Abstract
Background
Endoscopy within 24 h of admission (early endoscopy) is a quality standard in acute upper gastrointestinal bleeding (AUGIB). We aimed to audit time to endoscopy outcomes and identify factors affecting delayed endoscopy (>24 h of admission).
Methods
This prospective multicentre audit enrolled patients admitted with AUGIB who underwent inpatient endoscopy between November and December 2017. Analyses were performed to identify factors associated with delayed endoscopy, and to compare patient outcomes, including length of stay and mortality rates, between early and delayed endoscopy groups.
Results
Across 348 patients from 20 centres, the median time to endoscopy was 21.2 h (IQR 12.0–35.7), comprising median admission to referral and referral to endoscopy times of 8.1 h (IQR 3.7–18.1) and 6.7 h (IQR 3.0–23.1), respectively. Early endoscopy was achieved in 58.9%, although this varied by centre (range: 31.0–87.5%, p = 0.002). On multivariable analysis, lower Glasgow–Blatchford score, delayed referral, admissions between 7:00 and 19:00 hours or via the emergency department were independent predictors of delayed endoscopy. Early endoscopy was associated with reduced length of stay (median difference 1 d; p = 0.004), but not 30-d mortality (p = 0.344).
Conclusions
The majority of centres did not meet national standards for time to endoscopy. Strategic initiatives involving acute care services may be necessary to improve this outcome.
Keywords: Upper gastrointestinal bleeding, haemorrhage, time to endoscopy, endoscopy, quality
Key summary
International guidelines generally recommend early endoscopy within 24 h in all patients admitted with acute upper gastrointestinal bleeding (AUGIB).
Time to endoscopy (the time from admission with AUGIB to endoscopy) has been shown to be consistently suboptimal in previous UK audits.
The factors that affect time to endoscopy, including the role of the endoscopy referral, have not previously been studied.
There was significant variation in time to endoscopy following AUGIB between participating centres, with the majority failing to meet national standards.
Different factors determine time to endoscopy, and its components of time from admission to referral, and time from referral to endoscopy.
Strategic measures involving acute care services to expedite management and endoscopy referral are required to improve standards for time to endoscopy.
Introduction
Acute upper gastrointestinal bleeding (AUGIB) is a common medical emergency, with an annual incidence of 133/100,000,1 corresponding to approximately one presentation every 6 min in the UK. Despite advances in endoscopy, service provision, and guidelines,2–4 mortality following AUGIB has remained high over the last two decades and currently stands at approximately 10%.5
Endoscopy is the primary diagnostic and therapeutic modality for AUGIB. The time to endoscopy (i.e. interval between hospital admission to endoscopy) following AUGIB has been adopted by the National Institute for Health and Clinical Excellence (NICE),2 the European Society of Gastrointestinal Endoscopy (ESGE)4 and the Joint Advisory Group on Gastrointestinal Endoscopy (JAG)6 as a quality standard for both patients and endoscopy units. NICE and ESGE recommend early endoscopy (within < 24 h of admission) for all patients admitted with suspected AUGIB,2,4 whereas the JAG Global Rating Scale (GRS) stipulates that each endoscopy unit should perform early endoscopy in ≥75% of patients with AUGIB to achieve a Level B rating,6 which is the minimum rating for endoscopy unit accreditation.
In line with previous UK audits, the latest national audit (NCEPOD) in 2015 reported ongoing deficits in patient care,7 including delayed endoscopy (time to endoscopy > 24 h) in 35%. Organisational factors were cited as requiring improvement in 18.5% of cases reviewed. Amongst their recommendations, NCEPOD re-emphasised the need for all patients with AUGIB to have endoscopy within 24 h, and called for improved organisation and co-ordination of AUGIB services.
The organisational processes behind time to endoscopy have been poorly studied. Factors that may impact on time to endoscopy include: assessment of the patient, availability of test results, efficiency of referral pathways between clinical teams and the organisation of the patient journey. These factors inevitably vary among institutions, with areas of good practice and those needing development present in all. Single centre audits may not be comparable, limiting their value for shared learning and service improvement across the health service.
The aims of our study were to:
(1) Audit the rates of early/delayed endoscopy and identify the proportion of centres that met the JAG standard.
(2) Identify predictors of delayed endoscopy, which can be used to target service improvement.
(3) Assess the impact of delayed endoscopy on patient outcomes.
