Abstract
Background
Blue Light Imaging (BLI) is a new imaging technology that enhances mucosal surface and vessel patterns. A specific BLI classification was recently developed to enable better characterisation of colorectal polyps (BLI Adenoma Serrated International Classification (BASIC)). The aim of this study was to validate the diagnostic performance of BASIC in predicting polyp histology in experienced and trainee endoscopists.
Methods
Five experienced and five trainee endoscopists evaluated high-definition white light (HDWL) and BLI images from 45 small polyps to assess baseline accuracy, sensitivity, specificity, and positive and negative predictive values (NPVs) of polyp histology. Each endoscopist was trained with the BLI classification before repeating the exercise. Results were compared pre- and post-training.
Results
The overall pre-training accuracy improved from 87% to 94%. The sensitivity and NPV of adenoma diagnosis also improved significantly from 79% to 96% and 81% to 95% with BASIC training. This improvement was noted in both groups. The interobserver level of agreement was very good (K = 0.90) in the experienced cohort and good (K = 0.66) in the trainee group post-training.
Conclusions
BLI is a useful tool for optical diagnosis, and the use of BASIC with adequate training can significantly improve the accuracy, sensitivity and NPV of adenoma diagnosis.
Keywords: Adenoma, Blue Light Imaging, classification, colorectal polyps, optical diagnosis
Key summary
- Summarise the established knowledge on this subject:
- Accurate optical diagnosis of polyps may allow endoscopists to ‘resect and discard’ polyps with no malignant potential.
- Advanced endoscopic imaging technologies can facilitate polyp characterisation by enhancing mucosal pit and vessel patterns.
- Blue Light Imaging (BLI) is a new technology with a unique classification that has not yet been validated.
-
What are the significant and/or new findings of this study?
BLI improved the optical diagnostic performance in a range of endoscopists when compared to high-definition white light.
Experienced and inexperienced endoscopists alike could be trained to achieve high levels of accuracy, sensitivity and negative predictive value >90% using a bespoke classification.
Introduction
The pathway to development of cancer from adenomas is well recognised.1 Therefore, detection and removal of adenomas can reduce the risk of colorectal cancer.2–4 Various enhanced-imaging technologies have been developed to improve recognition of neoplastic lesions such as Narrow Band Imaging (NBI; Olympus, Tokyo, Japan), i-SCAN (Pentax, Tokyo, Japan) and Flexible Spectral Imaging Colour Enhancement (FICE; Fujifilm, Tokyo, Japan). The American Society for Gastrointestinal Endoscopy (ASGE) Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) initiative recommends that a technology achieve a threshold of 90% or greater negative predictive value (NPV) for adenomatous histology to guide the decision to leave suspected diminutive rectosigmoid hyperplastic polyps (HPs) in place.5 A recent meta-analysis calculating the pooled NPV of NBI, i-SCAN and FICE optical biopsy for predicting adenomatous histology showed that the NPV for NBI exceeded 90% (91%; 95% confidence interval (CI): 88% to 94%). This effect was pronounced in academic centres with experts.6
Blue Light Imaging (BLI; Fujifilm, Tokyo, Japan) is a new technology based on the direct emission of blue light with a short (410 nm) wavelength that is selectively absorbed by haemoglobin. Four individual light-emitting diodes are used to create bright, high-contrast imaging. This may improve optical diagnosis and adenoma detection.7,8–10 A suitable system of education and training in real-time diagnostics will need to be developed to enable endoscopists to characterise polyps accurately enough to implement a resect and discard strategy. Previous studies on training using NBI have shown diagnostic accuracy rates ranging from 81% to 90% among endoscopists of different experience levels following computer-based training.11–13
In vivo characterisation of polyps has been based on vascular and mucosal surface pit patterns. The NBI International Colorectal Endoscopic (NICE) classification was developed to differentiate between neoplastic and non-neoplastic polyps on NBI.14 However, a recent study demonstrated that NICE did not work optimally when used for optical diagnosis using a different technology (FICE).15
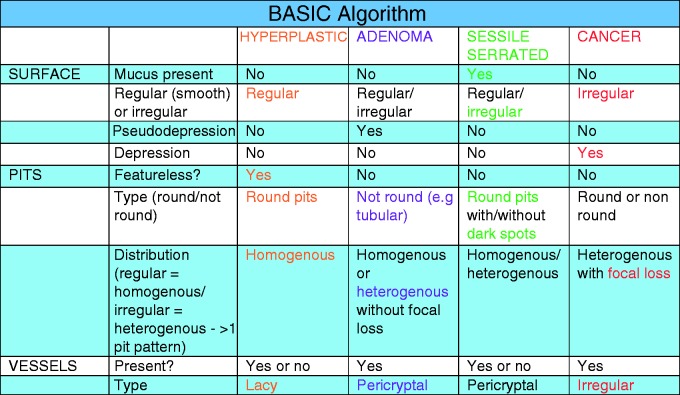
Therefore, a new bespoke classification system for differentiating among HPs, sessile-serrated and adenomatous polyps using BLI was recently developed.16 This classification (BLI Adenoma Serrated International Classification (BASIC)) incorporates the polyp morphology (surface) as well as pit and vessel characteristics. This has not been validated for clinical use.
