Abstract
We have previously shown that Gs-coupled adenosine receptors (A2a) are primarily involved in adenosine-induced human pulmonary artery endothelial cell (HPAEC) barrier enhancement. However, the downstream events that mediate the strengthening of the endothelial cell (EC) barrier via adenosine signaling are largely unknown. In the current study we tested the overall hypothesis that adenosine-induced Rac1 activation and EC barrier enhancement is mediated by Gs-dependent stimulation of cAMP-dependent Epac1-mediated signaling cascades. Adenoviral transduction of HPAEC with constitutively-active (C/A) Rac1 (V12Rac1) significantly increases transendothelial electrical resistance (TER) reflecting an enhancement of the EC barrier. Conversely, expression of an inactive Rac1 mutant (N17Rac1) decreases TER reflecting a compromised EC barrier. The adenosine-induced increase in TER was accompanied by activation of Rac1, decrease in contractility (MLC dephosphorylation), but not Rho inhibition. Conversely, inhibition of Rac1 activity attenuates adenosine-induced increase in TER. We next examined the role of cAMP-activated Epac1 and its putative downstream targets Rac1, Vav2, Rap1 and Tiam1. Depletion of Epac1 attenuated the adenosine-induced Rac1 activation and the increase in TER. Furthermore, silencing of Rac1 specific guanine nucleotide exchange factors (GEFs), Vav2 and Rap1a expression significantly attenuated adenosine-induced increases in TER and activation of Rac1. Depletion of Rap1b only modestly impacted adenosine-induced increases in TER and Tiam1 depletion had no effect on adenosine-induced Rac1 activation and TER. Together these data strongly suggest that Rac1 activity is required for adenosine-induced EC barrier enhancement and that the activation of Rac1 and ability to strengthen the EC barrier depends, at least in part, on cAMP-dependent Epac1/Vav2/Rap1-mediated signaling.
Keywords: Small GTPase, Adenosine, Rac1, pulmonary endothelium, barrier protection
Introduction
Pulmonary vascular endothelial cells (EC) function as a semi-permeable diffusion barrier between the circulating blood and surrounding tissues. EC regulate the trafficking of fluid, macromolecular transport and recruitment of leukocytes to sites of inflammation (Bazzoni and Dejana, 2004; Dudek and Garcia, 2001). Loss of endothelial barrier integrity is an important component of pulmonary vascular diseases that include acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (Matthay et al., 2012). Toxic or inflammatory agents such as thrombin, or the endotoxin lipopolysaccharide (LPS), promote cytoskeletal rearrangement, and contractile responses of EC that lead to disruption of intercellular contacts and ultimately increases in EC permeability (Dudek and Garcia, 2001; Pugin, 1999; Sukriti et al., 2014). Barrier destructive agents are opposed by mechanisms that maintain the integrity of the EC barrier. The breakdown product of ATP, adenosine, is an essential purine nucleoside that can protect against thrombin-induced EC permeability in vitro (Hassanian et al., 2014; Umapathy et al., 2010a) and LPS-induced vascular leak and inflammation in vivo (Gonzales et al., 2014).
The pulmonary vasculature is comprised of a heterogeneous population of EC. The function of the EC barrier, surface biochemistry, and morphology of confluent monolayers of microvascular and macrovascular endothelial cells are examples of two distinct types of EC (Kasa et al., 2015; Ren et al., 2013; Stevens, 2011). Microvascular cells are the focus of many studies, although pulmonary artery endothelial cells (macrovascular EC) are worthy of consideration in studies of vascular diseases studies as they crucially participate in blood homeostasis, blood-tissue exchange regulation under various conditions and are essential member of the vascular tree (Terramani et al., 2000).
Adenosine is an extracellular signaling molecule that plays an important role in numerous biological functions such as nucleotide biosynthesis and cellular energy metabolism (Hasko et al., 2008). In disease conditions such as inflammation, hypoxia or acute injury, extracellular adenosine is generated from the precursor molecules 5’-adenosine triphosphate (ATP) and 5’-adenosine monophosphate (AMP) in a two-step enzymatic reaction (Eltzschig et al., 2003). Adenosine elicits a wide range of physiological responses by binding to and activating four distinct cell surface adenosine receptors (ARs): A1, A2A, A2B, and A3 (Eltzschig, 2009). The four ARs are 7 transmembrane spanning receptors that couple to GTP-binding proteins. Activation of AR receptors inhibit inflammatory pathways (Hasko and Pacher, 2008). Different ARs can activate different signaling pathways in a cell- and tissue-specific manner (Hasko and Cronstein, 2004; Hasko et al., 1996). Previous studies have been shown that all four ARs are widely distributed in the pulmonary endothelium (Balestrieri et al., 1998; Eckle et al., 2008; Wilson and Batra, 2002). We have recently shown that HPAEC preferentially express the A2A and A2B adenosine receptors and that Gαs-coupled A2A is the major effector of adenosine-induced barrier enhancement (Umapathy et al., 2010a). It is widely accepted that adenosine-induced engagement of Gs leads to increases in cAMP (cyclic adenosine 3′,5′-monophosphate) followed by activation of PKA (protein kinase A) and Epac1 (exchange protein directly activated by cAMP) (Bogatcheva et al., 2009; Parnell et al., 2012; Patterson et al., 2000). We, and others, have also shown that activation of Epac1 is directly involved in EC barrier preservation (Bogatcheva et al., 2009; Lorenowicz et al., 2008), however, the molecular links between adenosine-induced EC barrier enhancement and Epac1 activation are not yet clear.
Rac1 (Ras-related C3 botulinum toxin substrate 1) is a member of the Rho family of small GTPases. Rac1 has been reported to influence numerous intracellular pathways and cellular functions such as cytoskeleton arrangement, gene expression, cell adhesion and proliferation, ROS (reactive oxygen species) production and inflammation (Bosco et al., 2009; Etienne-Manneville and Hall, 2002) (Marinkovic et al., 2015). Active, GTP-bound Rac1 is able to quickly activate its downstream effectors, while GDP-bound active Rac1 is inactive. Guanine nucleotide exchange factors (GEFs) facilitate the exchange of the GDP to GTP, thus activating Rac1. Conversely, GAPs (GTPase activating factors) and GDIs (guanine nucleotide dissociation inhibitors) reduce Rac1 activity (Hakoshima et al., 2003; Kaibuchi et al., 1999). In addition to these mechanisms, Kwon and colleagues have revealed that Rac1 can be regulated by phosphorylation, which inhibits its binding to GTP (Kwon et al., 2000). Epac1 is a cAMP binding protein with GEF activity (Grandoch et al., 2010) discovered in 1998 by deRooij and colleagues (de Rooij et al., 1998). Epac1 activation is able to couple cAMP production to the activation of Rap1a and Rap1b and also to PI3K (phosphoinositide 3-kinase) (Bos, 2003; de Rooij et al., 1998). The C-terminal of Epac1 is responsible for nucleotide exchange and the N-terminal contains the DEP (Dishevelled, Egl-10, Plekstrin) domain, responsible for the membrane attachment. Epac1 also has one cAMP-domain. In addition to binding Rap1-GTPase, Epac1 is able to interact with a number of Ras-GTPases (Bos, 2006; Holz et al., 2006). Tiam1 (T-lymphoma invasion and metastasis-inducing protein 1) and Vav2 are other Rac1-specific GEFs that are able to activate Rac1 without direct phosphorylation by PKA (Birukova et al., 2007c) (Lampugnani et al., 2002). Tiam1 regulates the organization of actin cytoskeleton and promotes the induction of membrane ruffling and invasion in T-lymphoma cells in fibroblasts via activating Rac1 (Michiels et al., 1995). Tiam1, induced by atrial natriuretic peptide (ANP), protects the pulmonary EC barrier against thrombin (Birukova et al., 2008), however, the role of Tiam1 in adenosine-induced EC barrier enhancement has not been described.