Methods
Study design
A prospective, multicentre audit was jointly undertaken by trainee-led gastroenterology trainee networks in the East (GARNet) and West Midlands (WMRIG) regions, covering a catchment population of approximately 10.5 million. Within each centre, patients admitted with suspected AUGIB who underwent endoscopy as inpatients between November and December 2017 were enrolled over a 30-d consecutive period. Exclusion criteria comprised: inpatients who developed AUGIB after being admitted for an unrelated condition, low-risk patients referred for outpatient endoscopy, paediatric cases (age < 16 yr) and bright red rectal bleeding.
Outcomes
The primary outcome studied was the time to endoscopy, defined as the time taken from first hospital presentation (to the emergency department (ED) or acute medical unit) to the start time of an endoscopy procedure. This was analysed using two approaches: (i) as a binary outcome (within 24/>24 h) to audit rates of delayed endoscopy; and (ii) as a continuous variable, which was additionally deconstructed into: time from admission to endoscopy referral (time to referral) and time from referral to endoscopy. Admission, endoscopy referral and procedure start times were preferentially collected using timestamped electronic records. For the endoscopy referral time, this represented the time when the electronic request was submitted.
Secondary outcomes included the length of stay and rates of rebleeding within 8 d of endoscopy (JAG standard). AUGIB was defined as haematemesis, melaena, coffee ground vomiting, which was deemed to require oesophagogastroduodenoscopy. Rebleeding was defined according to the clinician's impression of recurrent or ongoing AUGIB following index endoscopy. Post-discharge readmission rates and post-endoscopy mortality rates were also assessed, using a time to event approach.
Study covariates
A range of factors were studied in order to identify those associated with time to endoscopy. These included pre-endoscopic factors, e.g. suspicion of variceal bleeding, use of antithrombotics and empirical nil by mouth placement; haemodynamic parameters, shock index (first recorded heart rate divided by systolic blood pressure), haemodynamic instability (features of shock despite fluid resuscitation), and the Glasgow–Blatchford score (GBS). Where the GBS was not recorded, this was calculated using the GBS components. Endoscopic lesions, major haemorrhagic stigmata and application of endotherapy were retrieved from endoscopy reports. Vital case ascertainment for 30-d outcomes was verified using electronic case records.
Conduct
Each participating site involved a study team comprising:
(a) Site project lead: responsible for liaising with the local Clinical Governance and Audit department and clinical service lead as necessary, screening for eligible patients, providing quality assurance and security of data collection, and presenting the results at local level.
(b) Site consultant: Provides institutional oversight and responsibility, supporting the site project lead(s), and contributing to discussion of results and plan for quality improvement at site-level.
(c) Site project investigator(s): responsible for data collection and ensuring validity of collected data.
Data collection templates were disseminated to each site project lead. These maximised the utility of dropdown menus and automated data validation fields. Each collected variable was annotated with definitions in order to facilitate standardisation of data entries.
Study approval
This audit was registered centrally at Nottingham University Hospital (project code: 17-267c; approval date: 2 November 2017). Formal ethics approval and patient consent was not required as per departmental policy and Health Research Authority guidance. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's review board.
Statistical analysis
Initially, times to endoscopy were dichotomised into those that were early (≤24 h) or delayed (>24 h). Comparisons of delayed endoscopy rates were compared across centres using chi-square test. Univariable analyses were then performed to identify other factors associated with delayed endoscopy. Categorical variables were assessed using Fisher's exact tests, while ordinal and continuous variables were compared between patients with and without delayed endoscopy using Mann–Whitney tests. A multivariable analysis was then performed in order to identify independent predictors of delayed endoscopy. Prior to this analysis, Hosmer–Lemeshow tests were used to assess goodness of fit of continuous variables, which were categorised into ordinal groups where poor fit was detected. A multivariable binary logistic regression model was then produced, using a backwards stepwise approach to variable selection.
The times to endoscopy were then deconstructed into the time from admission to referral and from referral to endoscopy. These followed skewed distributions, and were therefore reported as medians and interquartile ranges (IQRs), and analysed using a non-parametric approach. Associations with ordinal or continuous variables were assessed using p-values from Spearman's rho correlation coefficients, with Mann–Whitney tests used for dichotomous factors. Patient outcomes were then compared between those patients with early and delayed endoscopies using a Mann–Whitney test for the length of stay, Fisher's exact test for the rebleed rate, and Kaplan–Meier curves with log-rank tests to assess the 30-d readmission and mortality rates.
All analyses were performed using IBM SPSS 22 (IBM Corp. Armonk, NY), with p<0.05 indicative of statistical significance throughout.
