Peripheral retinal drusen and reticular pigment changes are associated with AMD and with CFHY402H and CFHrs1410996 genotypes, adjusting for AMD grade. These phenotypes may be a marker of genetic susceptibility for patients with and without AMD.
Abstract
Purpose.
To evaluate the relationship between peripheral retinal drusen and reticular pigment changes and genotypes associated with age-related macular degeneration (AMD).
MEthods.
Using standard protocols, 2103 family members and twins were examined. Clinical and photographic data were graded according to the Clinical Age-Related Maculopathy Grading System (CARMS) as grade 1 (no AMD), grade 2 (small drusen and/or pigment irregularities), grade 3 (intermediate AMD), grade 4 (central or noncentral geographic atrophy), or grade 5 (neovascular disease). Peripheral drusen and reticular pigment were assessed with a standardized examination. Associations between six AMD genetic variants and retinal phenotypes were analyzed.
Results.
AMD grade was associated with peripheral drusen and reticular pigment (odds ratio [OR] 1.9 for advanced AMD; P < 0.001). Both peripheral retinal phenotypes were associated with AMD related genotypes. For CFHY402H, the OR was 2.8 for the CC genotype versus TT (P for trend < 0.001, with increase in peripheral drusen with each additional risk [C] allele). Similar results were seen for CFHrs1410996. Reticular pigment was related to CFHY402H, with OR 2.0 for the CC genotype versus TT (P for trend < 0.001, for increase in pigment with each risk allele) and to CFHrs1410996 (P for trend = 0.006). These findings were not seen for the LOC387715 A69S gene region, CFB, C2, or C3. Among individuals with no or minimal maculopathy, CFH variants were associated with more than a twofold increased risk of drusen and reticular pigment.
Conclusions.
Peripheral retinal drusen and reticular pigment are associated with AMD and with CFHY402H and CFHrs1410996 genotypes, adjusting for AMD grade. These phenotypes may be a marker of genetic susceptibility for patients with or without AMD.
Given that peripheral retinal drusen and reticular pigment changes have been observed and described in subjects with or without AMD,1we designed standardized clinical examination forms to ascertain these peripheral retinal findings in all our genetic and epidemiologic studies of AMD studies since 1989 (Seddon JM, etal IOVS 1997;38:ARVO Abstract 3172). Preliminary analyses of our data revealed an association between these peripheral retinal phenotypes and a family history of AMD (unpublished data, 2004). In recent years, several genetic variants have been associated with various forms of age-related macular degeneration (AMD) including CFHY402H, CFHrs1410996, LOC387715A69S gene region, complement factor 2 (CF2), complement factor B (CFB), and complement factor 3 (C3).2 3 4 5We expanded our analyses of family history of AMD to explore the association between these known genetic variants and the presence of peripheral drusen and pigment irregularities and to determine whether these phenotypes could represent another expression of genetic susceptibility among individuals with or without AMD.
Methods
Study Population
Participants in our Family Genetic Study of Age-Related Macular Degeneration (n = 1599) and our U.S. Twin Study (n = 504) included in this study were examined according to standardized examination and photography protocols and AMD grading systems designed for these studies. Baseline examinations were conducted from 1989 to 2007 by JMS and participating ophthalmologists throughout the United States. Follow-up ocular records were obtained, and follow-up examinations and photography were conducted during this same period.
This research complied with the tenets of the Declaration of Helsinki and was approved by the institutional review board; informed consent was obtained from all subjects.
Phenotypes
Grade of AMD.
AMD grade was determined based primarily on photographs of the macula (a 6000-μm diameter centered on the fovea) according to the Clinical Age-Related Maculopathy Grading System (CARMS) which has a five-step scale: grade 1, no AMD or only a few hard drusen; grade 2a, several hard drusen or a few intermediate size drusen; grade 2b, pigment irregularities; grade 2c, both hard drusen and pigment irregularities; grade 3a, large, soft drusen or several intermediate size drusen; grade 3b, drusenoid retinal pigment epithelial detachment (RPED); grade 4, geographic atrophy; and grade 5, neovascular disease or serous RPED (Seddon JM, etal IOVS 1997;38:ARVO Abstract 3172).6 7 8Both central foveolar and noncentral geographic atrophy were included in grade 4, and visual acuity was not a criteria for assigning AMD grade. The most recent grade in each eye was used in the analyses.