The aim of our study was to develop a simple training module and validate this novel BLI classification in a group of experienced and trainee endoscopists.
Methods
Image library
The images for this study were obtained from elective outpatient colonoscopies performed between December 2016 to February 2017 with Fujifilm colonoscope series ELUXEO TM 7000 (ELUXEO, VP-7000, BL-7000; Fujifilm, Tokyo, Japan) in three institutions (Portsmouth, Milan and Rome). All patients consented for the polyp images from their procedure to be used anonymously for educational purposes. For each polyp, a paired non-magnification, high-definition white light (HDWL) and BLI image was stored. The size, location and morphology (Paris classification)17 of the polyps were recorded. All polyps were resected and sent for histopathological examination. The histopathologist was not aware of the endoscopic optical diagnostic characteristics for each polyp and classified the polyp histology according to the revised Vienna classification.18 The histopathological diagnosis was used as the gold-standard true diagnosis.
Only high-quality and clear images of small (6–9 mm) and diminutive (1–5 mm) colorectal polyps were selected by one research fellow (S.S.) experienced in optical diagnosis. An equal number of adenomas and HPs were selected. Sessile-serrated polyps and poor-quality images were excluded.
Training module
Fifteen polyps (equally split by subtype) were used in the development of the training module. These images were not used in the testing. Microsoft PowerPoint (Microsoft Corporation, Redmond, WA, USA) was used as the training platform. The training module was delivered by an expert endoscopist (P.B.) and experienced research fellow in a face-to-face session. The module was structured as follows:
– Overview of the importance of endoscopic polyp characterisation to facilitate the recognition of HPs with high confidence that may be suitable for the resect and discard strategy.
– Review of ASGE PIVI thresholds.
– Evolution of advanced-imaging technology and BLI mode of action.
– Explanation of the individual descriptors used for BASIC including distinguishing between pseudodepression and truly depressed (i.e. Paris IIC)17 morphology.
– Algorithm for differentiation between polyp histological types using BASIC (Figure 1).
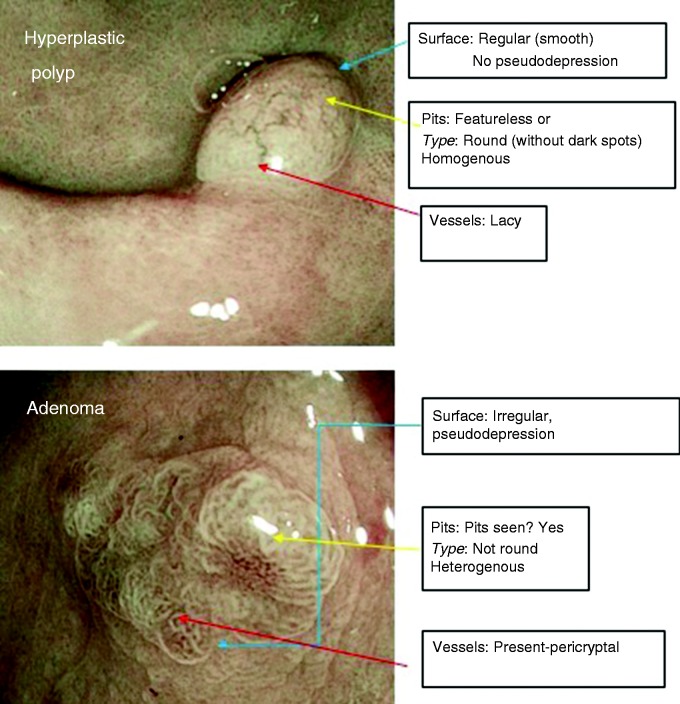
– Presentation of BLI images with illustration on the surface, pit and vessel pattern descriptors to formulate a diagnosis (Figure 2).
Figure 1.
Proposed algorithm to utilise Blue Light Imaging Adenoma Serrated International Classification (BASIC).
Figure 2.