Results
Patient characteristics
A total of 348 patients were enrolled from 20 sites, with time to referral captured in 64% (N = 226) of patients. The cohort had a median age of 70 years (IQR: 54–81), and 61% were male. The median GBS was 10 (IQR 6–13); 6 patients (1.7%) had a GBS of 0. The most common endoscopic diagnosis was peptic ulcer (24%), with normal endoscopy in 21% (Figure 1). Overall, 30% of patients required endotherapy.
Figure 1.
Endoscopic diagnoses.
At presentation, 20.9% of patients had a shock index >1, while 14.0% met criteria for haemodynamic instability. The majority of patients underwent endoscopy in the endoscopy unit (94.5%), with the remaining procedures performed in theatre (4.9%) and in the ED (0.6%). Patients with haemodynamic instability were more likely to receive endoscopy in theatre (22.2% vs 2.2% of those without haemodynamic instability, p<0.001).
Time to endoscopy
The median time to endoscopy was 21.2 h (IQR: 12.0–35.7 h), which differed significantly across centres (p<0.001), with medians ranging from 9.2 to 42.5 h (Figure 2). Delayed endoscopy occurred in 41.1% of patients; this varied across centres, ranging from 31.0% to 87.5% (p = 0.002). The JAG standard of early endoscopy in ≥75% of patients was achieved in 4/20 (20%) of the centres assessed.
Figure 2.
Time to endoscopy (h) for each participating centre. *Denotes centres achieving the JAG standard of early endoscopy in 75% + of patients.
The times to endoscopy were then deconstructed into the time from admission to referral and from referral to endoscopy for the 226 patients where referral times were available. The median time to referral was 8.1 h (IQR 3.7–18.1 h), without significant variation among centres (p = 0.710), with medians ranging from 1.7 to 18.1 h. However, the time from referral to endoscopy (median 6.7 h, IQR 3.0–23.1 h) differed among centres (p = 0.007), with medians ranging from 2.5 to 27.3 h.
Factors associated with delayed endoscopy
Univariable analysis of factors associated with the time to endoscopy is reported in Table 1. Patients with out-of-hours admissions (19:00 to 07:00), suspected variceal bleeding (p<0.001), shock index > 1 (p = 0.016), haemodynamic instability (p<0.001), higher GBS (p<0.001), and those placed nil by mouth (p = 0.001) at index assessment were less likely to have delayed endoscopies. A significant association between delayed endoscopy and the time from admission to referral was also detected (p<0.001).
Table 1.
Univariable analysis of predictors of delayed endoscopy.
| N | Delayed endoscopya | p-value | |
|---|---|---|---|
| Age (years) | 348 | 0.728c | |
| <55 | 92 | 39 (42.4%) | |
| 55–69 | 82 | 31 (37.8%) | |
| 70–79 | 73 | 31 (42.5%) | |
| 80+ | 101 | 42 (41.6%) | |
| Gender | 348 | 0.057 | |
| Male | 214 | 79 (36.9%) | |
| Female | 134 | 64 (47.8%) | |
| Referral source | 348 | 0.213 | |
| Acute medicine | 49 | 16 (32.7%) | |
| Emergency department | 299 | 127 (42.5%) | |
| Arrival day | 348 | 0.368 | |
| Weekday | 267 | 106 (39.7%) | |
| Weekend | 81 | 37 (45.7%) | |
| Arrival time | 348 | <0.001 | |
| Day | 234 | 113 (48.3%) | |
| Night (19:00 to 07:00) | 114 | 30 (26.3%) | |
| Suspected variceal bleed | 347 | 0.019 | |
| No | 280 | 124 (44.3%) | |
| Yes | 67 | 19 (28.4%) | |
| Antithrombotic therapy at arrival | 348 | 0.429 | |
| No | 221 | 87 (39.4%) | |
| Yes | 127 | 56 (44.1%) | |
| Haemodynamic instability | 319 | <0.001 | |
| No | 275 | 122 (44.4%) | |
| Yes | 44 | 6 (13.6%) | |
| Shock index | 347 | 0.016 | |
| ≤1 | 274 | 122 (44.5%) | |
| >1 | 73 | 21 (28.8%) | |
| Major haemorrhagic stigmata on endoscopy | 343 | <0.001 | |
| No | 235 | 116 (49.4%) | |
| Yes | 108 | 26 (24.1%) | |
| GBS | 346 | <0.001 c | |
| 0–6 | 103 | 57 (55.3%) | |
| 7–12 | 148 | 64 (43.2%) | |
| 13+ | 95 | 22 (32.2%) | |
| NBM ( < 1 hb) | 312 | 0.001 | |
| No | 191 | 89 (46.6%) | |
| Yes | 121 | 34 (28.1%) | |
| Admission to referral (h) | 226 | <0.001 c | |
| <4 | 63 | 12 (19.0%) | |
| 4–7 | 49 | 21 (42.9%) | |
| 8–15 | 44 | 11 (25.0%) | |
| 16+ | 70 | 50 (71.4%) |
Data are reported as N (%), with p-values from Fisher's exact tests, unless stated otherwise. Bold p-values are significant at p<0.05.