Peripheral Retinal Phenotypes.
Standardized clinical examination forms designed for the family and twin studies, as well as our other AMD studies, incorporate questions about the presence or absence of peripheral drusen and peripheral reticular pigment changes. Peripheral drusen were defined as hard or soft drusen located near the equator, usually small to medium in size. Reticular pigment was defined as “linear patterns of hyperpigmented lines which may have some branching, formation of incomplete geometric patterns or polygons, or complete polygons with marked pigmentation–five or six sided geometric patterns” located in the equatorial region to the ora, as described by Lewis etal1Example photographs for peripheral reticular pigment were also provided for the study examinations as shown in Figures 1 and 2 .
Figure 1.
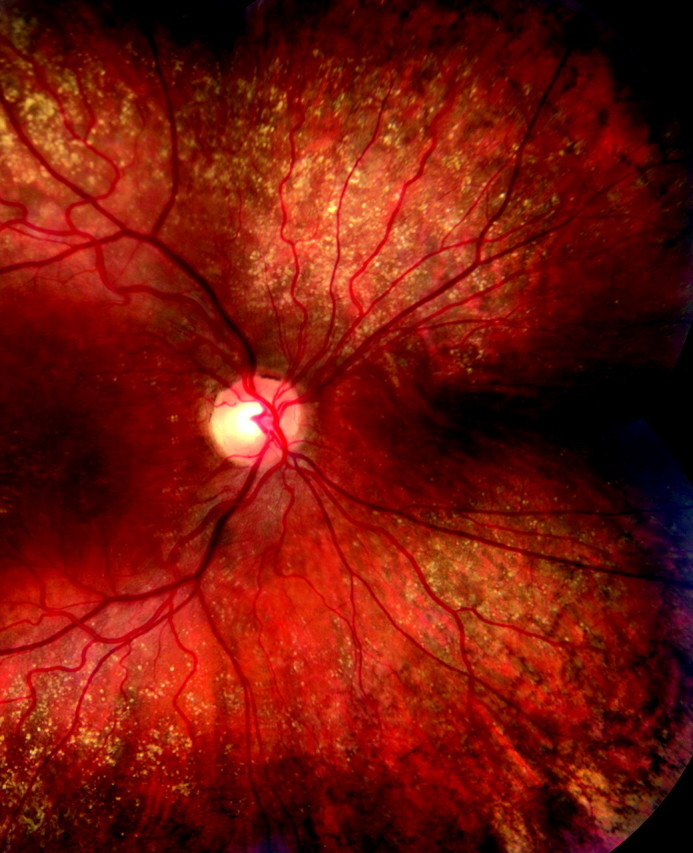
Fundus photograph demonstrating drusen and reticular pigment extending to the equator.
Figure 2.
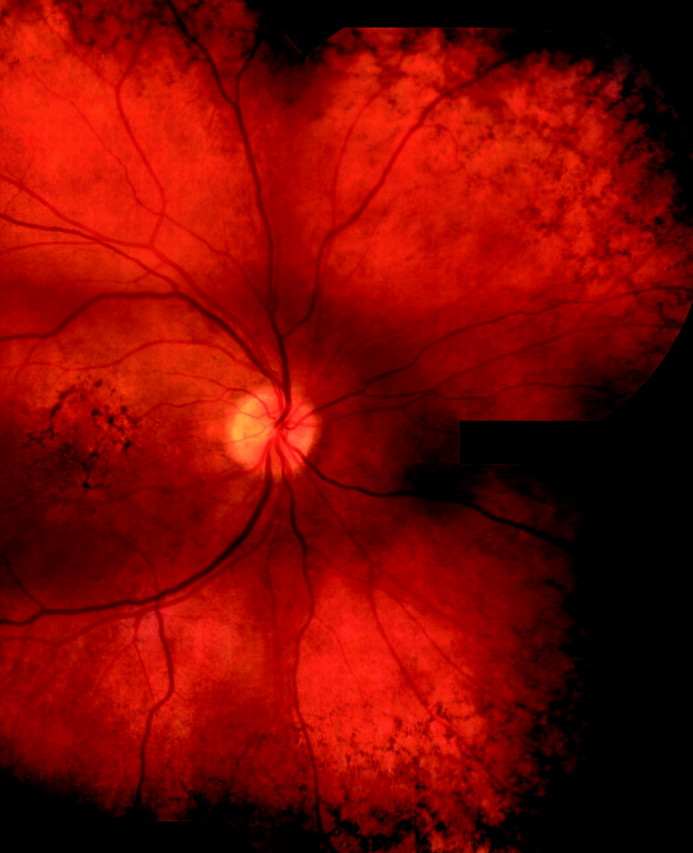
Fundus photograph demonstrating peripheral reticular pigment.
