of individuals with anxiety disorder (M = −10.37, s.d. = 5.66) did not differ in ERN from OCD patients (M = −9.62, s.d. = 4.58) and OCD relatives (M = −9.82, s.d. = 5.22; F2,190 = 0.72, p = 0.49, η2P = 0.008). Entering interactions between risk statuses does not significantly enhance the model fit (change in F = 0.75) and no significant interactions were observed.
Table 2.
Regression model for the ERN to examine effects of family history on error processing in unaffected comparison participants
| Variables | R2 | F | df1, df2 | p-value | B | s.e. | β | t-value | p value |
|---|---|---|---|---|---|---|---|---|---|
| DV: ERN | 0.03 | 0.70 | 5, 124 | 0.62 | |||||
| Age | 0.06 | 0.04 | −0.03 | −0.35 | 0.11 | ||||
| Gender | −0.29 | 0.83 | 0.14 | 1.60 | 0.73 | ||||
| Obsessive-compulsive inventory-R | −0.08 | 0.11 | −0.07 | −0.75 | 0.46 | ||||
| Beck depression inventory-II | −0.01 | 0.16 | 0.009 | −0.08 | 0.74 | ||||
| State-trait-anxiety-inventory trait | 0.06 | 0.07 | 0.10 | 0.77 | 0.44 | ||||
| DV: ERN | 0.13 | 2.29 | 8, 121 | 0.03 | |||||
| Age | 0.06 | 0.04 | 0.12 | 1.40 | 0.16 | ||||
| Gender | −0.27 | 0.80 | −0.03 | −0.34 | 0.74 | ||||
| Obsessive-compulsive inventory-R | −0.06 | 0.11 | −0.05 | −0.56 | 0.58 | ||||
| Beck depression inventory-II | −0.01 | 0.16 | −0.01 | −0.09 | 0.93 | ||||
| State-trait-anxiety-inventory trait | 0.05 | 0.07 | 0.07 | 0.65 | 0.52 | ||||
| Risk for SUD | 2.37 | 1.01 | 0.21 | 2.35 | 0.02 | ||||
| Risk for anxiety | −2.94 | 1.06 | −0.26 | −2.77 | 0.007 | ||||
| Risk for depression | 0.57 | 1.10 | 0.05 | 0.52 | 0.61 | ||||
| DV: ERN | 0.13 | 1.86 | 11, 118 | 0.05 | |||||
| Age | 0.06 | 0.04 | 0.13 | 1.48 | 0.14 | ||||
| Gender | −0.27 | 0.81 | −0.03 | −0.33 | 0.75 | ||||
| Obsessive-compulsive inventory-R | −0.07 | 0.11 | −0.06 | −0.66 | 0.51 | ||||
| Beck depression inventory-II | −0.05 | 0.17 | −0.04 | −0.29 | 0.78 | ||||
| State-trait-anxiety-inventory trait | 0.06 | 0.07 | 0.10 | 0.80 | 0.43 | ||||
| Risk for SUD | 2.77 | 1.32 | 0.25 | 2.11 | 0.04 | ||||
| Risk for anxiety | −3.09 | 1.38 | −0.33 | −2.67 | 0.009 | ||||
| Risk for depression | 1.41 | 1.70 | 0.13 | 0.83 | 0.41 | ||||
| Interaction risk for SUD × risk for anxiety | 2.97 | 2.78 | 0.15 | 1.07 | 0.29 | ||||
| Interaction risk for SUD × risk for depression | −3.09 | 2.50 | −0.20 | −1.23 | 0.22 | ||||
| Interaction risk for anxiety × risk for depression | 0.29 | 2.43 | 0.02 | 0.12 | 0.91 |
DV, dependent variable; SUD, substance use disorders.
Significant values are printed in bold.
Fig. 2.