Endoscopy > 24 h after arrival.
At index assessment, within 1 h of arrival.
p-value from a Mann–Whitney test, comparing the continuous variable between patients with and without delayed endoscopy. GBS: Glasgow-Blatchford score, NBM: nil by mouth.
Multivariable analysis was then performed to identify independent predictors of delayed endoscopy for the N = 189 (54.0%) patients with data available for all factors. Goodness-of-fit testing identified poor fit for time to referral as a continuous variable (Hosmer–Lemeshow test: p = 0.002), hence, this factor was divided into categories to ensure validity. The multivariable model (Table 2) found that patients admitted through acute medicine (p = 0.039), between 19:00 and 07:00 (p = 0.008) or with higher GBS (p<0.001) were significantly less likely to have a delayed endoscopy.After accounting for these factors, longer times to endoscopy referral (p<0.001) were found to be associated with higher rates of delayed endoscopy, with an odds ratio for delayed endoscopy of 2.66 and 1.44 for time to referral of 4–7 and 8–15 h, respectively, vs. < 4 h.
Table 2.
Multivariable analysis of delayed endoscopy.
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| Referral source (emergency department) | 3.11 (1.06–9.13) | 0.039 |
| Arrival day (weekend) | 0.38 (0.14–1.04) | 0.059 |
| Arrival time (1900 to 07:00) | 0.33 (0.15–0.75) | 0.008 |
| GBS (per unit increase) | 0.86 (0.79–0.94) | <0.001 |
| NBM ( < 1 ha) | 0.48 (0.22–1.06) | 0.071 |
| Admission to referral (hours) | <0.001 | |
| <4 | – | – |
| 4–7 | 2.66 (0.89–7.92) | 0.079 |
| 8–15 | 1.44 (0.42–4.98) | 0.563 |
| 16+ | 10.20 (3.65–28.53) | <0.001 |
Results are from a binary logistic regression model, with delayed endoscopy (>24 h from admission) as the dependent variable. Variables were selected using a backwards stepwise approach, and all factors in Table 1 were considered for inclusion. The final model is based on N = 189, after excluding cases with missing data.
Within 1 h of arrival. Bold p-values are significant at p < 0.05
Some factors were significantly associated with delayed endoscopy on univariable analysis, but not on multivariable analysis. This may have been due to correlations with other factors in the model. For example, patients with haemodynamic instability also had significantly higher GBS (median 12 vs. 9, p<0.001) and significantly shorter times from admission to referral (median 3.4 vs. 9.2 h, p = 0.003) than patients without haemodynamic instability, both of which were independently predictive of delayed endoscopy. Similar trends were also observed for the shock index, being placed nil by mouth at the index assessment and major haemorrhagic stigmata. As a result, the GBS and time from admission to referral effectively became surrogate markers of these factors, and superseded them in the multivariable model. Thus, the univariable analyses remain informative, reflecting the full gamut of factors associated with delayed endoscopy.
Factors associated with time to endoscopy
Univariable analysis was performed on time to endoscopy as a continuous outcome (Table 3), which yielded results that were consistent with the previously described analysis of delayed endoscopy. Additionally, this analysis deconstructed the times to endoscopy relative to the endoscopy referral. Some of the factors analysed were found to have differing impacts on these two periods. Patients admitted through acute medicine (p<0.001), daytime admission (p = 0.037), younger patients (p<0.001) and those on antithrombotic therapy (p = 0.004) had significantly shorter times from admission to referral. However, none of these factors was associated with significant differences in the times from referral to endoscopy. Conversely, time to referral was not affected by the day of admission (p = 0.410), but patients admitted at the weekend had significantly shorter times from referral to endoscopy than weekday admissions (p = 0.012). There was no significant difference between weekend/weekday admission status and whether endoscopy was performed in theatre or in the endoscopy unit (p = 0.341).
Table 3.