Illustrative polyp images included in training module to identify Blue Light Imaging Adenoma Serrated International Classification (BASIC) descriptors.
Direct feedback was given to the endoscopists during the session with emphasis on the interpretation of mucosal surface and vessel patterns using the training set. This was run as an in-training quiz with explanations provided on the correct use of BASIC descriptors.
Study participants
Two groups of five participants were involved. The first group consisted of five endoscopists who were experienced in using NBI for polyp characterisation during colonoscopy but had limited (<6 months) experience in BLI with no formal training in optical diagnosis. They had all performed >1000 colonoscopies.
The second group was made up of five gastroenterology trainees with minimal colonoscopy experience (<400 procedures) and no experience or training in any advanced endoscopic imaging.
Study phases
The study incorporated a pre- and post-training phase. In both phases, the diagnostic performance was assessed for each modality and each participant group by calculating the sensitivity, specificity, positive predictive value (PPV), NPV and accuracy with corresponding CIs.
Phase 1
The aim of this phase was to assess the baseline performance of both groups. Each group was shown separately a set of 45 non-magnified HDWL and BLI images (consisting of 23 adenomas and 22 HPs). The polyps were arranged randomly and participants were blinded to the proportion of histological subtypes, location, morphology and size of polyps in the set. The participants recorded the endoscopic diagnosis and level of confidence (high/low). Participants selected the high confidence option if they were at least 90% certain. No feedback on diagnostic accuracy was given following this phase.
Phase 2
This phase of the study was conducted three months following Phase 1 to minimise recall bias. All participants underwent face-to-face training session in the use of BASIC as previously described. The participants were then tested on the same 45-polyp image library presented in a different random order to the pre-training set. All participants were still blinded to the polyp characteristics as before. They rated each image using the BASIC descriptors and scored their level of confidence. Feedback on diagnostic accuracy was supplied following this phase.
In Phases 1 and 2, participants viewed the images on site using a high-definition screen and test conditions (discussion between the participants to reach a diagnosis was not permitted).
Ethical approval
This was an image-based, non-interventional endoscopic evaluation study with no patient identifiable data collected. Institutional review board approval was obtained (ICH 477/16, 1 December 2016) and the study was carried out in accordance with the 1975 Declaration of Helsinki.
Statistical analysis
The study was powered on the assumption that there would be a difference of 10% in diagnostic accuracy between the pre- and post-training tests. Using a power of 80% with 5% significance level, 200 observations were required in each phase. By recruiting five participants for each group, we generated 225 observations per group (450 in total), which satisfied the power calculations. All data were collected in Microsoft Excel (Microsoft Corporation, Redmond, Washington USA). Stata version 15.1 (StataCorp, College Station, TX, USA) was used for statistical analysis. To allow for the non-independence of the data, a bootstrapping approach was used to calculate CI for the differences between modalities. Multilevel logistic regression was used for the analysis.
The interobserver agreement between users pre- and post-training was made using the kappa statistic. A bootstrapping approach was used to calculate CI around the calculated value at each time point and also to compare between time points.
Results
The results are presented in a per-protocol fashion. There were no missing data and all ratings were included in the analysis. Table 1 shows the baseline characteristics of the 45 polyps included in the test phases. The proportions of adenomas and HPs were roughly equal (51.1% and 48.9%) with a majority (75%) deemed diminutive.
Table 1.
Characteristics of polyps included in the study.
| Polyp characteristics | N (%) | |
|---|---|---|
| Size | 1–5 mm | 34 (75.6%) |
| 6–9 mm | 11 (24.4%) | |
| Location | Rectum | 13 (28.9%) |
| Sigmoid | 15 (33.3%) | |
| Descending colon | 7 (15.5%) | |
| Transverse colon | 4 (8.9%) | |
| Ascending colon | 3 (6.7%) | |
| Caecum | 3 (6.7%) | |
| Morphology (Paris classification) | 0–IIa | 23 (51.1%) |
| 0–IIb | 10 (22.2%) | |
| 0–Is | 12 (26.7%) | |
| Histology | Adenoma | 23 (51.1%) |
| Hyperplastic polyp | 22 (48.9%) | |
Phase 1 results
Table 2 shows the performance of both groups on HDWL compared to BLI. The greatest improvement in sensitivity using BLI was observed in the experienced group (69% on HDWL, 95% CI: 60% to 77% vs 79% on BLI, 95% CI: 69% to 85%, p = 0.02). The proportion of high confidence predictions increased significantly in both groups when BLI was used (from 52% to 71% in the experienced cohort and 40% to 67% in the inexperienced group). When the results for both groups were combined, similar patterns were observed with increased sensitivity and high confidence with BLI, but no difference noted in the other parameters.