Photographs of seven fields were also obtained as part of the study protocol, to document extramacular retinal drusen and pigment changes. Drusen in the posterior pole (nasal to the disc, temporal to the macula and near the arcades) were graded separately and were not included in these analyses, unless more peripheral drusen were also present.
Genotyping
DNA samples were obtained from whole blood and stored in our blood repository. The following six common single-nucleotide polymorphisms (SNPs) associated with AMD were evaluated: (1) complement factor H (CFH) Y402H (rs1061170) in exon 9 of the CFH gene on 1q31, with change 1277T>C, resulting in a substitution of histidine for tyrosine at codon 402 of the CFH protein; (2) CFH rs1410996 which is an independently associated SNP variant within intron 14 of CFH; (3) LOC387715 A69S (rs10490924 in the LOC387715/HTRA1 region of chromosome 10), a nonsynonymous coding SNP variant in exon 1 of LOC387715, resulting in a substitution of the amino acid serine for alanine at codon 69; (4) complement factor 2 or C2 E318D (rs9332739), the nonsynonymous coding SNP variant in exon 7 of C2 resulting in the amino acid glutamic acid changing to aspartic acid at codon 318; (5) complement factor B or CFB R32Q (rs641153), the nonsynonymous coding SNP variant in exon 2 of CFB resulting in the amino acid glutamine changing to arginine at codon 32, (6) complement factor 3 or C3 R102G (rs2230199), the nonsynonymous coding SNP variant in exon 3 of C3 resulting in the amino acid glycine to arginine at codon 102. For the genetic variant on chromosome 10, LOC387715A69S (ARMS2), it remains uncertain whether the gene HTRA1 adjacent to it may in fact be the AMD susceptibility gene on 10q269 10 11 ; but the relevant SNPs in these two genes have been reported to be nearly perfectly correlated. Thus, although the other SNP is a promising candidate variant, rs10490924 can be considered a surrogate for the causal variant that resides in this region. For the C2/CFB genes, there are two independent associations to the C2/CFB locus, but because of linkage disequilibrium we do not know which of the two genes or both are functionally affected. Genotyping was performed with primer mass extension and MALDI-TOF MS analysis on a gene-analysis system (MassEXTEND; Sequenom, San Diego, CA) at the Broad Institute Center for Genotyping and Analysis (Cambridge, MA).
Statistical Analyses
Distributions of baseline demographic, genotypic, and ocular characteristics for the family, twin, and combined datasets were calculated and are displayed in Tables 1 and 2 . In Table 3 , the relationship between peripheral drusen and AMD grade as a categorical variable was assessed (PROC GENMOD; SAS Institute, Cary NC). Similar analyses were run for peripheral reticular pigment. Tables 4 and 5are based on GEE analyses (PROC GENMOD; SAS), with a logit link regressing the logit of the probability of peripheral drusen, with AMD grade and genotype as categories. In addition, a similar approach was used in tests for trend, which were calculated for the number of non-wild-type alleles for genes with more than two genotypes. For all analyses the eye was the unit of analysis, and the correlation between eyes was taken into account.
Table 1.
