Abstract
Background
Diabetic nephropathy (DN) is a potentially fatal complication of diabetes mellitus. While lifestyle changes can reduce diabetes risk, it is unclear whether improved lifestyle can slow or reverse DN progression. This study evaluated whether an intensive lifestyle intervention (IL-I) targeting weight loss and inflammation through increased physical activity and reduced caloric intake can delay DN progression.
Material/Methods
Patients were randomly divided into 2 groups. Both groups received diet and exercise guidelines, but one (IL-I) received more frequent external support than the other (control). We compared markers of metabolic and cardiovascular health, redox status, inflammation, and renal function between groups at 3 and 6 months. Metabolic and cardiovascular metrics included BMI, blood pressure, blood glycosylated hemoglobin (HbA1c), and serum HDL-cholesterol. Redox status was evaluated by serum superoxide dismutase (SOD) and the lipid oxidation product malondialdehyde (MDA), while inflammation was assessed by serum concentrations of IL-6 and TNF-α. Renal function was assessed by urine/serum 8-OHdG, albumin: creatinine ratio (ACR), and the renal fibrosis marker TGF-β1.
Results
Both groups demonstrated initial BMI reduction, lower HbA1c, and higher HDL-cholesterol, but changes were significantly larger in the IL-I group at 6 months. Blood pressure at 6 months was reduced only in the IL-I group. The IL-I group also achieved a greater sustained SOD increase and MDA reduction. Finally, only the IL-I group demonstrated significant reductions in urine ACR, serum/urine 8-OHdG, and plasma TGF-β1. These indicators deteriorated after IL-I was stopped.
Conclusions
Lifestyle changes including exercise and diet can delay renal damage and promote improvement from DN.
MeSH Keywords: Caloric Restriction, Diabetic Nephropathies, Fibrosis, Inflammation, Life Style
Background
Both type 1 and type 2 diabetes mellitus are associated with debilitating microvascular complications such as diabetic nephropathy (DN) [1,2]. Approximately 50% of patients with end-stage renal disease (ESRD) requiring dialysis are diabetic, and the growing incidence of DN is driving substantial increases in chronic kidney disease (CKD) and ESRD [3,4]. The annual mortality from ESRD (approaching 9%) is 10- to 20-fold higher than in the general population, even after stratification for factors such as age [5,6]. Therefore, delaying the progression of DN is of great value for reducing the incidence and mortality of ESRD.
The pathogenesis of DN is un clear. Traditionally, it has been thought of as a glomerular disease [7], as the proteinuria observed in many patients is a sign of glomerulopathy [8]. However, findings typical of glomerulopathy are present in only one-third of patients with type 2 diabetes [9], while the renal structure of another one-third is normal [10]. The remaining one-third of patients have minor or no glomerular changes, but have disproportionately severe tubulo-interstitial lesions [11,12]. Whether presenting with glomerular or tubulo-interstitial injury, many studies have confirmed an association of DN with oxidative stress and inflammation caused by hyperglycemia and renal hypoxia [10,13]. Therefore, effective improvement of the renal metabolic microenvironment may delay the development of DN.
Previous studies have confirmed that aerobic exercise and diet control are effective ways to treat diabetes [14] by improving the metabolic microenvironment. Action for Health in Diabetes (Look AHEAD), a multicenter randomized controlled clinical trial that featured 10 years of multidomain intervention, successfully induced long-term behavioral changes and improved diabetic cerebral vascular microcirculation [15]. Recent animal experiments have found that a diet mimicking the fasted state promotes islet cell regeneration and reverses diabetes [16]. It is therefore reasonable to speculate that intensive lifestyle intervention (IL-I) targeting weight loss by increasing physical activity and reducing caloric intake could improve DN prognosis.