Table 2.
Polyp diagnosis using HDWL and BLI in experienced and trainee groups.
| Variable | HDWL % (95% CI) | BLI % (95% CI) | Odds ratioa (95% CI) | p value |
|---|---|---|---|---|
| Experienced group | ||||
| Sensitivity | 69 (60, 77) | 79 (69, 85) | 2.52 (1.14, 5.59) | 0.02 |
| Specificity | 97 (92, 99) | 95 (90, 99) | 0.43 (0.10, 1.99) | 0.28 |
| PPV | 96 (93, 100) | 94 (90, 98) | 0.58 (0.14, 2.38) | 0.45 |
| NPV | 75 (69, 83) | 81 (73, 88) | 1.46 (0.81, 2.61) | 0.21 |
| Accuracy | 83 (78, 87) | 87 (82, 91) | 1.70 (0.86, 3.38) | 0.13 |
| High confidence | 52 (45, 58) | 71 (65, 77) | 2.80 (1.77, 4.43) | <0.001 |
| Trainee group | ||||
| Sensitivity | 75 (67, 82) | 79 (70, 85) | 1.43 (0.68, 3.02) | 0.35 |
| Specificity | 97 (93, 98) | 96 (93, 99) | 0.72 (0.15, 3.56) | 0.69 |
| PPV | 97 (91, 99) | 96 (89, 98) | 0.79 (0.17, 3.65) | 0.77 |
| NPV | 79 (72, 87) | 82 (74, 88) | 1.20 (0.65, 2.19) | 0.56 |
| Accuracy | 86 (81, 91) | 88 (83, 92) | 1.26 (0.65, 2.48) | 0.49 |
| High confidence | 40 (35, 48) | 67 (60, 72) | 2.95 (2.01, 4.33) | <0.001 |
| All | ||||
| Sensitivity | 72 (64, 77) | 79 (72, 84) | 1.87 (1.09, 3.20) | 0.02 |
| Specificity | 97 (94, 99) | 95 (92, 98) | 0.55 (0.19, 1.64) | 0.29 |
| PPV | 96 (92, 98) | 95 (91, 97) | 0.66 (0.24, 1.86) | 0.43 |
| NPV | 77 (71, 81) | 81 (77, 86) | 1.33 (0.87, 2.02) | 0.18 |
| Accuracy | 84 (81, 88) | 87 (84, 90) | 1.46 (0.91, 2.35) | 0.12 |
| High confidence | 46 (42, 50) | 69 (64, 73) | 2.70 (2.03, 3.58) | <0.001 |
BLI: Blue Light Imaging; CI: confidence interval; HDWL: high-definition white light; NPV: negative predictive value; PPV: positive predictive value.
When a subgroup analysis was performed stratifying results according to confidence level, the performance of BLI improved further (albeit not reaching statistical significance) as sensitivity and NPV reached 85% with corresponding accuracy rates of 90% (Table 3).
Table 3.
Polyp diagnosis on HDWL and BLI according to level of confidence in prediction.
| Variable | HDWL % (95% CI) | BLI % (95% CI) | Odds Ratioa (95% CI) | p value |
|---|---|---|---|---|
| High confidence | ||||
| Sensitivity | 81 (73, 89) | 85 (78, 90) | 2.32 (0.9, 5.83) | 0.07 |
| Specificity | 97 (93, 98) | 97 (94, 99) | 0.95 (0.19, 4.70) | 0.95 |
| PPV | 96 (92, 100) | 97 (92, 98) | 1.00 (0.23, 4.32) | 0.99 |
| NPV | 85 (76, 89) | 85 (79, 90) | 1.02 (0.54, 1.96) | 0.95 |
| Accuracy | 89 (86, 94) | 90 (87, 94) | 1.84 (0.84, 4.05) | 0.13 |
| Low confidence | ||||
| Sensitivity | 64 (56, 73) | 65 (55, 79) | 1.15 (0.57, 2.34) | 0.70 |
| Specificity | 97 (95, 100) | 93 (84, 97) | 0.40 (0.08, 1.98) | 0.26 |
| PPV | 96 (88, 99) | 90 (81, 98) | 0.31 (0.07, 1.38) | 0.13 |
| NPV | 71 (63, 78) | 75 (66, 83) | 1.27 (0.71, 2.23) | 0.42 |
| Accuracy | 80 (74, 84) | 80 (76, 87) | 1.11 (0.56, 5.72) | 0.61 |
BLI: Blue Light Imaging; CI: confidence interval; HDWL: high-definition white light; NPV: negative predictive value; PPV: positive predictive value.