In this study our goal was to elucidate the mechanisms underlying adenosine-induced endothelial barrier enhancement in human pulmonary artery EC focusing on the role of the Epac1/Rac1 axis. Herein we have uncovered an important role for Epac1-induced signaling cascades involved in the activation of Rac1 and strengthening of the endothelial barrier.
Materials and Methods
Chemicals and Reagents
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless indicated otherwise. Antibodies were from Cell Signaling Technology, Inc. (Danvers, MA): Epac1, Vav2, Rap1a, Rap1b, Tiam, diphospho-MLC (Thr18/Ser19) and phospho-MYPT1 (Thr853). All antibodies are rabbit monoclonal antibodies. HRP-linked anti-actin, and GAPDH were rabbit monoclonal antibodies. ECIS arrays (8W10E+) were from Applied BioPhysics (Troy, NY). Rac1 G-LISA Activation Assay Kit was purchased from Cytoskeleton Inc. (Denver, CO). LacZ adenoviral control vector was obtain from Invitrogen (Carlsbad, CA).
Cell Culture
Human pulmonary artery endothelial cells (HPAEC) obtained from Lonza Group Ltd. (Walkersville, MD) were propagated in culture medium EGM-2-MV (Lonza) supplemented with 5% (v/v) fetal bovine serum (FBS; HyClone, Waltham, MA) and used at passages 3–7. All cells were maintained at 37 °C in a humidified atmosphere of 5% CO2.
Production of adenovial Rac1 constructs
Adenoviruses encoding constitutively active (C/A) or dominant negative (D/N) mutant- of human Rac1 (GFP-V12 and GFP-N17, respectively) were generated using pADdest according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Adenoviral constructs were amplified in HEK293 cells and purified by CsCl banding and dialyzed and titer was estimated by spectrophotometric analysis as shown previously (Zhang et al., 2008). The resulting adenoviral Rac1 constructs were utilized for HPAEC infection at 1000 multiplicities of infection (MOI).
Small interfering RNA-mediated protein depletion
HPAEC were transfected with 50 nM of Epac1, Vav2, Rap1a, Rap1b and Tiam1 small interfering RNA (siRNA) (GE Dharmacon, Lafayette, CO) using SiPort Amine transfection reagent (Thermo Fisher Scientific Ambion, Grand Island, NY) according to the manufacturer’s instructions. Non-targeting siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a negative control.
Western blot analysis
Immunoblotting was performed as we have described previously (Kasa et al., 2013; Kovacs-Kasa et al., 2016). Membranes were probed with antibodies against proteins of interest, then scraped and re-probed with anti-β-actin or GAPDH to normalize protein loading.
Measurements of transendothelial electrical resistance of EC monolayer
Transendothelial electrical resistance (TER) was measured using an electrical cell substrate impedance sensing system (ECIS; Applied Biophysics, Troy, NY) as we have previously described (Kovacs-Kasa et al., 2016). HPAEC transfected with siRNAs of interest or infected with adenoviral Rac1 constructs were plated on gold microelectrodes and TER was measured 72 or 12 hrs later (after silencing or adenoviral infection, respectively).
Rac1 activity measurement
Rac1 activation was measured using Rac1 G-LISA Activation Assay Kit (Cytoskeleton, Denver, Co) according to the manufacturer’s description. Briefly, HPAEC were transfected either with control, non-targeting siRNA or with siRNAs specific for protein of interest (listed above). After 72 hours of post transfection time cells were treated with adenosine (50 μM) for different time periods (0, 5, 10, 15, 30 min). Cells were washed with PBS and harvested than snap frozen. Next day cells were lysed with G-LISA solution (Cytoskeleton, Denver, Co) and lysates were incubated on Rac1-GTP affinity plates for 30 minutes. After washing step the active, GTP bound Rac1 is detected with Rac1 specific primary antibody. Following another washing step Rac1 activation was measured with HRP detection reagents.
Rho kinase (ROCK) activity was measured using western blotting of cell lysates with anti-phospho MYPT1 (Thr853) antibody (Fukata et al., 2001).
Statistics
Values are expressed as mean ± SEM of at least 3 independent experiments. Analysis of variance (ANOVA) or Student’s t test was used, as appropriate. A p value of < 0.05 was considered statistically significant.
Results
Rac1 activity modulation affects transendothelial electrical resistance
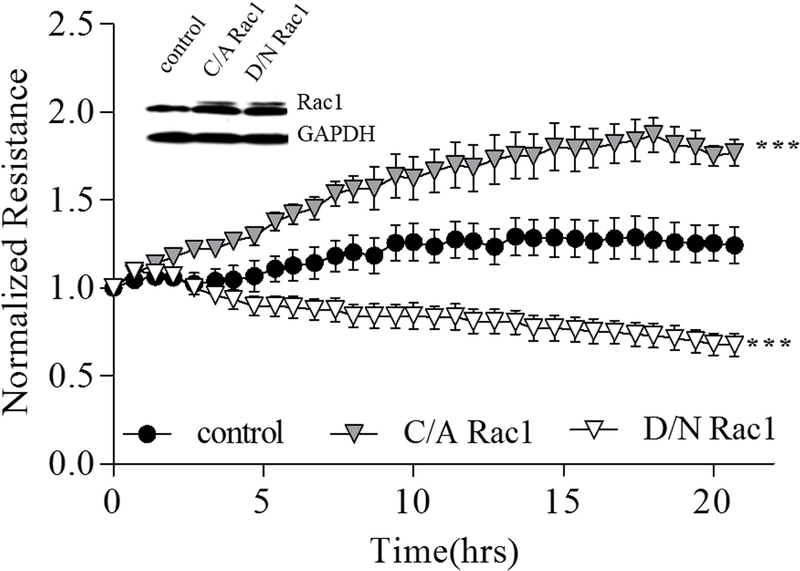
To elucidate the involvement of Rac1 activity in EC barrier regulation we infected HPAEC with C/A or D/N Rac1 adenoviral constructs and measured TER of EC monolayers. The active form of Rac1 significantly increased TER reflecting EC barrier enhancement. In contrast, infection of EC with the inactive form of Rac1 decreased basal TER reflecting EC barrier compromise (Figure 1). These data suggest that Rac1 activity is crucial in the maintenance of endothelial barrier integrity.
Figure 1. Rac1 signaling is involved in HPAEC barrier regulation.
To monitor the effect of Rac1 mutants on the resistance of HPAECs, cells were cultured on ECIS plate until they reached 80% confluence and incubated with basal medium for 8 hours before infection. Each well was infected with 1000 MOI of either vector (control), catalytically active (C/A) or dominant negative (D/N) Rac1 mutant adenoviral constructs. After overnight incubation with adenovirus, the medium was replaced with fresh complete EBM-2 medium containing 5% FBS, and the change of resistance was recorded for 20 hours. Normalized resistance was calculated and plotted as a function of time. The inserted western blot data shows the increased expression of Rac1 induced by infection with adenoviral constructs, and GAPDH was used as a loading control. *** p< 0.0001 vs. control; n=3.