Material and Methods
Participants and intervention
Participants for this study included 121 patients with DN and 47 normal non-diabetic healthy volunteers. The 47 healthy volunteers had no history of diabetes mellitus, nephropathy, heart disease, hepatitis, or tuberculosis, and no abnormalities in renal function, liver function, urine routine, or blood sugar were observed before admission. All participants recruited in the present study provided informed consent and the study was approved by the Ethics Committee of Yongchuan Hospital of Chongqing Medical University (approval number: sc2016). These patients included 72 men and 49 women (average age, 56±14.1 years) with a 7–12 history of diabetes (Table 1), age-normal cognitive and athletic ability, and the ability to arrange their work and free time independently. All were current DN out-patients who visited our hospitals and fulfilled one or more of the following criteria: 1) HbA1c level less than 9% at the time of enrollment and for at least 6 months prior to enrollment, 2) urinary albumin: creatinine ratio (ACR) higher than 30 mg albumin per g of creatinine [Cr]) (stage II DN or higher), 3) serum Cr less than 1.5 mg/dL and absence of hematuria, 4) absence of severe diabetic complications such as retinal hemorrhage and neuropathy, and 5) absence of severe hepatic damage and cerebrovascular disorders.
Table 1.
Patient characteristics.
| Characteristic | Control group n=61 | IL-I group n=60 | P value |
|---|---|---|---|
| Age, mean ±SD | 52.4±4.2 | 51.7±4.9 | 0.27 |
| Sex | 0.42 | ||
| Female | 25 | 24 | |
| Male | 36 | 36 | |
| BMI, mean ±SD | 28±1.4 | 28.6±1.8 | 0.68 |
| Smoker | 0.71 | ||
| Never | 13 | 11 | |
| Former | 31 | 37 | |
| Current | 17 | 12 | |
| Hypertension | 47 | 51 | 0.61 |
| History of cardiovascular disease | 5 | 6 | 0.91 |
| HDL-cholesterol, mean ±SD | 54.5±3.8 | 54.2±3.7 | 0.77 |
| Comorbidities | |||
| Peripheral neuropathy | 9 | 14 | 0.255 |
| Diabetic retinopathy | 13 | 8 | 0.338 |
| History of medication | |||
| ACEI/ARB | 11 | 13 | 0.654 |
| Spironolactone | 7 | 6 | 0.818 |
The 121 participants were randomly divided with equal probability into an IL-I group and a common lifestyle intervention (control) group. Both groups received diabetes support and education, and the following sports and diet goals. Participants were assigned a daily calorie goal of 1200–1800 calories based on initial weight, with fewer than 30% of total calories from fat (<10% from saturated fat) and a minimum of 15% of total calories from protein. The physical activity goal was more than 300 min of physical activity per week with intensity similar to brisk walking. The only difference in intervention between the 2 groups was that control group participants were contacted by telephone twice per week for the first 3 months and once per month for the next 3 months, while participants in the IL-I group were called twice per week for encouragement and supervision to help accomplish these goals throughout the 6-month trial. In the telephone interview, we emphasized the importance of exercise and diet control for diabetes and nephropathy, and encouraged patients to complete the established exercise and diet standards. Blood pressure (BP), body mass index (BMI), ACR, and plasma levels of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), HDL-cholesterol, and HbA1c were determined at baseline and after 3 months and 6 months of intervention.
Measurement of biological parameters
Plasma glucose was measured using the glucose oxidase method (Wako Chemical). Dedicated ELISA kits were used for quantification of plasma TGFβ1 and aldosterone (Kangsheng, Shanghai, China) and 24-h urinary 8-OHdG (8-OHdG Check; Nikken Seil, Shizuoka, Japan). Serum SOD activity was determined using the SOD Assay Kit-WST (Dojindo Molecular Technologies, Gaithersburg, MD, USA).
Analysis of renal function
Blood urea nitrogen (BUN) and Cr were measured to evaluate renal function using commercial kits (Sigma, St. Louis, MO) and a COBAS Mira chemical analyzer (Roche, Basel, Switzerland).
Statistical analysis
All data are expressed as mean ± standard deviation (SD) of 3 independent experiments with triplicate internal repeats. Multiple group means were compared by one-factor or two-factor analysis of variance (ANOVA) and paired means by use of the t test. A P<0.05 (two-tailed) was considered statistically significant.