Phase 2 results (pre- vs post-training)
HDWL
Table 4 shows the results of the pre- and post-training analysis using 45 HDWL images. When only experienced endoscopists are considered in the analysis, there was a highly significant improvement in the diagnostic sensitivity (from 69%, 95% CI: 60% to 77%, to 83%, 95% CI: 76% to 89%) and accuracy (from 83%, 95% CI: 78% to 87%, to 90%, 95% CI: 87% to 94%) following training. In the trainee group, smaller, non-statistically significant improvements were noted in sensitivity (75% to 84%), NPV (79% to 84%) and accuracy (86% to 87%). Notably, the specificity dropped in this group (97% to 89%), reflective of an increase in the false-positive rate of adenoma predictions.
Table 4.
Pre- and post-training results in experienced and trainee groups using HDWL.
| Variable | Pre-training % (95% CI) | Post-training % (95% CI) | Odds ratioa (95% CI) | p value |
|---|---|---|---|---|
| Experienced group | ||||
| Sensitivity | 69 (60, 77) | 83 (76, 89) | 3.35 (1.37, 8.16) | 0.008 |
| Specificity | 97 (92, 99) | 98 (94, 99) | 1.48 (0.23, 9.57) | 0.68 |
| PPV | 96 (93, 100) | 98 (95, 100) | 1.80 (0.29, 11.1) | 0.52 |
| NPV | 75 (69, 83) | 84 (7 9, 90) | 1.82 (0.99, 3.33) | 0.05 |
| Accuracy | 83 (78, 87) | 90 (87, 94) | 2.40 (1.25, 4.59) | 0.008 |
| High confidence | 52 (45, 58) | 75 (69, 80) | 3.26 (2.08, 5.11) | <0.001 |
| Trainee group | ||||
| Sensitivity | 75 (67, 82) | 84 (70, 85) | 2.03 (0.94, 4.38) | 0.07 |
| Specificity | 97 (93, 98) | 89 (83, 95) | 0.22 (0.06, 0.84) | 0.03 |
| PPV | 97 (91, 99) | 89 (83, 94) | 0.28 (0.08, 1.03) | 0.06 |
| NPV | 79 (72, 87) | 84 (78, 90) | 1.48 (0.77, 2.82) | 0.24 |
| Accuracy | 86 (81, 91) | 87 (82, 90) | 1.09 (0.61, 1.94) | 0.77 |
| High confidence | 40 (35, 48) | 31 (24, 37) | 0.57 (0.36, 0.90) | 0.02 |
| All | ||||
| Sensitivity | 72 (64, 77) | 83 (78, 87) | 2.53 (1.40, 4.61) | 0.002 |
| Specificity | 97 (94, 99) | 94 (90, 97) | 0.41 (0.16, 1.09) | 0.08 |
| PPV | 96 (92, 98) | 93 (89, 96) | 0.50 (0.19, 1.33) | 0.16 |
| NPV | 77 (71, 81) | 84 (80, 89) | 1.64 (1.06, 2.57) | 0.03 |
| Accuracy | 84 (81, 88) | 88 (85, 92) | 1.55 (1.01, 2.37) | 0.04 |
| High confidence | 46 (42, 50) | 53 (48, 58) | 1.43 (1.05, 1.94) | 0.02 |
CI: confidence interval; HDWL: high-definition white light; NPV: negative predictive value; PPV: positive predictive value.
BLI
The same analysis was carried out on the 45 BLI images assessed pre- and post-training (Table 5). The results for experienced raters suggested a much higher sensitivity post-training compared to pre-training, with an increase from 79% to 97% (p < 0.001). There was also an increase in NPV, accuracy and results made with high confidence post-training. There was no change in either specificity or PPV, but these were already high pre-training.
Table 5.
Pre- and post-training results in experienced and trainee groups using BLI.