Adenosine induces Rac1 activation in Rho-independent manner
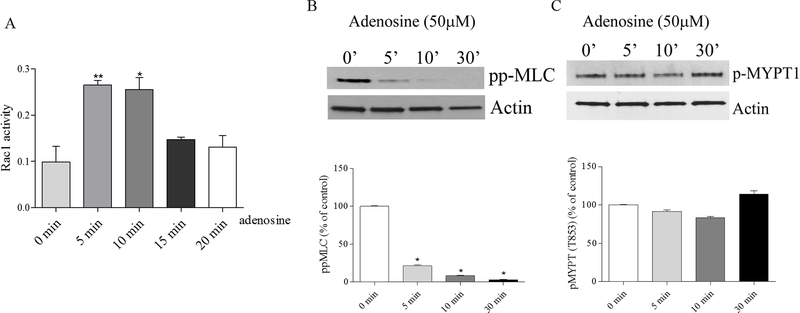
We have previously shown that extracellular purines, ATP and its degradation product, adenosine, enhances EC barrier in HPAEC, which accompanied by decrease in myosin light chain (MLC) phosphorylation with maximal effect at 30 min reflecting EC contractility inhibition (Kolosova et al., 2005; Umapathy et al., 2010a). ATP-induced EC barrier enhancement is dependent upon Rac1 activity (Jacobson et al., 2006). To analyze whether adenosine-induced HPAEC barrier enhancement correlates with Rac1 activation we analyzed Rac1 activity at different time points after adenosine stimulation. Figure 2A demonstrates that adenosine transiently increases Rac1 activity with a maximal effect at 5 min. This maximal activation of Rac1 is accompanied by decrease in MLC phosphorylation (an index of contractility), but not changes in MYPT1 phosphorylation (an index of Rho activation) (Fig. 2B), and precedes maximal adenosine-induced increase in TER (Figure 3) suggesting that Rac1 activation may be involved in the initiation of adenosine-induced EC barrier strengthening in Rho-independent manner.
Figure 2.
A. Adenosine stimulates Rac1 activity in HPAEC. To measure Rac1 activity in adenosine- treated HPAEC, cells were harvested at different time points (5, 10, 15, and 20 min) and Rac1 G-LISA Activation Assay Kit was used according to the manufacturer’s instructions. Cells were serum starved for 2 hours before adenosine treatment and Rac1 activity was measured, n=4; mean +/− SEM * p<0.05 compared to 0 min control. B and C. Adenosine decreases MLC, but not MYPT1 phosphorylation. HPAEC were treated with adenosine at different time points. Cells were harvested and western blots were performed to detect changes in phosphorylated MLC (Thr18/Ser19) and MYPT1 (Thr 853) levels (panels B and C, respectively). Actin was used as a loading control. Bar graphs show the changes of phospho- MYPT1 and diphospho-MLC quantified with scanning densitometry. Results are expressed as Mean and SEM (n=4 experiments). *<0.05 vs. control.
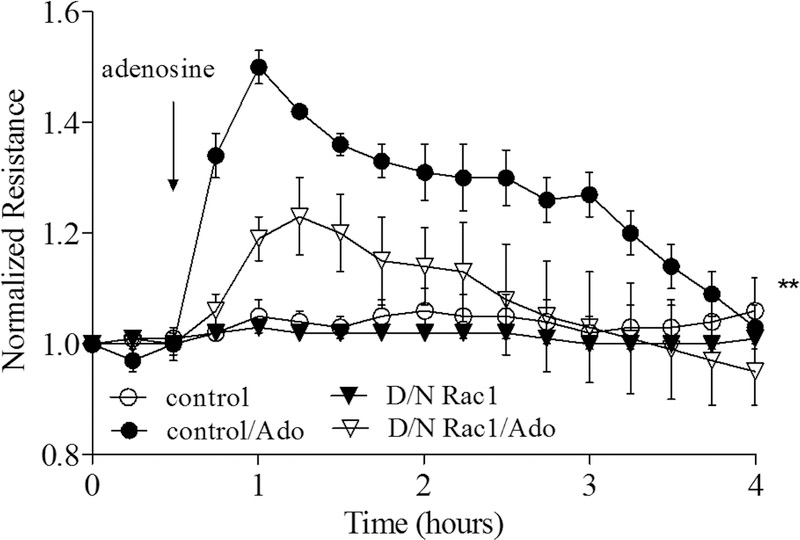
Figure 3. Attenuating Rac1 activity mitigates the increase in TER induced by adenosine in HPAEC.
To monitor the effect of D/N Rac1 on adenosine-induced TER increase of HPAECs, cells were infected with 1000 MOI of either vector (control) or D/N Rac1. After 24 hour incubation in complete cell culture medium, the change of resistance in the response to adenosine (50 μM) was measured. n=4; mean +/− SEM; **p < 0.01 vs. control/Ado. The arrow indicates the time that adenosine was added.
Involvement of Rac1 in adenosine-induced EC barrier enhancement
To directly access the role of Rac1 activation in endothelial permeability we transduced HPAEC with control LacZ or D/N Rac1 adenoviral constructs, then stimulated cells with adenosine while monitoring changes in TER. Figure 3 demonstrates that adenosine significantly increases TER in the control LacZ-transduced cells with a maximum effect observed at ~30 min after adenosine exposure. Depletion of Rac1 using siRNA-based approach does not affect basal TER (Supplemental Figure 1A), but markedly attenuates the adenosine-induced increase in TER (Figure 3) indicating the involvement of Rac1 activity in adenosine-induced endothelial barrier enhancement.
Epac1 is involved in adenosine-induced changes in EC permeability and Rac1 activation
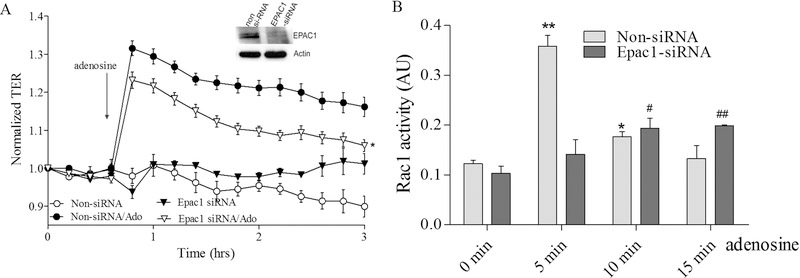
To elucidate signaling pathways involved in adenosine-induced Rac1-mediated EC barrier enhancement we initially clarified the role of the Rac1 cAMP-dependent GEF, Epac1. While down regulation of Epac1 has no effect on initial TER in control (Supplemental figure 1B), it significantly attenuates the positive effect of adenosine (Figure 4A) indicating the involvement of Epac1 in adenosine-induced EC barrier enhancement. Importantly, the depletion of Epac1 also attenuates the adenosine-mediated increase in Rac1 activity (Figure 4B) supporting the involvement of Epac1 in upstream events leading to Rac1 activation by adenosine.
Figure 4. Depletion of Epac1 attenuates adenosine-induced increase in TER and Rac1 activation in pulmonary endothelial cells.
A. HPAEC were treated with Epac1 specific siRNA or non-targeting siRNA and then challenged with 50 μM adenosine in TER measurement assay. Depletion of Epac1 significantly attenuated adenosine-induced endothelial barrier enhancement. n=3; mean +/− SEM; * p<0.05 vs. Non-siRNA/Ado. B. Effect of adenosine on Rac1 activation in Epac1 depleted cells. HPAEC were transfected with Epac1 specific siRNA or non-specific siRNA and then 72 hours later challenged with 50 μM adenosine at different time points in Rac1 activity assay. Cells were serum starved for 2 hours before adenosine treatment and Rac1 activity was measured according to the manufacturer’s protocol. n=4; mean +/− SEM; * p<0.05 vs. Non-siRNA/0 min, **P < 0.01 vs. Non-siRNA/0 min, # p<0.05 vs. Epac1-siRNA/0 min, ##p < 0.01 vs. Epac1-siRNA/0 min.