Univariable analysis of predictors of patient flow.
| Median (IQR) time in hours from: |
|||
|---|---|---|---|
| Admission to referral (N = 226) | Referral to endoscopy (N = 226) | Admission to endoscopy (N = 348) | |
| Age (years) | p<0.001 a | p = 0.539a | p = 0.069a |
| <55 | 5.7 (2.9–12.0) | 9.7 (3.8–20.2) | 20.5 (10.5–33.6) |
| 55–69 | 6.7 (3.2–14.6) | 5.9 (2.6–19.9) | 19.2 (9.2–28.9) |
| 70–79 | 10.1 (3.9–18.6) | 6.4 (3.2–25.2) | 21.9 (11.0–44.0) |
| 80+ | 14.4 (5.7–21.0) | 6.6 (3.6–23.4) | 22.5 (14.5–42.5) |
| Gender | p = 0.807 | p = 0.568 | p = 0.072 |
| Male | 7.9 (3.6–18.0) | 6.8 (3.2–22.0) | 20.3 (11.5–31.8) |
| Female | 9.5 (3.7–18.2) | 6.6 (3.0–23.6) | 23.2 (14.3–43.4) |
| Region | p = 0.217 | p = 0.124 | p = 0.493 |
| EM | 10.8 (3.7–18.8) | 6.1 (2.9–21.0) | 22.0 (15.0–32.3) |
| WM | 7.4 (3.7–15.6) | 9.7 (3.2–26.2) | 21.0 (11.3–38.8) |
| Admission | p<0.001 | p = 0.535 | p = 0.619 |
| Acute medicine | 3.7 (2.2–6.9) | 16.8 (3.7–22.7) | 21.6 (8.2–25.8) |
| Emergency department | 10.3 (4.4–18.3) | 6.2 (2.9–23.2) | 21.1 (12.0–38.3) |
| Arrival day | p = 0.410 | p = 0.012 | p = 0.770 |
| Weekday | 8.1 (3.6–17.0) | 8.3 (3.7–25.2) | 21.5 (11.9–33.2) |
| Weekend | 8.7 (3.9–21.9) | 4.3 (2.3–19.2) | 21.1 (12.0–44.3) |
| Arrival Time | p = 0.037 | p = 0.115 | p = 0.029 |
| Day | 6.5 (3.2–18.6) | 12.8 (3.2–23.6) | 23.6 (9.2–43.7) |
| Night (19:00 to 07:00) | 11.7 (7.1–16.3) | 4.7 (2.8–15.0) | 16.2 (12.6–32.3) |
| Variceal bleeding suspected | p = 0.032 | p = 0.009 | p = 0.001 |
| No | 9.3 (3.7–19.2) | 8.2 (3.7–24.5) | 22.3 (13.0–41.9) |
| Yes | 7.6 (2.3–11.9) | 3.9 (2.6–16.4) | 16.1 (7.1–27.0) |
| Antithrombotic therapy at arrival | p = 0.004 | p = 0.314 | p = 0.343 |
| No | 7.1 (3.4–15.9) | 6.8 (2.8–19.9) | 20.9 (11.7–32.3) |
| Yes | 11.9 (5.0–19.9) | 6.3 (3.6–25.4) | 22.3 (13.3–42.3) |
| Haemodynamically Unstable | p = 0.003 | p = 0.020 | p<0.001 |
| No | 9.2 (3.9–18.6) | 6.9 (3.1–23.4) | 22.3 (13.0–38.8) |
| Yes | 3.4 (1.5–10.2) | 3.9 (2.4–10.8) | 11.5 (5.8–17.6) |
| Shock index | p = 0.003 | p = 0.205 | p = 0.003 |
| ≤1 | 10.0 (4.0–19.6) | 7.1 (3.2–23.5) | 22.3 (13.3–42.3) |
| >1 | 5.4 (2.7–12.0) | 4.4 (2.7–19.9) | 16.5 (8.5–26.0) |
| GBS | p = 0.012 a | p<0.001 a | p<0.001 a |
| 0–6 | 11.7 (4.3–21.6) | 17.5 (4.3–28.8) | 26.4 (16.5–52.6) |
| 7–12 | 9.4 (3.9–18.0) | 6.5 (2.9–23.4) | 21.6 (12.5–40.7) |
| 13+ | 6.3 (2.8–14.4) | 4.1 (2.6–16.6) | 16.1 (7.9–23.9) |
| Major haemorrhagic stigmata on endoscopy | p = 0.006 | p<0.001 | p<0.001 |
| No | 10.1 (4.1–19.2) | 14.2 (3.8–26.2) | 24.0 (16.3–47.9) |
| Yes | 7.4 (2.2–12.0) | 4.0 (2.5–7.4) | 13.5 (7.0–22.9) |
| NBM ( < 1 hb) | p<0.001 | p = 0.015 | p<0.001 |
| No | 11.6 (5.0–21.6) | 8.3 (3.7–24.5) | 22.9 (15.1–45.2) |
| Yes | 5.4 (2.7–14.1) | 4.3 (2.6–17.7) | 15.4 (7.2–25.4) |
| Location of endoscopyc | p = 0.122 | p = 0.010 | p<0.001 |
| Endoscopy unit | 8.4 (3.7–18.1) | 6.9 (3.0–23.3) | 21.7 (12.6–38.3) |
| Theatres | 4.8 (1.6–8.4) | 2.8 (1.6–4.1) | 9.8 (4.3–16.6) |
p-values are from Mann-Whitney tests, unless stated otherwise, and bold p-values are significant at p<0.05.