Results
Intensive lifestyle intervention (IL-I) was more effective than common lifestyle intervention for sustaining reductions in BMI and glycosylated hemoglobin
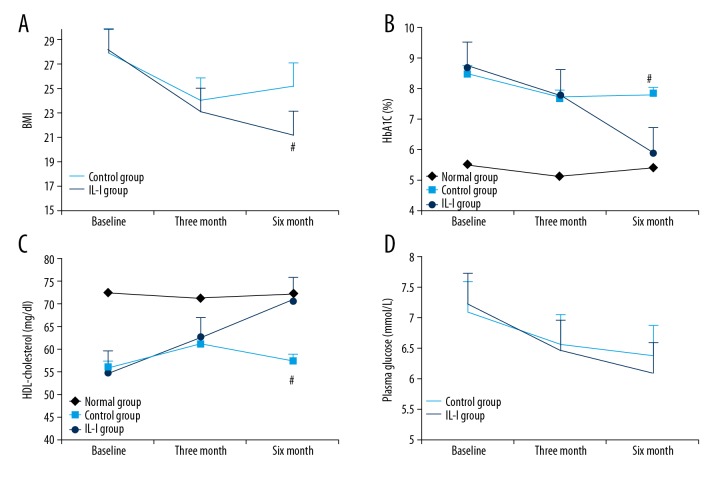
After 3 months, both the intensive lifestyle intervention (IL-I) group and common lifestyle intervention (control) group achieved significantly reduced BMI (Figure 1A, P<0.05) and increased HDL-cholesterol (Figure 1B, P<0.05). Exercise and caloric restriction also reduced blood glucose level, although the change from baseline did not reach statistical significance (Figure 1D, P>0.05). After 3 months, however, intervention compliance was significantly lower in the control group than at the start, while that of the IL-I group remained unchanged. In the control group, 6-month BMI was higher and HDL-cholesterol lower than at 3 months. On the contrary, the BMI in the IL-I group continued to decline and HDL-cholesterol remained elevated. In both groups, HbA1c continued to decline after 3 months, but HbA1c levels remained significantly lower in the IL-I group compared to the control group from month 3 to month 6 (Figure 1C, P<0.01).
Figure 1.
Effect of long-term intensive lifestyle intervention (IL-I) on metabolic health in diabetic nephropathy (DN) patients. (A–C) Compared to control intervention, IL-I produced a larger and more sustained (A) decrease in BMI (Pa, Pb, and Pd <0.05; Pc <0.01; Pab >0.05; Pcd <0.05), (B) increase in HDL-cholesterol (Pa and Pb <0.05; Pc <0.01; Pab and Pd >0.05; Pcd <0.05), and (C) reduction in HbA1c (Pa, Pb, and Pd >0.05; Pc <0.01, Pab >0.05; Pcd <0.05). (D) The 2 groups both demonstrated a downward trend in blood glucose that did not reach statistical significance (Pa, Pb, Pc, Pd, Pab, and Pcd >0.05). Pa, 3 months vs. baseline in the IL-I group; Pb, 3 months vs. baseline in the control group; Pab, IL-I vs. control group at 3 months; Pc, 6 months vs. baseline in the IL-I group; Pd, 6 months vs. baseline in the control group; Pcd, IL-I vs. control group at 6 months.
IL-I improved blood pressure in DN patients
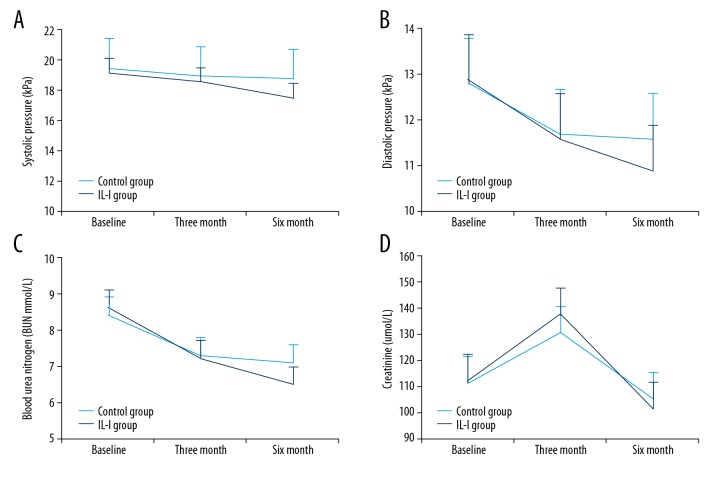
To evaluate the effects of IL-I on renal function, we measured creatinine and BUN in IL-I and controls, but found no significant group differences at 3 and 6 months (Figure 2C, 2D). Blood pressure is an independent risk factor for renal dysfunction. At 3 months, both groups achieved blood pressure reduction, but without statistical significance compared to baseline. At 6 months, however, both systolic and diastolic blood pressure were significantly lower in the IL-I group compared to baseline, but still were not significantly changed in the control group (Figure 2A, 2B).