Baseline Demographic and Genetic Characteristics of Participants
| Family (n = 1599) | Twin (n = 504) | Total (n = 2103) | |
|---|---|---|---|
| Mean age (mean ± SD) | 76.6 ± 9.4 | 77.8 ± 5.1 | 76.9 ± 8.6 |
| Sex | |||
| M | 654 (41) | 504 (100) | 1158 (55) |
| F | 945 (59) | 0 | 945 (45) |
| Genotype | |||
| CFH Y402H: rs1061170 | |||
| TT | 334 (21) | 156 (32) | 480 (24) |
| CT | 724 (47) | 220 (45) | 944 (46) |
| CC | 503 (32) | 112 (23) | 615 (30) |
| CFH: rs1410996 | |||
| TT | 113 (7) | 60 (13) | 173 (9) |
| CT | 561 (36) | 195 (42) | 756 (38) |
| CC | 868 (56) | 213 (45) | 1081 (54) |
| LOC387715:rs10490924(A69S) | |||
| GG | 578 (38) | 263 (54) | 841 (42) |
| GT | 670 (44) | 180 (38) | 850 (42) |
| TT | 278 (18) | 40 (8) | 318 (16) |
| C2:rs9332739(E318D) | |||
| GG | 1427 (93) | 447 (93) | 1874 (93) |
| CG/CC | 116 (7) | 35 (7) | 151 (7) |
| CFB:rs641153(R32Q) | |||
| CC | 1331 (87) | 427 (88) | 1780 (87) |
| CT/TT | 203 (13) | 57 (12) | 260 (13) |
| C3:rs2230199 (R102G) | |||
| CC | 703 (49) | 217 (48) | 920 (49) |
| CG | 623 (43) | 190 (42) | 813 (43) |
| GG | 115 (8) | 45 (10) | 160 (8) |
Frequency counts for some genotypes may not add up to the total sample size due to missing genotype information for some subjects.
Table 2.
Ocular Characteristics of Participants
| Family (n = 1599) | Twin (n = 504) | Total (n = 2103) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OD n (%) | OS n (%) | OD n (%) | OS n (%) | OD n (%) | OS n (%) | ||||
| AMD grade | |||||||||
| 1 | 362 (23) | 369 (23) | 217 (43) | 218 (43) | 579 (28) | 587 (28) | |||
| 2 | 229 (14) | 229 (14) | 122 (24) | 121 (24) | 351 (17) | 350 (17) | |||
| 3 | 293 (19) | 309 (19) | 89 (18) | 76 (15) | 382 (18) | 385 (18) | |||
| 4 | 241 (15) | 256 (16) | 30 (6) | 32 (6) | 271 (13) | 288 (14) | |||
| 5 | 458 (29) | 428 (27) | 44 (9) | 55 (11) | 502 (24) | 483 (23) | |||
| Total | 1583 (100) | 1596 (100) | 502 (100) | 502 (100) | 2085 (100) | 2093 (100) | |||
| Peripheral drusen | 147 (12) | 153 (13) | 25 (9) | 23 (8) | 172 (11) | 176 (12) | |||
| Reticular pigment | 306 (25) | 297 (25) | 61 (22) | 63 (23) | 367 (25) | 360 (24) | |||
Table 3.
Association between Peripheral Retinal Drusen and Reticular Pigment and AMD Grade
| AMD Grade, n (%) | P (trend) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||
| Peripheral drusen | 54/821 (7) | 31/437 (7) | 83/500 (17) | 52/406 (13) | 126/808 (16) | |||||
| OR (CI) | 1.0 | 1.3 (1.1–1.6) | 1.9 (1.4–2.5) | 1.9 (1.4–2.6) | 1.9 (1.4–2.5) | |||||
| P | 0.014 | <0.001 | <0.001 | <0.001 | 0.003 | |||||
| Peripheral pigment | 112/813 (14) | 67/433 (15) | 125/502 (25) | 141/403 (35) | 278/808 (34) | |||||
| OR (CI) | 1.0 | 1.2 (1.0–1.4) | 1.6 (1.3–2.0) | 2.0 (1.6–2.5) | 1.9 (1.5–2.3) | |||||
| P | 0.061 | <0.001 | <0.001 | <0.001 | 0.001 | |||||
The eye was the unit of analysis.
Table 4.