p-Value from Spearman's rho, using the continuous version of the factor.
Within 1 h of arrival. WM: West Midlands, EM: East Midlands, GBS: Glasgow-Blatchford score, NBM: nil by mouth, IQR: Interquartile range.
Impact of delayed endoscopy on patient outcomes
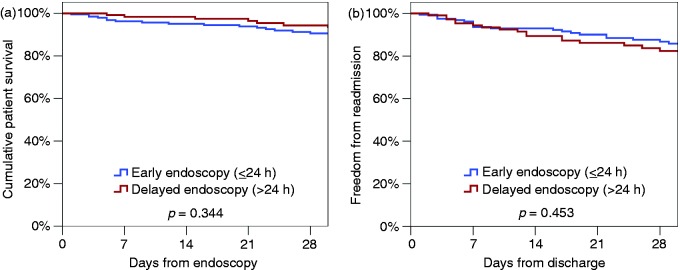
Patient outcomes, stratified according to early or delayed endoscopy, are presented in Table 4. The overall rate of 8-d rebleeding was 9.4%, which was higher in the early endoscopy cohort (12.3% vs. 5.6%, p = 0.041). Early endoscopy was also associated with a significantly shorter length of stay, with a difference of 1 d between the two groups (median: 4 vs. 5d, p = 0.004). On Kaplan–Meier analysis, no significant difference was detected between the early and delayed endoscopy groups, with 30-d mortality rates of 9.4% vs. 6.9% (p = 0.344, Figure 3(a)) and 30-d post-discharge readmission rates of 14.1% vs. 17.6% (p = 0.453, Figure 3(b)).
Table 4.
Outcomes of early and delayed endoscopy groups.
| Outcome | Time to endoscopy |
p-value | |
|---|---|---|---|
| Early (0–24 h) | Delayed (>24 h) | ||
| Rebleed within 8 d of endoscopy | 25 (12.3%) | 8 (5.6%) | 0.041 |
| Readmissions within 30 d of discharge | 20 (14.1%) | 17 (17.6%) | 0.453 |
| Deaths within 30 d of endoscopy | 16 (9.4%) | 7 (6.9%) | 0.344 |
| Length of stay (days) | 4 (2–7) | 5 (4–8) | 0.004 |
Length of stay is reported as median (interquartile range), with p-value from a Mann–Whitney test. Rebleeding is reported as N (%), with p-value from Fisher's exact test. Readmission/mortality are reported as N (Kaplan–Meier estimate), with p-values from log-rank tests. Analyses of length of stay and post-discharge readmissions are based on N = 330, after excluding N = 10 inpatient deaths and N = 8 patients where discharge dates were unavailable. Bold p-values are significant at p<0.05.
Figure 3.
Kaplan-Meier plots illustrating rates of 30-d survival (a) and readmission (b), stratified by time to endoscopy.
Discussion
This trainee-led audit of AUGIB has identified ongoing deficits in meeting time to endoscopy quality standards in the Midlands, UK. Delayed endoscopy occurred in 41% of patients, exceeding the 35% figure reported in the last NCEPOD audit.7 Only 20% of units met the early endoscopy standard set by JAG. Importantly, factors affecting time to endoscopy, and its components of time to referral and time from referral to endoscopy, were identified. To our knowledge, such analyses have not previously been performed either within or outside the National Health Service (NHS). As time to referral is primarily reliant on acute departments, and referral to endoscopy determined by endoscopy departments, this allows an arbitrary delineation of systems processes affecting time to endoscopy. Our data highlight potential mismatches in service demands between acute departments and endoscopy units and provide insight into areas that may be optimised. For instance, patients admitted through acute medicine, during the daytime, younger patients, and those on antithrombotic therapy had significantly lower referral times, but did not impact on referral to endoscopy times, whereas the opposite was true for weekend admissions. Reassuringly, both time to referral and time from referral to endoscopy were significantly lower in patients with more severe presentations, i.e. higher GBS, haemodynamic instability, suspected variceal bleeding, and those with haemorrhagic stigmata on endoscopy, suggesting alignment of priorities in sicker patients for endoscopy by acute departments and endoscopy units.