Figure 2.
Effects of IL-I on renal function and blood pressure. (A, B) IL-I reduces (A) systolic blood pressure (Pa, Pb, and Pd >0.05; Pc <0.01; Pab >0.05; Pcd >0.05) and diastolic blood pressure at 6 months (Pa, Pb, and Pd >0.05; Pc <0.01; Pab >0.05; Pcd >0.05). (C, D) There were no significant changes in creatinine and blood urea nitrogen at 3 and 6 months (Pa, Pb, Pc, Pd, Pab, and Pcd >0.05).
IL-I can enhance antioxidant capacity and reduce serum inflammatory cytokines concentrations
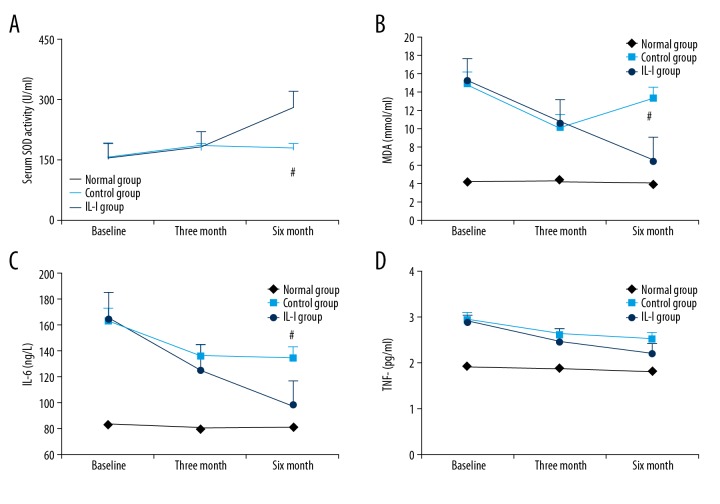
To evaluate the effects of exercise and caloric restriction on inflammatory status, we measured the serum concentrations of the antioxidant enzyme SOD, the membrane peroxidation product MDA, and the pro-inflammatory cytokines IL-6 and TNF-α. The serum concentration of SOD was significantly higher in both groups at 3 months, but continued to rise over the next 3 months only in the IL-I group, while SOD in the control group was actually lower at 6 months than at 3 months (Figure 3A). Also consistent with sustained improvement in systemic redox status, MDA levels was significantly reduced in both groups at 3 months, but was higher at 6 months compared to 3 months in the control group (Figure 3B). Serum IL-6 was also reduced significantly at 3 months in both groups, and remained significantly below baseline at 6 months (Figure 3C). Finally, TNF-α was also reduced at 3 and 6 months in both groups, but differences did not reach statistical significance (Figure 3D).
Figure 3.
Long-term intensive lifestyle intervention enhances antioxidant capacity and reduces inflammation in DN patients. (A, B) Both IL-I and control groups exhibited increased SOD and reduced MDA at 3 and 6 months, but the increase was greater and more sustained in the IL-I group (Pa and Pb <0.05; Pc <0.01; Pab and Pd >0.05; Pcd <0.05). (B) Both groups also demonstrated reduced MDA at 6 months, but the change was greater in the IL-I group (Pa and Pb <0.05, Pc <0.01, Pd and Pab >0.05, Pcd <0.01). (C) Both groups showed reduced serum IL-6 at 3 and 6 months (Pa, Pb, and Pd <0.05; Pc <0.01; Pab >0.05; Pcd <0.05). (D) Both groups exhibited a downward trend in plasma TNF-α, but the difference from baseline did not reach statistical significance (Pa, Pb, Pc, Pd, Pab, and Pcd >0.05).