(a) Grand average waveforms at electrode site FCz for ERN amplitude in healthy comparison participants without a family history for a major psychiatric disorder (gray line) compared to unaffected first-degree relatives of patients with OCD and OCD patients (first column), unaffected first-degree relatives of individuals with anxiety disorders (second column), unaffected first-degree relatives of individuals with depression (third column), and unaffected first-degree relatives of individuals with SUD (last column). (b) Bar chart depicting ERN amplitudes for healthy comparison participants, OCD patients, and unaffected first-degree relatives. Values are means, with error bars depicting standard errors.
Discussion
Neural error-signals were examined as an endophenotype for OCD by comparing it between patients with OCD, unaffected first-degree relatives of OCD patients and healthy participants without a family history for OCD. Furthermore, we evaluated whether neural error-signals may represent a transdiagnostic endophenotype by analyzing unaffected first-degree relatives of individuals with SUD, depression, and anxiety disorders. Three main findings were observed: first, both patients with OCD and unaffected first-degree relatives of OCD patients showed increased ERN amplitudes compared to healthy comparison participants. Second, the increase in ERN magnitude was not specific to OCD as unaffected first-degree relatives of individuals with anxiety disorders were also characterized by increased ERN amplitudes compared to healthy comparison participants. Third, unaffected first-degree relatives of individuals with SUD showed reduced ERN amplitudes compared to healthy comparison participants, OCD patients, and relatives of individuals with OCD or anxiety. Overall, these results support the notion that neural error-signals represent a potential endophenotype not only for OCD but across different psychiatric disorders.
A useful endophenotype is a robust, state-independent quantitative deviation that is found in patients and unaffected family members. It is heritable, easy and reliable to assess and informative about the pathophysiology of the disorder (Gottesman and Gould, 2003; Miller and Rockstroh, 2013). Enhanced neural error-signals in OCD fulfill most of these criteria. The ERN has been shown to have good psychometric properties including validity and reliability (Olvet and Hajcak, 2009; Weinberg and Hajcak, 2011; Riesel et al., 2013). Increased neural error-signals are robustly associated with OCD (Endrass and Ullsperger, 2014; Gillan et al., 2017) and can also be found in unaffected first-degree relatives of OCD patients (Riesel et al., 2011; Carrasco et al., 2013). Further, elevated ERN amplitudes in OCD persist after symptom reduction due to CBT (Hajcak et al., 2008; Huyser et al., 2011; Riesel et al., 2015). A twin study suggested a heritability of about 50% for ERN (Anokhin et al., 2008) and we derived comparable heritability estimates ranging between 22% and 48% (online Supplementary information SI 5). Altogether, these results highlight that increased neural error-signals represent a promising endophenotype for OCD.
The current study indicates that increased ERN amplitudes are also found in healthy participants at familial risk for anxiety (i.e. unaffected first-degree relatives of individuals with anxiety disorders). This is consistent with studies reporting increased ERN amplitudes for several anxiety disorders including GAD, social anxiety, and health anxiety (Manoach and Agam, 2013; Gillan et al., 2017; Riesel et al., 2017). These disorders are frequently comorbid and share symptoms that have been linked to elevated neural error signals such as repetitive behavior (Manoach and Agam, 2013), worry (Moser et al., 2013), and anxiety-proneness (Cavanagh and Shackman, 2014) pointing to shared pathophysiological mechanisms. The observed increase in ERN in unaffected relatives with a family history for anxiety disorders or OCD compared to healthy controls provides important evidence for the assumption that increased neural error-signals represent a transdiagnostic endophenotype indicating a shared vulnerability for OCD and anxiety. This is further supported by results indicating the state-independence of increased ERN amplitudes not only in OCD (Hajcak et al., 2008; Huyser et al., 2011; Riesel et al., 2015) but also in social anxiety disorder (Kujawa et al., 2016). Finally, increased ERN amplitudes prospectively predict the development of anxiety symptoms (Lahat et al., 2014; Lamm et al., 2014; Meyer et al., 2015; Meyer et al., 2018) highlighting the predictive validity of neural error signals.