Association between Genotypes and Peripheral Retinal Drusen and Reticular Pigment
| Genotype | Peripheral Drusen | Reticular Pigment | ||||
|---|---|---|---|---|---|---|
| n (%) | OR (CI) | n (%) | OR (CI) | |||
| CFHY402H: rs1061170 (Y402H) | ||||||
| TT | 39/616 (6) | 1.0 | 105/614 (17) | 1.0 | ||
| CT | 136/1355 (10) | 1.5 (0.9–2.4) | 304/1357 (22) | 1.3 (0.9–1.8) | ||
| CC | 165/897 (18) | 2.8 (1.7–4.6) | 288/886 (33) | 2.0 (1.4–2.9) | ||
| P (trend) | <0.001 | <0.001 | ||||
| CFH: rs1410996 | ||||||
| TT | 10/215 (5) | 1.0 | 36/213 (17) | 1.0 | ||
| CT | 108/1045 (10) | 2.0 (0.8–4.8) | 221/1044 (21) | 1.1 (0.7–1.9) | ||
| CC | 217/1596 (14) | 2.5 (1.1–6.0) | 450/1586 (28) | 1.6 (1.0–2.7) | ||
| P (trend) | 0.016 | 0.006 | ||||
| LOC387715: rs10490924 (A69S) | ||||||
| GG | 147/1099 (13) | 1.0 | 286/1092 (26) | 1.0 | ||
| GT | 126/1226 (10) | 0.7 (0.5–1.0) | 298/1220 (24) | 0.9 (0.7–1.1) | ||
| TT | 60/503 (12) | 0.8 (0.5–1.2) | 101/503 (20) | 0.6 (0.4–0.9) | ||
| P (trend) | 0.13 | 0.008 | ||||
| C2: rs9332739 (E318D) | ||||||
| GG | 308/2628 (12) | 1.0 | 650/2617 (25) | 1.0 | ||
| CG/CC | 27/225 (12) | 1.1 (0.6–2.0) | 49/225 (22) | 0.9 (0.6–1.5) | ||
| P (trend) | 0.69 | 0.77 | ||||
| CFB: rs641153 (R32Q) | ||||||
| CC | 281/2437 (12) | 1.0 | 596/2423 (25) | 1.0 | ||
| CT/TT | 42/374 (11) | 1.1 (0.8–1.5) | 90/376 (24) | 1.0 (0.7–1.5) | ||
| P (trend) | 0.84 | 0.83 | ||||
| C3: rs2230199 (R102G) | ||||||
| CC | 150/1266 (12) | 1.0 | 286/1267 (23) | 1.0 | ||
| CG | 118/1180 (10) | 0.8 (0.6–1.1) | 292/1169 (25) | 1.1 (0.9–1.5) | ||
| GG | 34/225 (15) | 1.3 (0.7–2.2) | 58/223 (26) | 1.2 (0.8–1.9) | ||
| P (trend) | 0.99 | 0.30 | ||||
The eye was the unit of analysis. OR (odds ratio) adjusted for AMD grade.
Table 5.
Association between Genotypes and Peripheral Drusen and Pigment for Grades 1 and 2
| Genotype | Peripheral Drusen | Reticular Pigment | ||||
|---|---|---|---|---|---|---|
| n (%) | OR (CI) | n (%) | OR (CI) | |||
| CFHY402H: rs1061170 (Y402H) | ||||||
| TT | 14/354 (4) | 1.0 | 33/352 (9) | 1.0 | ||
| CT | 46/567 (8) | 2.2 (1.0–4.9) | 89/563 (16) | 1.9 (1.1–3.4) | ||
| CC | 19/245 (8) | 2.0 (0.8–5.1) | 48/241 (20) | 2.4 (1.2–4.7) | ||
| P (trend) | 0.11 | 0.006 | ||||
| CFH: rs1410996 | ||||||
| TT | 4/140 (3) | 1.0 | 11/138 (8) | 1.0 | ||
| CT | 39/514 (8) | 2.7 (0.7–9.5) | 75/508 (15) | 1.9 (0.8–4.7) | ||
| CC | 33/496 (7) | 2.2 (0.6–8.0) | 83/495 (17) | 2.2 (0.9–5.3) | ||
| P (trend) | 0.52 | 0.010 | ||||
| LOC387715: rs10490924 (A69S) | ||||||
| GG | 47/577 (8) | 1.0 | 95/569 (17) | 1.0 | ||
| GT | 20/456 (4) | 0.5 (0.2–1.1) | 65/454 (14) | 0.9 (0.5–1.4) | ||
| TT | 8/109 (7) | 1.0 (0.4–2.6) | 6/107 (8) | 0.3 (0.1–1.0) | ||
| P (trend) | 0.37 | 0.055 | ||||
| C2: rs9332739 (E318D) | ||||||
| GG | 71/1013 (7) | 1.0 | 149/1003 (15) | 1.0 | ||
| CG/CC | 5/122 (4) | 0.6 (0.2–2.0) | 17/122 (14) | 0.9 (0.4–1.9) | ||
| P (trend) | 0.38 | 0.8 | ||||
| CFB: rs641153 (R32Q) | ||||||
| CC | 63/953 (7) | 1.0 | 139/943 (15) | 1.0 | ||
| CT/TT | 15/191 (8) | 1.2 (0.5–2.6) | 26/191 (14) | 0.9 (0.5–1.6) | ||
| P (trend) | 0.68 | 0.65 | ||||
| C3: rs2230199 (R102G) | ||||||
| CC | 40/535 (7) | 1.0 | 76/533 (14) | 1.0 | ||
| CG | 24/449 (5) | 0.7 (0.3–1.3) | 66/441 (15) | 1.0 (0.6–1.7) | ||
| GG | 6/79 (8) | 1.0 (0.3–3.1) | 8/79 (10) | 0.6 (0.2–1.8) | ||
| P (trend) | 0.51 | 0.59 | ||||
The eye was the unit of analysis. OR (odds ratio) adjusted for AMD grade.