| Variable | Pre-training % (95% CI) | Post-training % (95% CI) | Odds ratioa (95% CI) | p value |
|---|---|---|---|---|
| Experienced group | ||||
| Sensitivity | 79 (69, 85) | 97 (94, 100) | 17.1 (3.65, 79.8) | <0.001 |
| Specificity | 95 (90, 99) | 96 (94, 99) | 2.47 (0.43, 14.2) | 0.31 |
| PPV | 94 (90, 98) | 97 (92, 100) | 1.85 (0.51, 6.74) | 0.35 |
| NPV | 81 (73, 88) | 97 (93, 99) | 8.15 (2.38, 27.9) | 0.001 |
| Accuracy | 87 (82, 91) | 97 (95, 100) | 7.26 (2.72, 19.4) | <0.001 |
| High confidence | 71 (65, 77) | 88 (83, 91) | 3.46 (2.04, 5.89) | <0.001 |
| Trainee group | ||||
| Sensitivity | 79 (70, 85) | 94 (90, 98) | 5.49 (1.89, 15.9) | 0.002 |
| Specificity | 96 (93, 99) | 87 (79, 92) | 0.29 (0.08, 0.81) | 0.02 |
| PPV | 96 (89, 98) | 89 (80, 93) | 0.34 (0.11, 1.07) | 0.06 |
| NPV | 82 (74, 88) | 93 (86, 97) | 3.10 (1.28, 7.53) | 0.01 |
| Accuracy | 88 (83, 92) | 91 (85, 93) | 1.44 (0.76, 2.71) | 0.26 |
| High confidence | 67 (60, 72) | 80 (76, 86) | 4.00 (2.10, 7.62) | <0.001 |
| All | ||||
| Sensitivity | 79 (72, 84) | 96 (92, 97) | 8.44 (3.48, 20.4) | <0.001 |
| Specificity | 95 (92, 98) | 92 (86, 95) | 0.54 (0.23, 1.26) | 0.15 |
| PPV | 95 (91, 97) | 92 (89, 95) | 0.67 (0.30, 1.49) | 0.33 |
| NPV | 81 (77, 86) | 95 (92, 98) | 4.62 (2.27, 9.38) | <0.001 |
| Accuracy | 87 (84, 90) | 94 (92, 96) | 2.61 (1.55, 4.38) | <0.001 |
| High confidence | 69 (64, 73) | 84 (81, 88) | 3.73 (2.48, 5.62) | <0.001 |
BLI: Blue Light Imaging; CI: confidence interval; NPV: negative predictive value; PPV: positive predictive value.
Sensitivity (79% to 94%, p = 0.002) and high confidence results (67% to 80%, p < 0.001) also increased post-training in the trainee group. However, increases in sensitivity were offset by a significant decrease in specificity (96% pre-training to 87% post-training, p = 0.02). There was no change in overall accuracy between time periods.
When all raters were combined, there was a significant increase in sensitivity, NPV, accuracy and high confidence results (p < 0.001).
A subgroup analysis of high confidence predictions for all endoscopists on BLI images pre- and post-training showed an increase from 69% to 84% (p < 0.001). There was a significant improvement in sensitivity (85% to 97%, p < 0.001), NPV (85% to 96%, p = 0.002) and accuracy (90% to 96%, p = 0.006).
Interobserver agreement
The diagnostic agreement using BASIC on BLI images among experienced endoscopists showed an increase in the level of agreement from 0.67 (95% CI: 0.55 to 0.80) pre-training to 0.90 (95% CI 0.82 to 0.98) post-training (p = 0.003). There was no significant change between time points for the trainee endoscopists (K = 0.66 pre- and post-training). See Table 6.
Table 6.
Interobserver agreement on BLI.
| Observer group | Pre-training Kappa (95% CI) | Post-training Kappa (95% CI) | Differencea (95% CI) | p value |
|---|---|---|---|---|
| Experienced | 0.67 (0.55, 0.80) | 0.90 (0.82, 0.98) | 0.23 (0.08, 0.38) | 0.003 |
| Trainee | 0.66 (0.54, 0.79) | 0.66 (0.52, 0.79) | 0.00 (–0.19, 0.18) | 0.97 |
| All observers | 0.68 (0.57, 0.79) | 0.77 (0.69, 0.86) | 0.09 (–0.04, 0.23) | 0.18 |
BLI: Blue Light Imaging; CI: confidence interval.
Discussion
This study has shown that endoscopists with different levels of experience can be trained using a bespoke classification (BASIC) to achieve high levels of optical diagnostic accuracy (94%), sensitivity (96%) and NPV (95%) to differentiate between neoplastic and non-neoplastic polyps using a novel BLI technology.
In Phase 1 (baseline comparison between HDWL and BLI), there was a small improvement in sensitivity and NPV using BLI. The high degree of brightness and contrast on the HDWL image alone may account for its relatively good performance and therefore less incremental gain using BLI. Because BLI was a new tool for the endoscopists, we did not anticipate that there would be a significant difference between HDWL and BLI results without any training. The baseline performance of the experienced group was not significantly better than the trainees and, in fact, displayed a lower pre-training sensitivity on HDWL (69%) with high specificity. This may allude to inherent preconceived decisions on polyp diagnosis in experienced endoscopists who were also exposed to other technologies (NBI). The low sensitivity could also be a result of less risk-averse behaviour with fewer adenoma predictions resulting in a higher false-negative rate. It was evident that although BLI showed potential in improving optical diagnosis, both groups were unable to utilise it optimally to implement the resect and discard strategy.