The current study did not find support for altered ERN amplitudes in unaffected first-degree relatives of individuals with depression (i.e. healthy individuals at familial risk for depression). Results regarding increased ERN amplitudes in depression are inconsistent (Gillan et al., 2017), pointing toward increases (e.g. Chiu and Deldin, 2007), as well as decreases (e.g. Weinberg et al., 2016), or no differences (e.g. Schoenberg, 2014). Overall, neural error-signals in depression seem to be more affected by symptom state and severity (Schrijvers et al., 2009), as well as subtype (Weinberg et al., 2016). An increase was reported for mild to moderate depression, but not in severely depressed patients with melancholic or anhedonic features that even showed a blunted response to errors (Schrijvers et al., 2009; Weinberg et al., 2016). Further, alterations in error-monitoring disappear after symptom reduction (Schrijvers et al., 2009). Together with our results showing normal error-monitoring in individuals at familial risk for depression this suggests that ERN alterations seem not to be an endophenotypic trait marker of depression. Observed alterations may rather reflect a state marker or a result from overlap/comorbidity with relevant transdiagnostic phenotypes. This supports some specificity and suggests that increased neural error-signals may distinguish vulnerability for OCD and anxiety from depression.
Finally, unaffected first-degree relatives of individuals with SUD showed reduced error-related brain activity compared to healthy participants without a family history for SUD. This supports that a blunted neural response to errors may not only be found in patients with SUD (Luijten et al., 2014; Gillan et al., 2017) but can also be seen in individuals at increased risk for SUD. This confirms a previous study in adolescents at high-risk for SUD that were characterized by diminished neural error-signals (Euser et al., 2013). Further, the suitability of reduced error-related brain activity as an endophenotype for SUD is supported by longitudinal studies showing that it predicts relapse (Luo et al., 2013; Marhe et al., 2013) and the initiation of tobacco use (Anokhin and Golosheykin, 2015). Besides SUD, several studies suggest that reduced ERN amplitudes are also found in schizophrenia (Martin et al., 2018), bipolar disorder (Minzenberg et al., 2014), and inconsistently in autism and attention-deficit/hyperactivity disorder (Manoach and Agam, 2013; Luijten et al., 2014; Gillan et al., 2017). This suggests that a blunted neural response to errors is common to different disorders and may reflect a shared pathophysiological mechanism.
In summary, the present results support the utility of neural error-signals as a dimensional, transdiagnostic endophenotype with an enhancement in ERN reflecting vulnerability for OCD and anxiety, and a reduction indicating risk for SUD. OCD and anxiety disorders are frequently comorbid (Ruscio et al., 2010) and share prominent clinical features such as heightened harm avoidance (Kampman et al., 2014), worry (Yook et al., 2010; Dar et al., 2015), increased intolerance of uncertainty (Tolin et al., 2003; Holaway et al., 2006), as well as reassurance seeking and checking behavior (Kobori and Salkovskis, 2013; Weinberg et al., 2015b; Halldorsson and Salkovskis, 2017). These symptoms have also been related to hyperactive neural error signals that are observed across OCD and anxiety disorders (e.g. Moser et al., 2013; Cavanagh and Shackman, 2014; Weinberg et al., 2015b). Collectively, similarities in symptoms, neural substrate, and comorbidity point to a partly shared etiology involving alterations in error-monitoring. More specifically, differences in neural error signals that are generated by the ACC (Debener et al., 2005; Manoach and Agam, 2013; Grutzmann et al., 2016), are assumed to trigger adaptations in cognition, affect, and control implementation to avoid future negative outcomes (Shackman et al., 2011; Cavanagh and Shackman, 2014). In this regard, hyperactive neural error-signals (i.e. increased sensitivity to errors) in OCD and anxiety may reflect over-controlled responses and an error- and harm-avoidant response style. Moreover, elevated neural error-signals are not only found in patients with these disorders but seem to indicate vulnerability for OCD and anxiety that persists independent of symptom status and are present in individuals at risk. On the other hand, reduced neural error-signals may indicate under-controlled behavior (i.e. reduced responsiveness to errors/negative consequences and deficits in adaptive control) and seem to be related to risk for substance use. Overall, our results add to numerous findings pointing to common etiological factors shared between different psychiatric disorders which encompass structural abnormalities in ACC and disruptions in cognitive control (Goodkind et al., 2015; McTeague et al., 2017). Error-monitoring dysfunctions fit nicely into this picture since they have been linked to cognitive control (Cavanagh and Shackman, 2014) and are assumed to depend on activity in the ACC (Debener et al., 2005; Manoach and Agam, 2013). The ACC is implicated in the processing and integration of negative affect, cognitive conflict, and pain, as well as the implementation of adaptive control (Shackman et al., 2011) and its function seems to be critically involved in mental health.