The role of Epac1 downstream targets in adenosine-induced changes in EC permeability and Rac1 activation
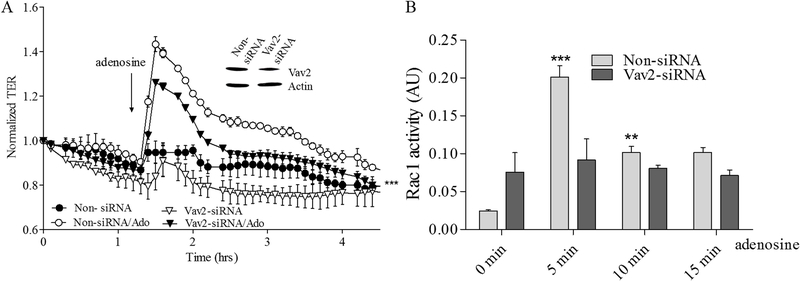
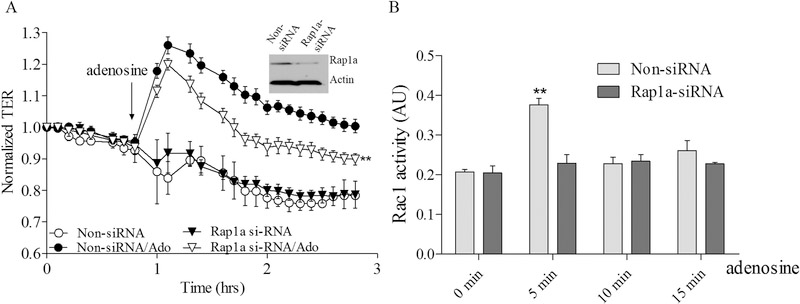
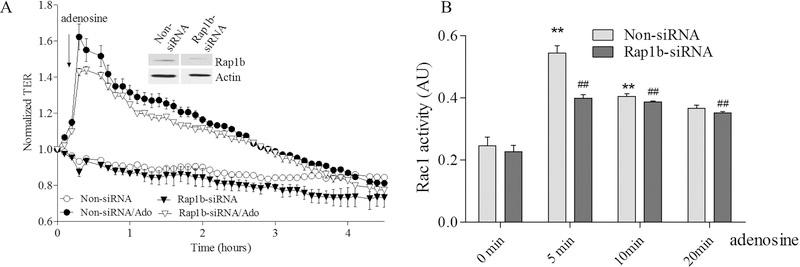
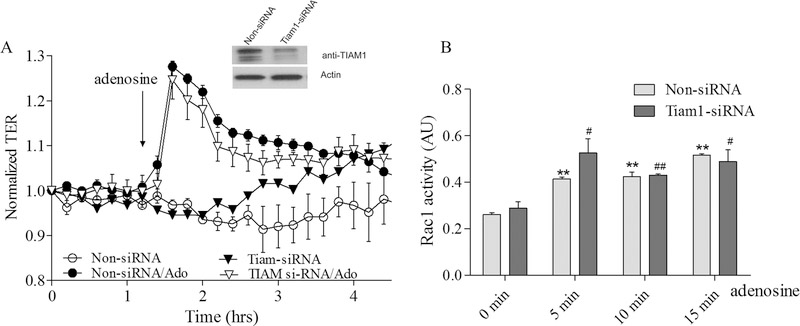
To further clarify the role of Epac1-mediated signaling in adenosine-induced EC barrier enhancement and Rac1 activation we sequentially depleted the putative downstream Epac1 targets Vav2, Rap1a, Rap2b and Tiam1 again using an siRNA approach and determine the effects on adenosine-induced changes in TER and Rac1 activity. Our data on Supplemental figures 1 C–F demonstrate that depletion of either one of these proteins has no effect on initial TER values in control cells. The down-regulation of Vav2 significantly attenuates both the adenosine-mediated increase in TER (Figure 5A) and Rac1 activation (Figure 5B). Similarly the down-regulation of Rap1a also attenuated both parameters (Figure 6A&B) indicating that both Vav2 and Rap1a are involved in adenosine-induced EC barrier enhancement likely through inhibition of adenosine-induced Rac1 activation. Interestingly, Rap1b depletion only modestly affected maximal adenosine-induced TER changes while substantially affected Rac1 activation (Figure 7A&B) supporting distinct role of Rap1 isoforms in EC barrier regulation. Surprisingly, depletion of Tiam1 was ineffective towards both adenosine-induced changes in TER and Rac1 activation (Figure 8A&B) suggesting that Tiam1 is not involved in adenosine-induced Rac1 mediated EC barrier strengthening.
Figure 5. Depletion of Vav2 attenuates adenosine-induced increase in TER and Rac1 activation in pulmonary endothelial cells.
A. HPAEC were treated with Vav2, specific siRNA or non-specific siRNA and then challenged with 50 μM adenosine in the TER measurement assay. Depletion of Vav2 significantly attenuated adenosine-induced endothelial barrier enhancement. Arrow indicates the time point when adenosine was added. n=4; mean +/− SEM;*** p< 0.0001 vs. Non-siRNA/Ado B. Effect of adenosine on Rac1 activation in Vav2 depleted cells. HPAEC were transfected with Vav2 specific siRNA or non-specific siRNA and then 72 hours later challenged with 50 μM adenosine at different time points in Rac1 activity assay. Cells were serum starved for 2 hours before adenosine treatment and Rac1 activity was measured according to the manufacturer’s protocol. n=4; mean +/− SEM; **p < 0.01 vs. Non-siRNA/0 min.
Figure 6. Depletion of Rap1a attenuates adenosine-induced increase in TER and Rac1 activation in pulmonary endothelial cells.
A. HPAEC were treated with Rap1a specific siRNA or non-specific siRNA and then challenged with 50 μM adenosine in TER measurement assay. Depletion of Rap1a significantly attenuated adenosine-induced endothelial barrier enhancement. Arrow indicates the time point when adenosine was added. n=4; mean +/− SEM; **p < 0.01 vs. Non-siRNA/Ado. B. Effect of adenosine on Rac1 activation in Rap1a depleted cells. HPAEC were transfected with Rap1a specific siRNA or non-specific siRNA and then 72 hours later challenged with 50 μM adenosine at different time points in Rac1 activity assay. Cells were serum starved for 2 hours before adenosine treatment and Rac1 activity was measured according to the manufacturer’s protocol. n=4; mean +/− SEM; **p < 0.01 vs. Non-siRNA/0 min.
Figure 7. Depletion of Rap1b modestly affects adenosine-induced increase in TER while substantially decreases Rac1 activation in pulmonary endothelial cells.
A. HPAEC were treated with Rap1b specific siRNA or non-specific siRNA and then challenged with 50 μM adenosine in TER measurement assay. Depletion of Rap1b modestly affected maximal adenosine-induced endothelial barrier enhancement. Arrow indicates the time point when adenosine was added. n=4; mean +/− SEM; B. Effect of adenosine in Rac1 activation in Rap1b depleted cells. HPAEC were transfected with Rap1b specific siRNA or non-specific siRNA and then 72 hours later challenged with 50 μM adenosine at different time points in Rac1 activity assay. Cells were serum starved for 2 hours before adenosine treatment and Rac1 activity was measured according to the manufacturer’s protocol. n=4; mean +/− SEM; **p < 0.01 vs. Non-siRNA/ 0 min; ##p < 0.01 vs. Rap1b-siRNA/0 min.