IL-I can reduce the expression of TGF-β1 and oxidative stress
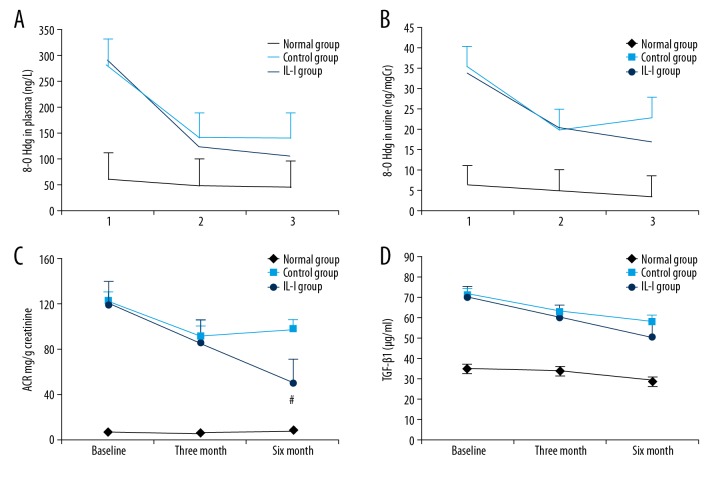
Oxidative damage to DNA and subsequent repair results in excretion of 8-hydroxy-2′-deoxyguanosine (8-OHdG), and reduced excretion is a marker for improvement of oxidative stress. Therefore, we also examined 8-OHdG in plasma and urine. The IL-I group had reduced concentrations of 8-OHdG in both plasma and urine (Figure 4A, 4B). As ACR is a marker of early renal injury, reduced ACR also can reflect improvement of renal function, so we also measured ACR. We found that the IL-I group demonstrated significantly reduced ACR compared to baseline at 3 and 6 months, while the early decrease in the control group at 3 months was not maintained at 6 months (Figure 4C). Renal fibrosis is the final common pathway for various forms of renal injury, and TGF-β1 is an important fibroblast-promoting factor. At 3 months, plasma TGF-β1 was reduced in both groups, but the change from baseline did not reach statistical significance. At 6 months, however, plasma TGF-β1 was significantly below baseline in the IL-I group but not in the control group (Figure 4D).
Figure 4.
Long-term intensive lifestyle intervention improves renal function in diabetic nephropathy. (A, B) IL-I reduced both plasma (A) and 24-h urine (B) 8-OHdG (in A: Pa, Pb, and Pd <0.05; Pc <0.01; Pab and Pcd >0.05; in B: Pa, Pb, and Pd <0.05; Pc <0.01; Pab and Pcd >0.05). (C) Both IL-I and control groups demonstrated reduced 24-h urine ACR at 3 and 6 months, but the decrease was larger in the IL-I group (Pa, Pb, and Pab <0.05; Pc <0.01; Pab >0.05; Pcd <0.05). (D) IL-I also induced a more sustained reduction in TGF-β1 (Pa, Pb, and Pd >0.05; Pc <0.05; Pab >0.05; Pcd >0.05).
Discussion
Diabetes and its complications have become a major threat to human health and a great economic burden on society [17]. Effective control of blood glucose and the prevention or delay of complications are the main goals of diabetes treatment and research. Previous studies have shown that exercise and caloric restriction play an important role in controlling glycemia and chronic inflammation in diabetics [18]. However, it is not clear whether exercise and caloric restriction can slow or stop the progression of diabetic nephropathy. Our study found that continuous and effective exercise and caloric restriction are of great benefit for reducing the inflammatory state of DN and retarding the development of renal failure by postponing renal fibrosis.