Some limitations have to be noted. Groups differ in age, however, results are corrected for age differences. Some patients were medicated and some had current comorbid disorders. But in line with previous reports, our findings were not affected by medication or comorbidity in patients (Stern et al., 2010; Riesel et al., 2015). Family history methods have high specificity (above 0.9), but rather low sensitivity and false negative diagnoses are common (Rougemont-Buecking et al., 2008; Vandeleur et al., 2008; Vandeleur et al., 2015). This may have led to an underestimation of family history for depression, SUD, and anxiety. Further, in the current study diagnostic confidence differs between unaffected first-degree relatives of OCD patients and unaffected first-degree relatives of individuals with disorders other than OCD. All unaffected first-degree relatives of patients with OCD were recruited via patients of the outpatient clinic for OCD at Humboldt-Universität zu Berlin, where they were carefully diagnosed using the SCID. Thus, the OCD diagnosis of the affected first-degree relative was verified by a direct SCID. Family history information for disorders other than OCD was assessed with a family history interview (Weissman et al., 2000). Due to restrictions by data protection regulations, diagnostic information was not verified with a direct interview of the affected family member. Further, prevalence rates were too small to separately analyze the effects of specific anxiety disorders. Finally, the endophenotype concept is not without criticism (Kendler and Neale, 2010; Miller and Rockstroh, 2013; Iacono et al., 2017). The success of the endophenotype strategy to identify predisposing genes has been demonstrated in non-psychiatric disorders, such as cardiac syndromes (Keating et al., 1991; Vincent et al., 1992; Keating and Sanguinetti, 2001), but remains limited for psychiatric disorders (Iacono et al., 2017). The genetic basis of endophenotypes is complex (Iacono et al., 2017). For the ERN results regarding its genetic foundation are inconsistent, rely on rather small samples, and replications are needed (Manoach and Agam, 2013). Furthermore, as Kendler and Neale (2010) have noted, endophenotypes can either causally mediate between genes and the clinical phenotype, or be risk-indicators that share genes with the phenotype. Nevertheless, even complex endophenotypes are presumably simpler than the complex disorder. Besides pointing out genetic influences, endophenotypes can help to illuminate disorder mechanisms and familial environmental risk factors (Kendler and Neale, 2010) and potentially provide targets for interventions. Further, endophenotypes and especially profiles of different potential endophenotypes have the power to differentiate disorders that overlap symptomatically and in pathophysiological mechanisms, as has been shown for psychotic disorders including schizophrenia, bipolar, and schizoaffective disorder (e.g. Benes, 2007; Braff and Tamminga, 2017; Ivleva et al., 2017). Thus, they may inform new ways of categorizing disorders (Insel et al., 2010) and can foster individualized and precision medicine (Insel and Cuthbert, 2015). Finally, the clinical relevance of neural error-signals as a risk indicator or endophenotype is further supported by studies demonstrating the predictive validity for the onset of disorders or symptoms (Lahat et al., 2014; Lamm et al., 2014; Anokhin and Golosheykin, 2015; Meyer et al., 2015, 2018; Kessel et al., 2016), as well as treatment outcome (Luo et al., 2013; Marhe et al., 2013) across different mental disorders.