Results
Table 1displays the demographic and genetic characteristics of the study population. Only Caucasian participants were included in these analyses. The mean age for the total population was 76.9 ± 8.6 years, with 55% men and 45% women.
Ocular characteristics were similar for OD and OS as shown in Table 2 . Of the eyes studied, 28% were grade 1 (no AMD) and approximately 37% had advanced AMD (grade 4 or 5). Peripheral drusen were present in 11% to 12% of the eyes, and peripheral reticular pigment was present in 24% to 25% of them.
Table 3displays the association between AMD grade and peripheral retinal drusen and reticular pigment. The presence of peripheral drusen increased from 7% for grades 1 and 2, to between 13% and 17% for grades 3 to 5 (P for trend = 0.003). The presence of reticular pigment increased from 14% to 15% for grades 1 and 2 to 25% for grade 3 and 34% to 35% for grades 4 and 5 (P for trend = 0.001).
Compared to grade 1, odds ratios (OR) were 1.9 for grade 3 to 5 for peripheral drusen, and 1.6 to 2.0 for peripheral pigment, indicating that maculopathy is associated with approximately a twofold increased risk of peripheral retinal findings.
Table 4displays the association between genotypes and prevalence of peripheral drusen and pigment, adjusting for AMD grade. The CFHY402H genotype was associated with peripheral drusen, with P for trend < 0.001 for an increase in drusen with each additional risk allele (C) compared with the TT genotype (drusen present in 6% of TT, 10% of CT, and 18% of CC genotypes). The CC genotype was associated with a 2.8-fold increased risk of peripheral drusen (OR, 2.8; 95% confidence interval [CI] 1.7–4.6). Results were similar for CFHrs1410996, with a P for trend = 0.016 for the increase in drusen with each additional risk allele (C), compared with the TT genotype (drusen present in 5% of TT, 10% of CT, and 14% of CC genotypes), and a 2.5-fold increased risk (95% CI, 1.1–6.0) for the CC genotype. The CFHY402H genotype was also related to peripheral reticular pigment changes with P for trend < 0.001 for the increase in pigment with each risk allele (reticular pigment present in 17% of TT, 22% of CT, and 33% of CC genotypes), with a 2.0-fold increased risk for the CC genotype. For CFHrs1410996, the association was similar for peripheral pigment (P for trend = 0.006), which was present in 17% of TT, 21% of CT, and 28% of CC genotypes, with a 1.6-fold increased risk for the CC genotype. For the LOC387715 A69S gene region, there was a significant inverse trend for less peripheral pigment with each T (risk) allele. No associations were seen for the C2 or CFB genotypes. For C3, there was a slight trend in the same direction as CFH for both peripheral drusen and pigment, which was not significant.
Table 5shows the association between genotype and prevalence of peripheral drusen and pigment among individuals without AMD or with only minimal signs of maculopathy (grades 1 and 2 only), adjusting for AMD grade. For both of the CFH genotypes, reticular pigment was significantly associated with an increasing number of risk alleles (P = 0.006 for CFHY402H, and P = 0.010 for CFHrs1410996), with a 2.2- to 2.4-fold increased risk of peripheral reticular pigment for the CC genotype. There was also over a twofold increased risk for having peripheral drusen with the presence of one or two risk alleles for both CFH genotypes, but the trend for this association was not statistically significant. Similar associations were not seen for the other genotypes among individuals with early or no disease.