Attainment of time to endoscopy targets is dependent on close integration between acute departments and the endoscopy unit. Although time to endoscopy is a quality metric that potentially determines the JAG accreditation status of an endoscopy unit, the time to referral is largely dependent on frontline services, and may be beyond the control of the endoscopy department. Patients admitted via ED waited longer before being referred compared to those admitted via acute medicine. From our experience within the NHS, patients who present with AUGIB to the ED are triaged and empirically managed, before being transferred to an acute medical ward for ongoing care. Often, the decision to refer for endoscopy in non-life-threatening cases of AUGIB is undertaken by the acute medical rather than the ED teams, which can incur delays. While it is possible to streamline the referral pathways through AUGIB protocols, improved access to the duty endoscopist, acute gastroenterologist liaison services etc., earlier endoscopy referral at the ED level could be considered to reduce time to endoscopy. Nonetheless, there may also be scope to optimise the referral to endoscopy window. The observation that time to endoscopy was longer for daytime admissions, despite earlier referral times, could reflect difficulties of endoscopy units to accommodate additional cases when operating at full capacity. This is consistent with the finding of shorter referral to endoscopy times for weekend admissions, when endoscopy units typically have less planned activity.
Previous studies have assessed the impact of early endoscopy (<24 h),8–12 urgent endoscopy (6–12 h)13–15 and very early endoscopy (<2–6h)16,17 on patient outcomes. The impact of early endoscopy on mortality is conflicting; some have shown no significant difference,8,9 while two recent studies11,12 reported this to be protective of mortality. Several retrospective studies have associated early endoscopy with increased likelihood of haemorrhagic stigmata.14,17 However, this interpretation may be confounded by reverse causality, as patients with severe AUGIB are likely to undergo early endoscopy. Therefore, the preponderance of high-risk endoscopic stigmata and rebleeding in our early endoscopy group may denote such selection bias. Despite this, we did not show an association between early endoscopy and mortality, but did uncover a modest but statistically significant reduction in length of stay, which can benefit patient satisfaction, healthcare costs and hospital bed availability.
Several limitations require acknowledgement. First, referral times and 30-d outcomes were not fully available, which may have reduced the statistical power of our analyses. Second, as the study was undertaken between November and December, where acute departments are vulnerable to ‘winter pressures’, our data may not be representative of practice during less intense periods. Third, investigators were not physically assigned to acute departments to record the timings of specific interventions. Although electronic timestamps provide accurate records of time to endoscopy outcomes, desirable data fields to further evaluate the referral pathway, e.g. time of first decision for endoscopy or telephone discussion/referral to endoscopy team were inconsistently recorded within case records and excluded. This prevented accurate process mapping of the patient journey. Fourth, inpatients with AUGIB while hospitalised for another condition were excluded; this cohort is associated with higher mortality and may benefit from earlier endoscopy. Consequently, our time to endoscopy may be longer and rates of mortality and rebleeding lower than previous audits. Finally, our analyses were centred at patient-level. The involvement of 20 centres was insufficient to permit unit-level analysis. While it is acknowledged that there was heterogeneity in care offered, potentially confounding parameters relevant to endoscopy units (e.g. endoscopy volume, accreditation status, access to 24-h on-site endoscopy, endoscopy rotas, type of hospital, availability of dedicated inpatient lists) and acute departments (e.g. ED waiting times, referral protocols) were not studied, which should be considered for future research.
Moving forward, the British Society of Gastroenterology (BSG) Endoscopy Quality Improvement Project comprises a series of initiatives to improve standards of care in endoscopy. Amongst these is the development of a national AUGIB quality bundle, which can be implemented in acute departments to facilitate early management, risk stratification and referral, with the objective of improving time to endoscopy and patient outcomes. Such interventions may ultimately pave way for the attainment of quality standards in AUGIB.
Conclusion
In conclusion, the majority of centres did not meet national standards for time to endoscopy. Factors associated with delayed endoscopy included time and route of admission, severity scoring and the timing of endoscopy referral. Early endoscopy may reduce length of stay, but is dependent on prompt assessment and referral. Strategic initiatives involving acute care services are likely to be required to improve this outcome.