The current study is the first to examine error-monitoring in unaffected individuals with familial risk for several disorders simultaneously. A diagnosis of OCD, familial risk for OCD or anxiety disorders were associated with increased error-related brain activity compared to healthy control participants without a family history for psychopathology. In contrast, familial risk for SUD was associated with a reduced neural response to errors compared to the healthy control group as well as patients with OCD and relatives with a family history of OCD or anxiety. The results provide important evidence for variations in neural error-signals being a promising transdiagnostic endophenotype reflecting vulnerability for the development of OCD, anxiety, or SUD. Further, they add to results suggesting that structural ACC alterations and deficits in cognitive control represent a shared neurobiological substrate across mental disorders.
Acknowledgements
This work was funded by the German Research Foundation, with grants awarded to Norbert Kathmann (Grant KA815/6-1) and Michael Wagner (Grant WA731/10-1). The funding source had no further influence on study conduction and publication. The authors thank Thomas Pinkpank and Rainer Kniesche for technical support. We thank Ulrike Bunzenthal, Sarah Dreßel, Alexandra Günther, Marvin Groh, Anna Unger-Nübel, and Verena Wüllhorst for their help in data acquisition. We thank Dr Eva Kischkel and Dr Benedikt Reuter for their assistance in patient recruitment. Further, we are grateful for the help of Prof. Sebastian Market in performing the heritability analyses.
Conflict of interest
All authors assure to have no competing financial interests and conflict of interest regarding the presented work.
The notes appear after the main text.
Notes
A smaller negative component, the correct-related negativity (CRN, Ford, 1999), can be seen following correct responses. Similarities in topography, timing, and source suggest that both components reflect similar and overlapping neuronal and cognitive processes (Vidal et al., 2000; Hoffmann & Falkenstein, 2010; Klawohn et al., 2014). Overall, anxiety and OCD have more closely and consistently been related to hyperactive error-monitoring. The primary focus of the present paper is on the ERN and related CRN results are presented in the online Supplementary information (SI 1).
Number of healthy control participants with a family history for other disorder: schizophrenia (n = 1), bipolar disorder (n = 3), attention-deficit/hyperactivity disorder (n = 7), anorexia nervosa (n = 3), tic disorder (n = 1), excoriation disorder (n = 2), and body dysmorphic disorder (n = 3).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291719000199.
click here to view supplementary material
References
- Anokhin AP and Golosheykin S (2015) Neural correlates of error monitoring in adolescents prospectively predict initiation of tobacco use. Developmental Cognitive Neuroscience 16, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin A, Golosheykin S and Heath A (2008) Heritability of frontal brain function related to action monitoring. Psychophysiology 45, 524–534. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R and Brown G (1996) Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Benes FM (2007) Searching for unique endophenotypes for schizophrenia and bipolar disorder within neural circuits and their molecular regulatory mechanisms. Schizophrenia Bulletin 33, 932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL and Tamminga CA (2017) Endophenotypes, epigenetics, polygenicity and more: Irv Gottesman's dynamic legacy. Schizophrenia Bulletin 43, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Harbin S, Nienhuis J, Fitzgerald K, Gehring W and Hanna G (2013) Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depression and Anxiety 30, 39–46. [DOI] [PubMed] [Google Scholar]
- Cavanagh J and Shackman A (2014) Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. Journal of Physiology-Paris 109, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P and Deldin P (2007) Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry 164, 608. [DOI] [PubMed] [Google Scholar]
- Dar KA and Iqbal N, (2015) Worry and rumination in generalized anxiety disorder and obsessive compulsive disorder. The Journal of Psychology 149, 866–880. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY and Engel AK (2005) Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience 25, 11730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker W and Gonner S (2008) Incompleteness and harm avoidance in OCD symptom dimensions. Behaviour Research and Therapy 46, 895–904. [DOI] [PubMed] [Google Scholar]