Figure 8. Tiam1 depletion has no effect on adenosine-induced increase in TER and Rac1 activation in pulmonary endothelial cells.
A. HPAEC were treated with Tiam1 specific siRNA or non-specific siRNA and then challenged with 50 μM adenosine in TER measurement assay. Depletion of Tiam1 did not affect adenosine-induced endothelial barrier enhancement. Arrow indicates the time point when adenosine was added. n=4; mean +/− SEM; B. Effect of adenosine on Rac1 activation in Tiam1 depleted cells. HPAEC were transfected with Tiam1 specific siRNA or non-specific siRNA and then 72 hours later challenged with 50 μM adenosine at different time points in Rac1 activity assay. Cells were serum starved for 2 hours before adenosine treatment and Rac1 activity was measured according to the manufacturer’s protocol. n=4; mean +/− SEM; **P < 0.01 vs. Non-siRNA/ 0 min; #p < 0.05 vs. Tiam1-siRNA/0 min, ##p < 0.01 vs. Tiam1-siRNA/0 min.
Discussion
It is well known that the morphology and motility of EC are regulated by the actin cytoskeleton which is highly dependent upon the activity of Rho family GTPases, especially RhoA and Rac1 (Hall, 1998; Lampugnani et al., 2002). These two small GTPases have opposing effects on EC barrier function with the majority of the literature indicating that RhoA negatively regulates EC permeability and cortical actin arrangement, while Rac1 increases and stabilizes junctional connections between ECs to maintain barrier integrity (Curry and Adamson, 2013). Rac1 activity is also required to maintain the stability of the actin cytoskeleton in EC to preserve the barrier (Jacobson et al., 2006; Lampugnani et al., 2002; Tzima et al., 2002; Vandenbroucke et al., 2008). Lampugnani and colleagues have reported that expression of inactive Rac1 (N17Rac1) in EC results in disassembled actin stress fibers (Lampugnani et al., 2002). It has also been reported that phosphoinositide 3-kinase (PI3K) selectively activates Rac1 to regulate endothelial barrier function by promoting the assembly of a protein complex (VE-Cadherin, Pyk2, Rac1 and Tiam1) which coordinates EC adherens junctions and barrier integrity (Cain et al., 2010). However, there is substantial body of literature that suggests that Rac1 can have a negative influence on the regulation of the EC barrier (Chatterjee et al., 2012; Hordijk, 2006; Marei and Malliri, 2016; Vazquez-Medina et al., 2016). Rac1 is a component of the NADPH oxidase 2 (NOX2) enzyme which is responsible for high output ROS generation, mainly O·̄2 (Frey et al., 2009; Lassegue and Griendling, 2010). Various stimuli including cytokines, growth factors, and shear stress in ECs can increase ROS production triggering changes in gene expression, cell proliferation and cell migration, apoptosis and angiogenesis. These changes can contribute to the development of many pathophysiological syndromes like pneumonia, ALI and ARDS. Based on existing controversies on the exact role of Rac1 in endothelial barrier regulation, it is reasonable to assume that the balance between EC barrier-disrupting and enhancing effects of Rac1 is EC type-specific and depends upon disease stage. In the present study we have shown that introduction of a C/A Rac1 construct into the HPAEC significantly increases TER reflecting enhancement of the EC barrier. Conversely, transduction of HPAEC with an inactive Rac1 adenoviral construct significantly decreases basal TER reflecting EC barrier compromise. Overall, these data strongly suggest that Rac1 activity has an overall positive role in maintaining the barrier integrity of HPAEC, at least in vitro.
Adenosine is an endogenous purine nucleoside and an important mediator of cardiovascular signaling. Cellular signaling by adenosine occurs through four known adenosine receptor subtypes (A1, A2a, A2b, A3) (Eltzschig, 2009; Hasko and Cronstein, 2004; Hasko et al., 2008). Extracellular adenosine concentrations from normal cells are in the range of approximately 300 nM; however, in response to cellular damage and stress, these concentrations can be quickly exceeded (600–1,200 nM). Local adenosine concentrations can reach ~100 μM. In the context of stress or injury, a major function of adenosine is cytoprotection and to prevent tissue damage during events such as hypoxia and ischemia (Hasko and Pacher, 2008; Hassanian et al., 2014). Our current results demonstrate that adenosine-induced HPAEC barrier enhancement is accompanied by activation of Rac1. Inhibition of Rac1 activity leads to attenuation of adenosine-induced increases in TER and indicate that Rac1 is necessary for adenosine-induced EC barrier enhancement.
Our data indicate that Rac1-mediated adenosine-induced EC barrier strengthening is accompanied by MLC dephosphorylation. We have previously shown that decreases in MLC phosphorylation is attributed to increase in MLC phosphatase (MLCP) activity, which is critically involved in EC barrier strengthening induced by extracellular purines (Kim et al., 2012; Kolosova et al., 2005; Umapathy et al., 2010a). It is well known that MLCP activity may be regulated through Rho pathway (Fukata et al., 2001; Kamm and Stull, 2001; Matsumura and Hartshorne, 2008). Downstream Rho effector, Rho kinase (ROCK), directly phosphorylates MLCP regulatory subunit, MYPT1 leading to MLCP inhibition (Fukata et al., 1998). Further, the level of MYPT1 phosphorylation at Thr696 and Thr853 reflects Rho/ROCK activity level (Fukata et al., 2001). Data of literature suggested that Rac1 may down-regulate Rho pathway via ROCK inhibition (Saito et al., 2012). However, our data demonstrate that adenosine-induced Rac 1 activation does not accompanied by MYPT1 dephosphorylation at Thr 853 suggesting that Rac1 is involved in adenosine-induced HPAEC barrier strengthening in Rho/ROCK-independent manner.
We, and others, have previously demonstrated the barrier enhancing/protective effects of extracellular adenosine in vitro as well as in murine models of ALI (Gonzales et al., 2014; Lu et al., 2010; Umapathy et al., 2010a). Our group has previously shown that HPAEC predominantly express A2a and A2b receptors, but not A1 or A3 (Umapathy et al., 2010a). Further, the A2a, but not 2B receptor mediates adenosine-induced strengthening of the HPAEC barrier (Umapathy et al., 2010a). In contrast, Lu and colleagues have shown that both adenosine A2a and 2B receptors mediate EC barrier enhancement in bovine PAEC. This discrepancy is most likely explained by species-specific differences in EC barrier regulation. For example, differences in expression of myosin light chain isoforms have been previously described in different species (Garcia et al., 1997). Vessel- and species-specific differences in EC purinergic receptors subtypes were also noted (Burnstock, 2017). A2a is Gs-coupled while A1 and A3 are Gi, and A2b Gs and Gq coupled adenosine receptors (Eltzschig, 2009). We have shown that depletion of Gαs, but not the Gαq or Gαi significantly attenuate adenosine-induced increases in HPAEC, suggesting that adenosine-induced EC barrier enhancement is cAMP-dependent (Umapathy et al., 2010a).