However, upon implementation of face-to-face training using BASIC, the optical diagnostic parameters using BLI in both groups of endoscopists improved significantly, surpassing 90% in sensitivity, NPV and accuracy. These improvements were greater in the experienced cohort with no decrease in specificity. BASIC training allowed this group to adapt prior optical diagnostic knowledge and apply it to BLI images, achieving thresholds that would meet PIVI criteria. Furthermore, they achieved a very good level of agreement in their responses. Inexperienced endoscopists applying BASIC post-training also achieved high sensitivity and NPV for adenoma diagnosis. Similar results have been demonstrated in studies of trainees taught to interpret NBI for which accuracy or NPV of >90% for adenomatous histology was achieved.19,20 In our cohort, this was offset by a significant decrease in specificity (i.e. HPs were mistaken for adenomas) reflective of the degree of caution that inexperienced endoscopists may have in predicting HPs with high confidence. However, the learning curve of virtual chromoendoscopy is likely to improve over time with practice, as shown in a previous study in which accuracy rates of 94.3% were obtained when at least 89 polyp images had been viewed.21 The sustainability of optical diagnosis results should also be reinforced by standardised and ongoing training.22
Training in BASIC also led to significant improvement in HDWL predictions, with overall sensitivity reaching 83%, NPV 84% and accuracy 88%. We believe that this is partly due to the unique surface morphology feature incorporated into the classification and the training delivered, which allowed the endoscopists to develop a structured method of distinguishing polyps.
While the use of BLI before training increased the cohort's confidence level, the adoption of BASIC further improved the proportion of high confidence BLI predictions from 69% to 84%. This encouraging finding lends weight to the importance of a structured training module with direct feedback to enhance the learning effect when using a novel technology. Nevertheless, we still have some way to go before optical diagnosis can be recommended for use in routine practice. A large multicentre study (DISCARD 2) demonstrated that optical diagnosis using NBI cannot currently be recommended for use outside academic medical centres because diagnostic accuracy parameters were low.23
Our study has several limitations. Primarily, it did not incorporate real-time in vivo characterisation of polyps. We used still images rather than videos to simulate real-life colonoscopy and the majority of endoscopists captured a still image when encountering a polyp to photograph and analyse its surface and vessel patterns without interference from movement artefact. The proportion of adenomatous histology in this cohort was higher than in an average surveillance population to validate both dichotomous responses though it is important to note that all participants were blinded to the proportion of histology. We did not include sessile-serrated polyps although BASIC does incorporate its descriptors because the overall prevalence is low in the general population and the training focus was on differentiating between adenomas and HPs. We used the same set of images both pre- and post-training but mitigated the effect of any recall bias by introducing a time gap between both phases in the study (three months) and assigning a different random order to the images in the post-training phase as well as keeping the endoscopists blinded to the true polyp histology until both phases were completed.
In conclusion, we have demonstrated that BLI is a useful tool in optical diagnosis of small and diminutive colorectal polyps and its utility can be improved by training and adoption of a recently developed bespoke classification system (BASIC). This study is the first validation of the only existing colorectal polyp classification for BLI. The overall post-training NPV of 93% and 97% respectively both in experienced and inexperienced endoscopists reaches the PIVI threshold for optical diagnosis. However, these results need to be validated in a prospective, multicentre, real-time, in vivo optical characterisation study before any recommendation can be made on its widespread adoption.
Acknowledgements
The authors acknowledge Matthew Stammers and Rebecca Smith for their contribution to validating the images used in this study.
Declaration of conflicting interests
Emmanuel Coron has received speaker fees from Fujifilm, Olympus, Cook and Norgine and is a consultant for Medtronic. Oliver Pech has received speaker fees from Fujifilm, Olympus, Boston Scientific, Medtronic and Creo Medical. Cesare Hassan has received speaker fees from Fujifilm, Olympus, Boston Scientific, Medtronic and Creo Medical. Helmut Neumann is a consultant for and has received research support from Fujifilm, Pentax and Olympus. Raf Bisschops is a consultant for and has received speaker fees and research support from Fujifilm. Alessandro Repici is a consultant and has received speaker fees from Fujifilm. Pradeep Bhandari has received research support from Fujifilm, Pentax and Olympus. All other authors have nothing to declare.