- Endrass T and Ullsperger M (2014) Specificity of performance monitoring changes in obsessive-compulsive disorder. Neuroscience & Biobehavioral Reviews 46, 124–138. [DOI] [PubMed] [Google Scholar]
- Endrass T, Riesel A, Kathmann N and Buhlmann U (2014) Performance monitoring in obsessive-compulsive disorder and social anxiety disorder. Journal of Abnormal Psychology 123, 705–714. [DOI] [PubMed] [Google Scholar]
- Euser AS, Evans BE, Greaves-Lord K, Huizink AC and Franken IHA (2013) Diminished error-related brain activity as a promising endophenotype for substance-use disorders: evidence from high-risk offspring. Addiction Biology 18, 970–984. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M and Williams JB (1996) Structured Clinical Interview for DSM-IV Axis I Disorders, SCID, Clinician Version: User's Guide. Washington, DC: Psychiatric Press. [Google Scholar]
- Foa E, Huppert J, Leiberg S, Langner R, Kichic R, Hajcak G and Salkovskis P (2002) The obsessive-compulsive inventory: development and validation of a short version. Psychological Assessment 14, 485–496. [PubMed] [Google Scholar]
- Ford JM (1999) Schizophrenia: the broken P300 and beyond. Psychophysiology 36, 667–682. [PubMed] [Google Scholar]
- Gillan CM, Fineberg NA and Robbins TW (2017) A trans-diagnostic perspective on obsessive-compulsive disorder. Psychological Medicine 47, 1528–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT and Etkin A (2015) Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR and Charney DS (1989) The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Archives of General Psychiatry 46, 1006–1011. [DOI] [PubMed] [Google Scholar]
- Gottesman II and Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry 160, 636. [DOI] [PubMed] [Google Scholar]
- Grutzmann R, Endrass T, Kaufmann C, Allen E, Eichele T and Kathmann N (2016) Presupplementary motor area contributes to altered error monitoring in obsessive-compulsive disorder. Biological Psychiatry 80, 562–571. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin M, Foa E and Simons R (2008) Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. American Journal of Psychiatry 165, 116. [DOI] [PubMed] [Google Scholar]
- Halldorsson B and Salkovskis PM (2017) Why do people with OCD and health anxiety seek reassurance excessively? An investigation of differences and similarities in function. Cognitive Therapy and Research 41, 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S and Falkenstein M (2010) Independent component analysis of erroneous and correct responses suggests online response control. Human Brain Mapping 31, 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaway RM, Heimberg RG and Coles ME (2006) A comparison of intolerance of uncertainty in analogue obsessive-compulsive disorder and generalized anxiety disorder. Journal of Anxiety Disorders 20, 158–174. [DOI] [PubMed] [Google Scholar]
- Huyser C, Veltman DJ, Wolters LH, de Haan E and Boer F (2011) Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive-compulsive disorder: a fMRI study with a flanker task before and after CBT. Journal of Child Psychology and Psychiatry 52, 1251–1260. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM and Vrieze SI (2017) Endophenotype best practices. International Journal of Psychophysiology 111, 115–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR and Cuthbert BN (2015) Medicine. Brain disorders? Precisely. Science 348, 499–500. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine D, Quinn K, Sanislow C and Wang P (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry 167, 748–751. [DOI] [PubMed] [Google Scholar]
- International Obsessive Compulsive Disorder Foundation Genetics, C. & Studies, O. C. D. C. G. A (2017) Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Molecular Psychiatry 23, 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Clementz BA, Dutcher AM, Arnold SJM, Jeon-Slaughter H, Aslan S, Witte B, Poudyal G, Lu H, Meda SA, Pearlson GD, Sweeney JA, Keshavan MS and Tamminga CA (2017) Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biological Psychiatry 82, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman O, Viikki M, Jarventausta K and Leinonen E (2014) Meta-analysis of anxiety disorders and temperament. Neuropsychobiology 69, 175–186. [DOI] [PubMed] [Google Scholar]
- Keating M and Sanguinetti MC (2001) Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104, 569–580. [DOI] [PubMed] [Google Scholar]
- Keating M, Atkinson D, Dunn C, Timothy K, Vincent GM and Leppert M (1991) Linkage of a cardiac-arrhythmia, the long Qt syndrome, and the Harvey Ras-1 gene. Science 252, 704–706. [DOI] [PubMed] [Google Scholar]