It is widely accepted that the cAMP-activated guanine nucleotide exchange factor (GEF) for Ras-like GTPases, Epac is involved in the regulation of the EC barrier (Birukova et al., 2008; Birukova et al., 2007c; Kooistra et al., 2005; Lorenowicz et al., 2008; Parnell et al., 2012; Umapathy et al., 2010a). Two Epac isoforms, Epac1 and 2, are expressed in mammalian cells with Epac1 being the major isoform form in vascular endothelial endothelium (Bos, 2003). Epac1 was recently reported to be an upstream of Rac1 signaling (Birukova et al., 2008; Waschke, 2017). The Krüppel-like factor 2 (KLF2) transcription factor, which controls endothelial homeostasis, can activate Rac1 signaling via Epac1 (Atkins and Jain, 2007; Huang et al., 2016; Parmar et al., 2006). We have previously shown that Epac1/Rac1-dependent reorganization of F-actin is involved in extracellular β-nicotinamide adenine dinucleotide (β-NAD)-induced EC barrier enhancement (Umapathy et al., 2010b). Our current study demonstrates that adenosine-induced increases in TER are accompanied by Rac1 activation. The role of Rac1 is revealed by experiments showing that its down-regulation attenuates adenosine-induced increases in TER. Furthermore, depletion of Epac1 also attenuates the effect of adenosine on TER and Rac1 activity implicating the involvement of an Epac1/Rac1 signaling axis in adenosine-induced HPAEC barrier enhancement. Indeed, there is a growing body of evidence showing that Rac1 protects the EC barrier, at least in part, via an Epac1-Rap1 mediated signaling pathway (Aslam et al., 2014; Baumer et al., 2009; Birukova et al., 2008). Activation of Epac-Rap1 by its agonists enhances EC barrier function, reduces the destructive effects of inflammatory mediators on EC permeability and promotes cell junction stability (Cullere et al., 2005; Kooistra et al., 2005; Lezoualc’h et al., 2016). Rap1-mediated Rac1 activation may involve Rho A inhibition through activation of ARAP3, Rap-dependent RhoGAP (Jeon et al., 2010a; Jeon et al., 2010b; Jeon et al., 2012). Down-regulation of Rho/ROCK pathway may lead to decreased phosphorylation (inhibition) of Rac-specific GAP, FilGAP, resulting in Rac1 activation (de-inhibition) (Saito et al., 2012). However, our data do not support the involvement of Rho inhibition in Rac1-mediated adenosine-induced HPAEC barrier strengthening, therefore, the mechanisms of Rap1-mediated adenosine-induced Rac1 activation remain to be determined.
Rap 1 is a Ras-related protein that exists in 2 highly homologous isoforms, Rap1a and Rap1b, with the b isoform being the major isoform expressed in EC (Kitayama et al., 1989; Lakshmikanthan et al., 2011). While Rap1b is important for maintaining vascular tone and blood pressure in murine model, Rap1a appears to have a greater role in the regulation of EC permeability, at least in vitro (Lakshmikanthan et al., 2014; Wittchen et al., 2011). Consistent with these data, our results demonstrate that down-regulation of Rap1a markedly increases adenosine-induced increases in TER and activation of Rac1 in HPAEC. In contrast, the depletion of Rap1b, while decreasing the ability of adenosine to maximally activate Rac1, only modestly affects adenosine-induced EC barrier enhancement. The functional difference between these two isoforms may be explained by their different intracellular localization. For example, ectopically expressed Rap1a, but not Rap1b can co-localized with VE-cadherin at cellular junctions in human endothelium (Wittchen et al., 2011). However, a greater understanding of the isoform-specific effects of Rap1 on EC barrier will have to wait for future studies. Lampugnani and colleagues reported that VE-Cadherin expression increases the total amount of Tiam1 which activates Rac1 (Lampugnani et al., 2002). Furthermore, Birukova et al revealed the involvement of Tiam1 in the barrier protective role of hepatocyte growth factor (HGF) in ECs (Birukova et al., 2007a). They have also demonstrated that Tiam1 is a mediator of Rac1 activation induced by Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) which leads to cytoskeletal remodeling, and barrier protective responses in pulmonary endothelium (Birukova et al., 2007b). However, we found that Tiam1 depletion did not affect adenosine-induced TER increase or Rac1 activity. This apparent discrepancy might be explained by agonist-specific regulation of Rac1 activity, however, clarification of this will require future studies. In contrast to Tiam1, our data clearly demonstrate that another Rac1 GEF, Vav2, is involved in adenosine-induced HPAEC barrier enhancement and the increase in Rac1 activity. The mechanistic link between Epac1 and Vav2 is currently unclear, but it was shown that cAMP/Epac1-induced Vav2-mediated Rac1 activation may involve Vav2-Rap1 association (Li et al., 2012). Activated Rap1a (Rap1a.GTP) binds to Vav2 via its catalytic DH-PH module targeting Vav2 to specific intracellular locale (Arthur et al., 2004).
In conclusion, our data demonstrate, for the first time, the direct involvement of Rac1 activity in the regulation of the HPAEC barrier. Furthermore, the ability of adenosine to increase EC barrier function depends on Rac1 activation via a cAMP-dependent Epac1/Rap1/Vav2-mediated signaling pathway. However, precise mechanisms involved in adenosine-induced EC Rac1 activation and barrier strengthening remain to be determined.
Supplementary Material
Supplemental figure 1. Depletion of signaling proteins examined in the study does not affect initial TER in non-stimulated cells. Shown are non-normalized resistance values of HPAEC monolayers in ECIS arrays treated with either nsRNA-control or siRNAs specific for proteins of interest prior to adenosine stimulation. See text for further explanation.
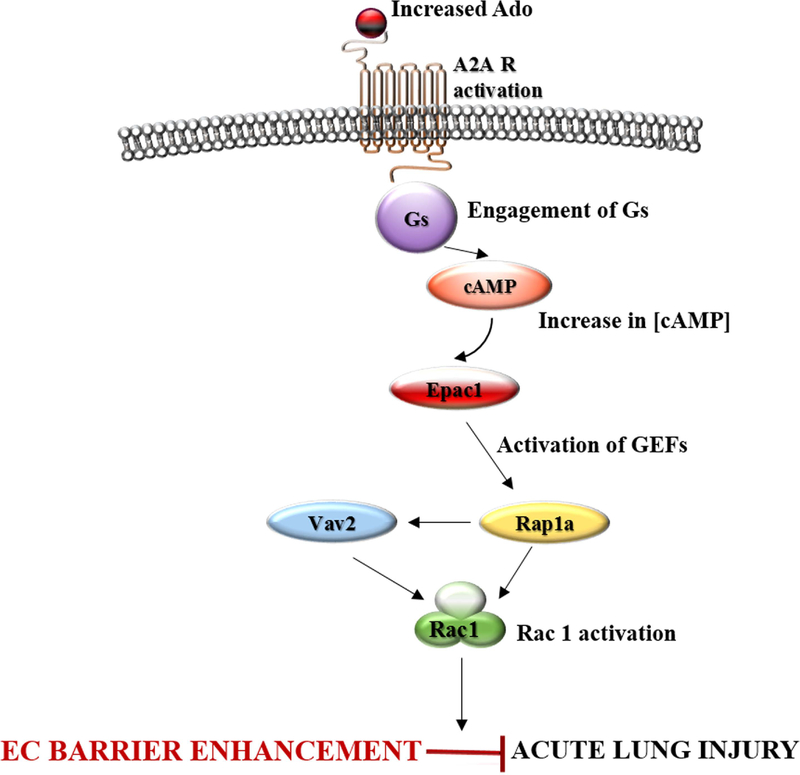
Figure 9. Schematic representation of adenosine-induced Rac1 signaling.