Authorship statement
This study was jointly performed by WMRIG and GARNet trainee collaborative networks. All authors contributed to the study and approved the manuscript.
Declaration of conflicting interests
Keith Siau is a research fellow for the Joint Advisory Group on Gastrointestinal Endoscopy.
Ethics approval
Formal ethics approval was not required as per departmental policy and Health Research Authority guidance. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's review board.
Funding
WMRIG and GARNet have been awarded a research network grant by the charity Guts UK.
Informed consent
Patient consent was not required as per departmental policy and Health Research Authority guidance.
References
- 1.Button LA, Roberts SE, Evans PA, et al. Hospitalized incidence and case fatality for upper gastrointestinal bleeding from 1999 to 2007: a record linkage study. Aliment Pharmacol Ther 2011; 33(1): 64–76. [DOI] [PubMed] [Google Scholar]
- 2.NICE. Acute upper gastrointestinal bleeding in over 16s: management. Available from: https://www.nice.org.uk/guidance/cg141. (2012, accessed 20 February 2018).
- 3.SIGN. Management of acute upper and lower gastrointestinal bleeding. Available from: http://www.sign.ac.uk/assets/sign105.pdf (2008, accessed 20 February 2018).
- 4.Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015; 47(10): a1–46. [DOI] [PubMed] [Google Scholar]
- 5.Hearnshaw SA, Logan RF, Lowe D, et al. Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: results of a nationwide audit. Gut 2010; 59(8): 1022–1029. [DOI] [PubMed] [Google Scholar]
- 6.Joint Advisory Group on Gastrointestinal Endoscopy. Joint Advisory Group on Gastrointestinal Endoscopy (JAG) Global Rating Scale (GRS): Version for acute services (all nations) – April 2016; 2016: 10. [Google Scholar]
- 7.NCEPOD. Time to Get Control? A review of the care received by patients who had a severe gastrointestinal haemorrhage. Available from: http://www.ncepod.org.uk/2015report1/downloads/TimeToGetControlFullReport.pdf (2015, accessed 19 October 2018).
- 8.Lanas A, Aabakken L, Fonseca J, et al. Variability in the management of nonvariceal upper gastrointestinal bleeding in Europe: an observational study. Adv Ther 2012; 29(12): 1026–1036. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel BM, Vakil NB, Ofman JJ. Endoscopy for acute nonvariceal upper gastrointestinal tract hemorrhage: is sooner better? A systematic review. Arch Intern Med 2001; 161(11): 1393–1404. [DOI] [PubMed] [Google Scholar]
- 10.Lim LG, Ho KY, Chan YH, et al. Urgent endoscopy is associated with lower mortality in high-risk but not low-risk nonvariceal upper gastrointestinal bleeding. Endoscopy 2011; 43(4): 300–306. [DOI] [PubMed] [Google Scholar]
- 11.Garg SK, Anugwom C, Campbell J, et al. Early esophagogastroduodenoscopy is associated with better outcomes in upper gastrointestinal bleeding: a nationwide study. Endosc Int Open 2017; 5(5): E376–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong N, Kim KS, Jung YS, et al. Delayed endoscopy is associated with increased mortality in upper gastrointestinal hemorrhage. Am J Emerg Med. Epub ahead of print 1 June 2018. DOI: 10.1016/j.ajem.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 13.Lin HJ, Wang K, Perng CL, et al. Early or delayed endoscopy for patients with peptic ulcer bleeding. A prospective randomized study. J Clin Gastroenterol 1996; 22(4): 267–271. [DOI] [PubMed] [Google Scholar]
- 14.Bjorkman DJ, Zaman A, Fennerty MB, et al. Urgent vs. elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastrointest Endosc 2004; 60(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 15.Targownik LE, Murthy S, Keyvani L, et al. The role of rapid endoscopy for high-risk patients with acute nonvariceal upper gastrointestinal bleeding. Can J Gastroenterol 2007; 21(7): 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JG, Turnipseed S, Romano PS, et al. Endoscopy-based triage significantly reduces hospitalization rates and costs of treating upper GI bleeding: a randomized controlled trial. Gastrointest Endosc 1999; 50(6): 755–761. [DOI] [PubMed] [Google Scholar]
- 17.Schacher GM, Lesbros-Pantoflickova D, Ortner MA, et al. Is early endoscopy in the emergency room beneficial in patients with bleeding peptic ulcer? A “fortuitously controlled” study. Endoscopy 2005; 37(4): 324–328. [DOI] [PubMed] [Google Scholar]