- Kendler KS and Neale MC (2010) Endophenotype: a conceptual analysis. Molecular Psychiatry 15, 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel EM, Meyer A, Hajcak G, Dougherty LR, Torpey-Newman DC, Carlson GA and Klein DN (2016) Transdiagnostic factors and pathways to multifinality: the error-related negativity predicts whether preschool irritability is associated with internalizing versus externalizing symptoms at age 9. Development and Psychopathology 28, 913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawohn J, Riesel A, Grutzmann R, Kathmann N and Endrass T (2014) Performance monitoring in obsessive-compulsive disorder: a temporo-spatial principal component analysis. Cognitive, Affective, & Behavioral Neuroscience 14, 983–995. [DOI] [PubMed] [Google Scholar]
- Kobori O and Salkovskis PM (2013) Patterns of reassurance seeking and reassurance-related behaviours in OCD and anxiety disorders. Behavioural and Cognitive Psychotherapy 41, 1–23. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Weinberg A, Bunford N, Fitzgerald KD, Hanna GL, Monk CS, Kennedy AE, Klumpp H, Hajcak G and Phan KL (2016) Error-related brain activity in youth and young adults before and after treatment for generalized or social anxiety disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry 71, 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA and Fox NA (2014) Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child and Adolescent Psychiatry 53, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, McDermott JM and Fox NA (2014) Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Developmental Science 17, 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L and Franken IH (2014) Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. Journal of Psychiatry and Neuroscience 39, 149–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Zhang S, Hu S, Bednarski SR, Erdman E, Farr OM, Hong KI, Sinha R, Mazure CM and Li CSR (2013) Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain 136, 1231–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS and Agam Y (2013) Neural markers of errors as endophenotypes in neuropsychiatric disorders. Frontiers in Human Neuroscience 7, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, van de Wetering BJM and Franken IHA (2013) Error-related brain activity predicts cocaine Use after treatment at 3-month follow-up. Biological Psychiatry 73, 782–788. [DOI] [PubMed] [Google Scholar]
- Martin EA, McCleery A, Moore MM, Wynn JK, Green MF and Horan WP (2018) ERP indices of performance monitoring and feedback processing in psychosis: a meta-analysis. International Journal of Psychophysiology 132, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB and Etkin A (2017) Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. American Journal of Psychiatry 174, 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A and Klein DN (2015) Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. Journal of Abnormal Psychology 124, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Nelson B, Perlman G, Klein DN and Kotov R (2018) A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. Journal of Child Psychology and Psychiatry 59, 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA and Rockstroh B (2013) Endophenotypes in psychopathology research: where do we stand? Annual Review of Clinical Psychology 9, 177–213. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Gomes GC, Yoon JH, Swaab TY and Carter CS (2014) Disrupted action monitoring in recent-onset psychosis patients with schizophrenia and bipolar disorder. Psychiatry Research 221, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA and Asberg M (1979) A new depression scale designed to be sensitive to change. The British Journal of Psychiatry 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB and Yeung N (2013) On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in Human Neuroscience 7, Article 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet D and Hajcak G (2009) The stability of error-related brain activity with increasing trials. Psychophysiology 46, 957–961. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Abramovitch A, Rauch SL and Geller DA (2014) Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nature Reviews Neuroscience 15, 410–424. [DOI] [PubMed] [Google Scholar]
- Riesel A (2019) The erring brain: error-related negativity as an endophenotype for obsessive-compulsive disorder – A review and meta-analysis. Psychophysiology e13348, 10.1111/psyp.13348. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C and Kathmann N (2011) Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. American Journal of Psychiatry 168, 317–324. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Meyer A and Hajcak G (2013) The ERN is the ERN is the ERN? convergent validity of error-related brain activity across different tasks. Biological Psychology 93, 377–385. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Auerbach LA and Kathmann N (2015) Overactive performance monitoring as an endophenotype for obsessive-compulsive disorder: evidence from a treatment study. American Journal of Psychiatry 172, 665–673. [DOI] [PubMed] [Google Scholar]