Rac1 activity is directly linked to EC barrier enhancement. Barrier protective effect of adenosine involved in Rac1 activation. Adenosine induces Rac1 activation and EC barrier enhancement, at least in part, via cAMP-dependent Epac1/Vav2/Rap1a-mediated signaling
Acknowledgments
This work was supported by the NIH HL101902.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the contents of this article.
References
- Arthur WT, Quilliam LA, Cooper JA. 2004. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol 167(1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Tanislav C, Troidl C, Schulz R, Hamm C, Gunduz D. 2014. cAMP controls the restoration of endothelial barrier function after thrombin-induced hyperpermeability via Rac1 activation. Physiol Rep 2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins GB, Jain MK. 2007. Role of Kruppel-like transcription factors in endothelial biology. Circulation research 100(12):1686–1695. [DOI] [PubMed] [Google Scholar]
- Balestrieri ML, Malik KU, Balestrieri C, Lee TC. 1998. Types of purinoceptors and phospholipase A2 involved in the activation of the platelet-activating factor-dependent transacetylase activity and arachidonate release by ATP in endothelial cells. Prostaglandins Other Lipid Mediat 56(5–6):363–375. [DOI] [PubMed] [Google Scholar]
- Baumer Y, Spindler V, Werthmann RC, Bunemann M, Waschke J. 2009. Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. Journal of cellular physiology 220(3):716–726. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. 2004. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84(3):869–901. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. 2007a. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. Faseb J 21(11):2776–2786. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. 2007b. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. Journal of cellular physiology 211(3):608–617. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. 2008. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. Journal of cellular physiology 215(3):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. 2007c. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313(11):2504–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogatcheva NV, Zemskova MA, Kovalenkov Y, Poirier C, Verin AD. 2009. Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvascular endothelial cells (HLMVEC) hyperpermeability. Journal of cellular physiology 221(3):750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. 2003. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4(9):733–738. [DOI] [PubMed] [Google Scholar]
- Bos JL. 2006. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31(12):680–686. [DOI] [PubMed] [Google Scholar]
- Bosco EE, Mulloy JC, Zheng Y. 2009. Rac1 GTPase: a “Rac” of all trades. Cell Mol Life Sci 66(3):370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G 2017. Purinergic Signaling in the Cardiovascular System. Circulation research 120(1):207–228. [DOI] [PubMed] [Google Scholar]
- Cain RJ, Vanhaesebroeck B, Ridley AJ. 2010. The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol 188(6):863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Browning EA, Hong N, DeBolt K, Sorokina EM, Liu W, Birnbaum MJ, Fisher AB. 2012. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol 302(1):H105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. 2005. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105(5):1950–1955. [DOI] [PubMed] [Google Scholar]
- Curry FR, Adamson RH. 2013. Tonic regulation of vascular permeability. Acta Physiol (Oxf) 207(4):628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396(6710):474–477. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. 2001. Cytoskeletal regulation of pulmonary vascular permeability. Journal of applied physiology 91(4):1487–1500. [DOI] [PubMed] [Google Scholar]
- Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. 2008. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111(4):2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK. 2009. Adenosine: an old drug newly discovered. Anesthesiology 111(4):904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. 2003. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. The Journal of experimental medicine 198(5):783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. 2002. Rho GTPases in cell biology. Nature 420(6916):629–635. [DOI] [PubMed] [Google Scholar]
- Frey RS, Ushio-Fukai M, Malik AB. 2009. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 11(4):791–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Amano M, Kaibuchi K. 2001. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 22(1):32–39. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Kimura K, Oshiro N, Saya H, Matsuura Y, Kaibuchi K. 1998. Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J Cell Biol 141(2):409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Schaphorst KL, Shi S, Verin AD, Hart CM, Callahan KS, Patterson CE. 1997. Mechanisms of ionomycin-induced endothelial cell barrier dysfunction. The American journal of physiology 273(1 Pt 1):L172–184. [DOI] [PubMed] [Google Scholar]
- Gonzales JN, Gorshkov B, Varn MN, Zemskova MA, Zemskov EA, Sridhar S, Lucas R, Verin AD. 2014. Protective effect of adenosine receptors against lipopolysaccharide-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 306(6):L497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandoch M, Roscioni SS, Schmidt M. 2010. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol 159(2):265–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakoshima T, Shimizu T, Maesaki R. 2003. Structural basis of the Rho GTPase signaling. Journal of biochemistry 134(3):327–331. [DOI] [PubMed] [Google Scholar]
- Hall A 1998. Rho GTPases and the actin cytoskeleton. Science 279(5350):509–514. [DOI] [PubMed] [Google Scholar]
- Hasko G, Cronstein BN. 2004. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25(1):33–39. [DOI] [PubMed] [Google Scholar]
- Hasko G, Linden J, Cronstein B, Pacher P. 2008. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7(9):759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Pacher P. 2008. A2A receptors in inflammation and injury: lessons learned from transgenic animals. Journal of leukocyte biology 83(3):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. 1996. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. Journal of immunology 157(10):4634–4640. [PubMed] [Google Scholar]
- Hassanian SM, Dinarvand P, Rezaie AR. 2014. Adenosine regulates the proinflammatory signaling function of thrombin in endothelial cells. Journal of cellular physiology 229(9):1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. 2006. Cell physiology of cAMP sensor Epac. The Journal of physiology 577(Pt 1):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk PL. 2006. Regulation of NADPH oxidases: the role of Rac proteins. Circulation research 98(4):453–462. [DOI] [PubMed] [Google Scholar]
- Huang RT, Wu D, Meliton A, Oh MJ, Krause M, Lloyd JA, Nigdelioglu R, Hamanaka RB, Jain MK, Birukova A, Kress JP, Birukov KG, Mutlu GM, Fang Y. 2016. Experimental Lung Injury Reduces KLF2 to Increase Endothelial Permeability via Regulation of RAPGEF3-Rac1 Signaling. American journal of respiratory and critical care medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG. 2006. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol 291(2):L289–295. [DOI] [PubMed] [Google Scholar]
- Jeon CY, Kim HJ, Lee JY, Kim JB, Kim SC, Park JB. 2010a. p190RhoGAP and Rap-dependent RhoGAP (ARAP3) inactivate RhoA in response to nerve growth factor leading to neurite outgrowth from PC12 cells. Exp Mol Med 42(5):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CY, Kim HJ, Morii H, Mori N, Settleman J, Lee JY, Kim J, Kim SC, Park JB. 2010b. Neurite outgrowth from PC12 cells by basic fibroblast growth factor (bFGF) is mediated by RhoA inactivation through p190RhoGAP and ARAP3. Journal of cellular physiology 224(3):786–794. [DOI] [PubMed] [Google Scholar]
- Jeon CY, Moon MY, Kim JH, Kim HJ, Kim JG, Li Y, Jin JK, Kim PH, Kim HC, Meier KE, Kim YS, Park JB. 2012. Control of neurite outgrowth by RhoA inactivation. Journal of neurochemistry 120(5):684–698. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. 1999. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol 11(5):591–596. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. 2001. Dedicated myosin light chain kinases with diverse cellular functions. The Journal of biological chemistry 276(7):4527–4530. [DOI] [PubMed] [Google Scholar]