Discussion
The associations between six known AMD genetic variants and peripheral retinal phenotypes were evaluated. The associations between peripheral retinal phenotypes and AMD were also determined. The results of this study newly quantify (1) the association between peripheral retinal drusen and peripheral reticular pigment and presence of AMD, (2) the association of these peripheral phenotypes with CFHrs1410996 controlling for the presence of AMD; (3) the magnitude of the relationship between peripheral retinal drusen and CFHY402H adjusting for AMD grade; and (4) the association between these peripheral retinal phenotypes and CFH genotypes, even among individuals without a diagnosis of AMD or with minimal signs of maculopathy.
We found approximately a twofold increased risk of peripheral retinal drusen and peripheral reticular pigment among individuals with intermediate to advanced maculopathy, which indicates that the central macular and peripheral retinal phenotypes are related. After adjustment for AMD grade, both CFH genotypes were significantly associated with peripheral drusen and peripheral reticular pigment. The risk was 1.6- to 2.8-fold higher for the presence of the peripheral phenotypes with the CC genotype for both variants. The genetic variant CFHrs1410996,4a SNP lying within an intron of CFH, is related to AMD—more strongly than CFHY402H in some populations.12Even among individuals with little or no maculopathy, these associations between peripheral retinal phenotypes and both CFH genotypes were present, with similar magnitude of effect. These results indicate that the peripheral retinal phenotypes are associated with AMD genotypes. Furthermore, among those with little or no central macular disease, these extramacular phenotypes may be a sign of genetic susceptibility.
Individuals with the LOC387715A69S TT (risk) genotype were somewhat less likely to have peripheral retinal reticular pigment. The exact biological mechanism related to this AMD gene region is unknown but appears to differ from CFH and other complement factor genes. We have previously shown that the LOC387715 gene is more strongly related to progression to neovascular disease compared with geographic atrophy,13whereas the CFH genotype is related to both advanced forms with somewhat lower magnitude of effect. Additional studies with large numbers of subjects are needed to evaluate further the relationship between this gene region on chromosome 10 and peripheral retinal phenotypes.
One study showed a relationship between the CFHY402H genotype and peripheral pigment changes,14and another showed no relationship with LOC387715,15using our protocols. However, they did not report an association between the two genotypes and peripheral drusen. Another study with 420 cases and 50 controls showed an association between CFHY402H and the presence of “peripheral drusen” defined as located “outside the temporal vascular arcades,”16but it is uncertain whether drusen in the periphery at or near the equator were evaluated separately from drusen in the posterior pole and whether the presence of AMD was controlled for in the analyses. Our analyses quantified the magnitude of the relationships between central and peripheral phenotypes, demonstrated and quantified the relationship of peripheral retinal findings with AMD and also assessed the independent associations between the central and peripheral phenotypes with six genotypes related to AMD.
Lewis etal1studied 396 donor eyes obtained postmortem and 50 patients and described the histopathology of peripheral reticular pigment. Their results showed a correlation between these peripheral pigment changes and macular degenerative abnormalities. Our results among 2103 subjects support that association, and also quantify the magnitude of that relationship: about a twofold increased likelihood of having AMD with either peripheral drusen or reticular pigment compared with eyes without those findings. The reason for the geometric pattern of hypertrophy and hyperplasia of the pigment epithelium is unknown. Lewis etal1described it as a “zonal degeneration related to ischemia in the watershed area of the anteroposterior circulation and the gradual decline in the metabolic activities of the pigment epithelial cells.” They did not find the pigment to be related to the choroidal vascular lobules.
Rudolf etal 17studied nine AMD-affected eyes and reported the characteristics of drusen in the macula and in the retinal periphery. They found differences in morphology, composition, and physical properties between the drusen types in the two locations. Soft drusen appeared in the macula only, and the periphery had hard and compound drusen. Despite the morphologic differences, our results suggest that genotypes for macular disease are also associated with the more peripheral types of drusen.
Given the peripheral nature of the phenotypes which may be difficult to capture by photography, peripheral retinal phenotypes were based mainly on the standardized study examination, which specifically asked for presence or absence of these findings as defined in the protocol. Therefore, reliability measures involving repeated examinations were not available. The validity and reliability of the AMD grading system has been described previously.6Advantages of this study include the large number of subjects (N = 2103), assessment of six known single-nucleotide polymorphisms (SNPs), use of standardized protocols, analyses of the magnitude of the effects seen, assessment of the independent associations controlling for AMD grade, and stratification according to AMD grade allowing estimation of the effect of the peripheral findings among eyes with little or no macular disease.