- Riesel A, Goldhahn S and Kathmann N (2017) Hyperactive performance monitoring as a transdiagnostic marker: results from health anxiety in comparison to obsessive-compulsive disorder. Neuropsychologia 96, 1–8. [DOI] [PubMed] [Google Scholar]
- Rougemont-Buecking A, Rothen S, Jeanpretre N, Lustenberger Y, Vandeleur CL, Ferrero F and Preisig M (2008) Inter-informant agreement on diagnoses and prevalence estimates of anxiety disorders: direct interview versus family history method. Psychiatry Research 157, 211–223. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT and Kessler RC (2010) The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Molecular Psychiatry 15, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KH and Metzler P (1992) WST. Wortschatztest. Göttingen: Beltz Test GmbH. [Google Scholar]
- Schoenberg PL (2014) The error processing system in major depressive disorder: cortical phenotypal marker hypothesis. Biological Psychology 99, 100–114. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, De Bruijn E, Maas Y, Vancoillie P, Hulstijn W and Sabbe B (2009) Action monitoring and depressive symptom reduction in major depressive disorder. International Journal of Psychophysiology 71, 218–224. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ and Davidson RJ (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience 12, 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonite M, Bates AT, Groom MJ, Jackson GM, Hollis C and Liddle PF (2012) Error processing-associated event-related potentials in schizophrenia and unaffected siblings. International Journal of Psychophysiology 84, 74–79. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg P and Jacobs G (1983) Manual for the State-Trait Anxiety Inventory. Palo Alto, California: Consulting Psychologists Press. [Google Scholar]
- Stern ER, Liu Y, Gehring WJ, Lister JJ, Yin G, Zhang J, Fitzgerald KD, Himle JA, Abelson JL and Taylor SF (2010) Chronic medication does not affect hyperactive error responses in obsessive-compulsive disorder. Psychophysiology 47, 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Abramowitz JS, Brigidi BD and Foa EB (2003) Intolerance of uncertainty in obsessive-compulsive disorder. Journal of Anxiety Disorders 17, 233–242. [DOI] [PubMed] [Google Scholar]
- Vandeleur CL, Rothen S, Jeanpretre N, Lustenberger Y, Gamma F, Ayer E, Ferrero F, Fleischmann A, Besson J, Sisbane F and Preisig M (2008) Inter-informant agreement and prevalence estimates for substance use disorders: direct interview versus family history method. Drug and Alcohol Dependence 92, 9–19. [DOI] [PubMed] [Google Scholar]
- Vandeleur CL, Rothen S, Lustenberger Y, Glaus J, Castelao E and Preisig M (2015) Inter-informant agreement and prevalence estimates for mood syndromes: direct interview vs. Family history method. Journal of Affective Disorders 171, 120–127. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J and Bonnet M (2000) Is the ‘error negativity’ specific to errors? Biological Psychology 51, 109–128. [DOI] [PubMed] [Google Scholar]
- Vincent GM, Timothy KW, Leppert M and Keating M (1992) The spectrum of symptoms and Qt intervals in carriers of the gene for the long-Qt syndrome. New England Journal of Medicine 327, 846–852. [DOI] [PubMed] [Google Scholar]
- Weinberg A and Hajcak G (2011) Longer term test–retest reliability of error related brain activity. Psychophysiology 48, 1420–1425. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R and Riesel A (2015a) Error-related brain activity in the age of RDoC: a review of the literature. International Journal of Psychophysiology 98, 276–299. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Kotov R and Proudfit GH (2015b) Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. Journal of Abnormal Psychology 124, 172–185. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Liu H and Shankman SA (2016) Blunted neural response to errors as a trait marker of melancholic depression. Biological Psychology 113, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H and Olfson M (2000) Brief screening for family psychiatric history: the family history screen. Archives of General Psychiatry 57, 675–682. [DOI] [PubMed] [Google Scholar]
- Yook K, Kim KH, Suh SY and Lee KS, (2010) Intolerance of uncertainty, worry, and rumination in major depressive disorder and generalized anxiety disorder. Journal of Anxiety Disorders 24, 623–628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291719000199.
click here to view supplementary material