Ethical approval
Institutional review board approval was obtained (ICH 477/16, 1 December 2016) and the study was carried out in accordance with the 1975 Declaration of Helsinki.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Informed consent
All patients consented for the polyp images from their procedure to be used anonymously for educational purposes.
References
- 1.Hill MJ, Morson BC, Bussey HJ. Aetiology of adenoma-carcinoma sequence in large bowel. Lancet 1978; 311: 245–247. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993; 329: 1977–1981. [DOI] [PubMed] [Google Scholar]
- 3.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010; 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 4.Kaminski MF, Wieszczy P, Rupinski M, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology 2017; 153: 98–105. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Kahi C, O'Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2011; 73: 419–422. [DOI] [PubMed] [Google Scholar]
- 6.ASGE Technology Committee, Abu Dayyeh BK, Thosani N, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2015; 81: 502.e1–502.e16. [DOI] [PubMed] [Google Scholar]
- 7.Ikematsu H, Sakamoto T, Togashi K, et al. Detectability of colorectal neoplastic lesions using a novel endoscopic system with Blue Laser Imaging: A multicenter randomized controlled trial. Gastrointest Endosc 2017; 86: 386–394. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida N, Yagi N, Inada Y, et al. Ability of a novel Blue Laser Imaging system for the diagnosis of colorectal polyps. Dig Endosc 2014; 26: 250–258. [DOI] [PubMed] [Google Scholar]
- 9.Togashi K, Nemoto D, Utano K, et al. Blue Laser Imaging endoscopy system for the early detection and characterization of colorectal lesions: A guide for the endoscopist. Therap Adv Gastroenterol 2016; 9: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano A, Hirooka Y, Yamamura T, et al. Comparison of the diagnostic ability of Blue Laser Imaging magnification versus pit pattern analysis for colorectal polyps. Endosc Int Open 2017; 5: E224–E231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rastogi A, Rao DS, Gupta N, et al. Impact of a computer-based teaching module on characterization of diminutive colon polyps by using narrow-band imaging by non-experts in academic and community practice: A video-based study. Gastrointest Endosc 2014; 79: 390–398. [DOI] [PubMed] [Google Scholar]
- 12.Ignjatovic A, Thomas-Gibson S, East JE, et al. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc 2011; 73: 128–133. [DOI] [PubMed] [Google Scholar]
- 13.Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: Narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc 2010; 72: 572–576. [DOI] [PubMed] [Google Scholar]
- 14.Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012; 143: 599–607.e1. [DOI] [PubMed]
- 15.Repici A, Ciscato C, Correale L, et al. Narrow-band Imaging International Colorectal Endoscopic Classification to predict polyp histology: REDEFINE study (with videos). Gastrointest Endosc 2016; 84: 479–486.e3. . [DOI] [PubMed] [Google Scholar]
- 16.Bisschops R, Hassan C, Bhandari P, et al. BASIC (BLI Adenoma Serrated International Classification) classification for colorectal polyp characterization with Blue Light Imaging. Endoscopy 2018; 50: 211–220. [DOI] [PubMed] [Google Scholar]
- 17.Participants in the Paris Workshop P in the P, Aiko T, Sasako M, et al. The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003; 58(6 Suppl): S3–S43. [DOI] [PubMed]
- 18.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SG, Rastogi A, Austin G, et al. Gastroenterology trainees can easily learn histologic characterization of diminutive colorectal polyps with Narrow Band Imaging. Clin Gastroenterol Hepatol 2013; 11: 997–1003.e1. [DOI] [PubMed] [Google Scholar]
- 20.Higashi R, Uraoka T, Kato J, et al. Diagnostic accuracy of narrow-band imaging and pit pattern analysis significantly improved for less-experienced endoscopists after an expanded training program. Gastrointest Endosc 2010; 72: 127–135. [DOI] [PubMed] [Google Scholar]
- 21.Neumann H, Vieth M, Fry LC, et al. Learning curve of virtual chromoendoscopy for the prediction of hyperplastic and adenomatous colorectal lesions: A prospective 2-center study. Gastrointest Endosc 2013; 78: 115–120. [DOI] [PubMed] [Google Scholar]
- 22.McGill SK, Soetikno R, Rastogi A, et al. Endoscopists can sustain high performance for the optical diagnosis of colorectal polyps following standardized and continued training. Endoscopy 2015; 47: 200–206. [DOI] [PubMed] [Google Scholar]
- 23.Rees CJ, Rajasekhar PT, Wilson A, et al. Narrow Band Imaging optical diagnosis of small colorectal polyps in routine clinical practice: The Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut 2016; 66: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]