Results of this study may have clinical relevance in that peripheral drusen and reticular pigment changes appear to be signs of genetic susceptibility, even among those who do not have AMD.
Acknowledgments
The authors thank all the families, twins, and participating ophthalmologists throughout the United States; Mark Daly for valuable advice and comments; Jesen Fagerness, Julian Maller, and Daniel Mirel for assistance with genotyping; and Marion McPhee (Harvard University Channing Laboratory, Boston, MA) for programming assistance.
Footnotes
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, April 2008.
Supported in part by Grant R01-EY11309 from the National Eye Institute, unrestricted grants from Research to Prevent Blindness Inc., New York, NY; the Foundation Fighting Blindness, Owing Mills, MD; the Massachusetts Lions Eye Research Fund Inc., Northboro, MA; and the Macular Degeneration Research Fund of the Ophthalmic Epidemiology and Genetics Service, the New England Eye Center, Tufts Medical Center, Tufts University School of Medicine.
Submitted for publication July 1, 2008; revised August 19, 2008; accepted December 4, 2008.
Disclosure: J.M. Seddon, None; R. Reynolds, None; B. Rosner, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Corresponding author: Johanna M. Seddon, Tufts Medical Center, New England Eye Center, 800 Washington Street, No. 450 Boston, MA 02111; jseddon@tuftsmedicalcenter.org.
References
- 1.Lewis H, Straatsma BR, Foos RY, Lightfoot DO. Reticular degeneration of the pigment epithelium. Ophthalmology. 1985;92:1485–1495. doi: 10.1016/s0161-6420(85)33829-0. [DOI] [PubMed] [Google Scholar]
- 2.Klein RJ, Zeiss C, Chew E et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol. 2006;50:306–363. doi: 10.1016/j.survophthal.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Maller J, George S, Purcell S et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Gen. 2006;38:1055–1059. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 5.Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Gen. 2007;39:1200–1201. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- 6.Seddon JM, Sharma S, Adelman SL, Seddon RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmol. 2006;113:260–266. doi: 10.1016/j.ophtha.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Seddon JM, Santangelo SL, Book K, Chong S, Cote J. A genome-wide scan for age-related macular degeneration provides evidence for linkage to several chromosomal regions. Am J Hum Gen. 2003;73:780–790. doi: 10.1086/378505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seddon JM, Cote J, Page W, Aggen SH, Neale MC. The U.S. age-related macular degeneration twin study: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123:323–327. doi: 10.1001/archopht.123.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Dewan A, Liu M, Hartman S et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Camp NJ, Santangelo SL, Sun H et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 11.Kanda A, Chen W, Othman M et al. A variant of mitochondrial protein LOC387715/ ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci USA. 2007;104:16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori K, Gehlbach PL, Kabasawa S et al. Coding and noncoding variants in the CFH gene and cigarette smoking influence the risk of age-related macular degeneration in a Japanese population. Invest Ophthalmol Vis Sci. 2007;48:5315–5319. doi: 10.1167/iovs.07-0426. [DOI] [PubMed] [Google Scholar]
- 13.Seddon JM, Francis PJ, George S, Schultz DW, Rosner B, Klein ML. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297:1793–1800. doi: 10.1001/jama.297.16.1793. [DOI] [PubMed] [Google Scholar]
- 14.Shuler RK, Jr, Schmidt S, Gallins P et al. Peripheral reticular pigmentary change is associated with complement factor H polymorphism (Y402H) in age-related macular degeneration. Ophthalmology. 2008;115:520–524. doi: 10.1016/j.ophtha.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Shuler RK, Jr, Schmidt S, Gallins P et al. Phenotype analysis of patients with the risk variant LOC387715 (A69S) in age-related macular degeneration. Am J Ophthalmol. 2007;145:303–307. doi: 10.1016/j.ajo.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Droz I, Mantel I, Ambresin A, Faouzi M, Schorderet DF, Munier FL. Genotype-phenotype correlation of age-related macular degeneration: influence of complement factor H polymorphism. Br J Ophthalmol. 2008;92:513–517. doi: 10.1136/bjo.2007.127811. [DOI] [PubMed] [Google Scholar]
- 17.Rudolf M, Clark ME, Chimento MF, Li CM, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]