- Kasa A, Csortos C, Verin AD. 2015. Cytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injury. Tissue barriers 3(1–2):e974448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasa A, Czikora I, Verin AD, Gergely P, Csortos C. 2013. Protein phosphatase 2A activity is required for functional adherent junctions in endothelial cells. Microvascular research 89:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Csortos C, Czikora I, Fulton D, Umapathy NS, Olah G, Verin AD. 2012. Molecular characterization of myosin phosphatase in endothelium. Journal of cellular physiology 227(4):1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. 1989. A ras-related gene with transformation suppressor activity. Cell 56(1):77–84. [DOI] [PubMed] [Google Scholar]
- Kolosova IA, Mirzapoiazova T, Adyshev D, Usatyuk P, Romer LH, Jacobson JR, Natarajan V, Pearse DB, Garcia JG, Verin AD. 2005. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circulation research 97(2):115–124. [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Corada M, Dejana E, Bos JL. 2005. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS letters 579(22):4966–4972. [DOI] [PubMed] [Google Scholar]
- Kovacs-Kasa A, Gorshkov BA, Kim KM, Kumar S, Black SM, Fulton DJ, Dimitropoulou C, Catravas JD, Verin AD. 2016. The protective role of MLCP-mediated ERM dephosphorylation in endotoxin-induced lung injury in vitro and in vivo. Sci Rep 6:39018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T, Kwon DY, Chun J, Kim JH, Kang SS. 2000. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. The Journal of biological chemistry 275(1):423–428. [DOI] [PubMed] [Google Scholar]
- Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M. 2011. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alphavbeta(3). Blood 118(7):2015–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmikanthan S, Zieba BJ, Ge ZD, Momotani K, Zheng X, Lund H, Artamonov MV, Maas JE, Szabo A, Zhang DX, Auchampach JA, Mattson DL, Somlyo AV, Chrzanowska-Wodnicka M. 2014. Rap1b in smooth muscle and endothelium is required for maintenance of vascular tone and normal blood pressure. Arteriosclerosis, thrombosis, and vascular biology 34(7):1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. 2002. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Molecular biology of the cell 13(4):1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassegue B, Griendling KK. 2010. NADPH oxidases: functions and pathologies in the vasculature. Arteriosclerosis, thrombosis, and vascular biology 30(4):653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezoualc’h F, Fazal L, Laudette M, Conte C. 2016. Cyclic AMP Sensor EPAC Proteins and Their Role in Cardiovascular Function and Disease. Circulation research 118(5):881–897. [DOI] [PubMed] [Google Scholar]
- Li Y, Kim JG, Kim HJ, Moon MY, Lee JY, Kim J, Kim SC, Song DK, Kim YS, Park JB. 2012. Small GTPases Rap1 and RhoA regulate superoxide formation by Rac1 GTPases activation during the phagocytosis of IgG-opsonized zymosans in macrophages. Free radical biology & medicine 52(9):1796–1805. [DOI] [PubMed] [Google Scholar]
- Lorenowicz MJ, Fernandez-Borja M, Kooistra MR, Bos JL, Hordijk PL. 2008. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol 87(10):779–792. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhu Y, Li Y, Lu W, Hu L, Niu B, Qing P, Gu L. 2010. Prediction of interaction between enzymes and small molecules in metabolic pathways through integrating multiple classifiers. Protein Pept Lett 17(12):1536–1541. [DOI] [PubMed] [Google Scholar]
- Marei H, Malliri A. 2016. Rac1 in human diseases: The therapeutic potential of targeting Rac1 signaling regulatory mechanisms. Small GTPases:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic G, Heemskerk N, van Buul JD, de Waard V. 2015. The Ins and Outs of Small GTPase Rac1 in the Vasculature. J Pharmacol Exp Ther 354(2):91–102. [DOI] [PubMed] [Google Scholar]
- Matsumura F, Hartshorne DJ. 2008. Myosin phosphatase target subunit: Many roles in cell function. Biochemical and biophysical research communications 369(1):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Ware LB, Zimmerman GA. 2012. The acute respiratory distress syndrome. The Journal of clinical investigation 122(8):2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. 1995. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375(6529):338–340. [DOI] [PubMed] [Google Scholar]
- Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA Jr., Garcia-Cardena G 2006. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. The Journal of clinical investigation 116(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell E, Smith BO, Palmer TM, Terrin A, Zaccolo M, Yarwood SJ. 2012. Regulation of the inflammatory response of vascular endothelial cells by EPAC1. Br J Pharmacol 166(2):434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CE, Lum H, Schaphorst KL, Verin AD, Garcia JG. 2000. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 7(4):287–308. [DOI] [PubMed] [Google Scholar]
- Pugin J 1999. Sepsis and the immune response. Intensive care medicine 25(9):1027–1028. [DOI] [PubMed] [Google Scholar]
- Ren Y, Guo L, Tang X, Apparsundaram S, Kitson C, Deguzman J, Fuentes ME, Coyle L, Majmudar R, Allard J, Truitt T, Hamid R, Chen Y, Qian Y, Budd DC. 2013. Comparing the differential effects of LPA on the barrier function of human pulmonary endothelial cells. Microvascular research 85:59–67. [DOI] [PubMed] [Google Scholar]
- Saito K, Ozawa Y, Hibino K, Ohta Y. 2012. FilGAP, a Rho/Rho-associated protein kinase-regulated GTPase-activating protein for Rac, controls tumor cell migration. Molecular biology of the cell 23(24):4739–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T 2011. Functional and molecular heterogeneity of pulmonary endothelial cells. Proceedings of the American Thoracic Society 8(6):453–457. [DOI] [PubMed] [Google Scholar]
- Sukriti S, Tauseef M, Yazbeck P, Mehta D. 2014. Mechanisms regulating endothelial permeability. Pulm Circ 4(4):535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terramani TT, Eton D, Bui PA, Wang Y, Weaver FA, Yu H. 2000. Human macrovascular endothelial cells: optimization of culture conditions. In vitro cellular & developmental biology Animal 36(2):125–132. [DOI] [PubMed] [Google Scholar]
- Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. 2002. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J 21(24):6791–6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathy NS, Fan Z, Zemskov EA, Alieva IB, Black SM, Verin AD. 2010a. Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement. Vascul Pharmacol 52(5–6):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathy NS, Zemskov EA, Gonzales J, Gorshkov BA, Sridhar S, Chakraborty T, Lucas R, Verin AD. 2010b. Extracellular beta-nicotinamide adenine dinucleotide (beta-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement. Journal of cellular physiology 223(1):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke E, Mehta D, Minshall R, Malik AB. 2008. Regulation of endothelial junctional permeability. Annals of the New York Academy of Sciences 1123:134–145. [DOI] [PubMed] [Google Scholar]
- Vazquez-Medina JP, Dodia C, Weng L, Mesaros C, Blair IA, Feinstein SI, Chatterjee S, Fisher AB. 2016. The phospholipase A2 activity of peroxiredoxin 6 modulates NADPH oxidase 2 activation via lysophosphatidic acid receptor signaling in the pulmonary endothelium and alveolar macrophages. Faseb J 30(8):2885–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke J 2017. Epac1 - a tonic stabilizer of the endothelial barrier. Acta Physiol (Oxf) 219(2):339–340. [DOI] [PubMed] [Google Scholar]
- Wilson CN, Batra VK. 2002. Lipopolysaccharide binds to and activates A(1) adenosine receptors on human pulmonary artery endothelial cells. J Endotoxin Res 8(4):263–271. [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Aghajanian A, Burridge K. 2011. Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small GTPases 2(2):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. 2008. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arteriosclerosis, thrombosis, and vascular biology 28(9):1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Depletion of signaling proteins examined in the study does not affect initial TER in non-stimulated cells. Shown are non-normalized resistance values of HPAEC monolayers in ECIS arrays treated with either nsRNA-control or siRNAs specific for proteins of interest prior to adenosine stimulation. See text for further explanation.