Many chronic diseases are associated with unhealthy lifestyles [19], and changing lifestyle may be important for recovery from disease [20,21]. However, without continuous supervision and management, it is difficult to change unhealthy habits. In our research, we found that effective supervision is of great importance for maintaining changes in patient lifestyle. In the control group, after 3 months of intensive supervision (2 times a week), the BMI of the patients declined and HDL-cholesterol increased from baseline. However, after the next 3 months of low intensity surveillance (1 call per month), BMI began to rebound and HDL-cholesterol decreased again. On the contrary, in the IL-I group receiving continuous high-intensity surveillance (called twice per week for 6 months), BMI continued to decline and HDL-cholesterol continued to rise over the final 3 months. This intensive surveillance had similar effects on glycosylated hemoglobin.
Rising serum HbA1c is indicative of diabetes progression [22]. Glycosylated hemoglobin initiates the expression of inflammatory factors [23], promotes oxidative stress [24] via ROS production [25], induces cell apoptosis [26], and ultimately leads to impairment of target organ function in diabetes [27]. The production of ROS promotes glomerular podocyte apoptosis and proteinuria, leading to deterioration of renal function [28]. Continuous inflammation can damage renal microvessels, resulting in ischemia and anoxia of renal tubules, atrophy of tubular epithelial cells, and functional defects, which further activate the renin-angiotensin-aldosterone (RAA) system and increase the progression of inflammation [29,30]. Therefore, effective reduction of inflammation can delay the deterioration of renal function.
Our subsequent findings further verified these assumptions. We found that inflammation index and redox index were better in the IL-I group than in the control group. As inflammation subsided, oxidative damage in the kidneys was restored and microalbuminuria and 8-OHdG were significantly reduced. Previous studies have confirmed that the improvement of kidney function is closely related to inhibition of the RAA system, and is more conducive to controlling blood pressure [31]. Our study confirms these findings and shows that lifestyle changes reinforced by continuous monitoring are more beneficial to blood pressure control than is simple distribution of health information.
Renal fibrosis is the end result of kidney injury, and TGF-β1 is an important fibroblast-promoting factor [32]. Thus, inhibition of TGF-β1 expression can effectively delay renal fibrosis [33,34]. Our study found that the expression of TGF-β1 was decreased continuously in DN patients under a long-term intensive lifestyle intervention, which may be related to the reduction of renal inflammation and improvement of tubule function. Previous studies have shown that inflammation, ischemia, and hypoxia can initiate renal fibrosis, and inhibition of fibrosis is critical for delaying uremia.
Conclusions
In this study, we confirmed that exercise and caloric control can delay renal damage and dysfunction. More importantly, changing patient lifestyle using a continuous intervention is of great value for improvement from DN. However, once the supervision is weakened, the patients may return to old habits, thereby reversing treatment effects. This may also be the limitation of “exercise and energy restriction”, which relies on patient self-management. However, our research period was relatively short (6 months), and it is not yet clear if life-long intervention will help patients form stable, healthy habits. Further research is needed on this issue.
Acknowledgments
We are grateful to Dr. Pan Yu for critically reading the manuscript and for valuable comments.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81470985) and Natural Science Foundation of Jiangsu Province (No. bk20150557) and the Scientific and Technological Research Projects of Chongqing Education Commission (KJQN201800412)
References
- 1.Furukawa M, Gohda T, Tanimoto M, Tomino Y. Pathogenesis and novel treatment from the mouse model of type 2 diabetic nephropathy. ScientificWorldJournal. 2013;9:281–97. doi: 10.1155/2013/928197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Wang Y, Zhang R, et al. Serum fibrinogen predicts diabetic ESRD in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;141:1–9. doi: 10.1016/j.diabres.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 3.Walker R, Marshall MR, Morton RL, et al. The cost-effectiveness of contemporary home haemodialysis modalities compared with facility haemodialysis: A systematic review of full economic evaluations. Nephrology (Carlton, Vic) 2014;19:459–70. doi: 10.1111/nep.12269. [DOI] [PubMed] [Google Scholar]
- 4.Beaudry A, Ferguson TW, Rigatto C, et al. Cost of dialysis therapy by modality in Manitoba. Clin J Am Soc Nephrol. 2018;13(8):1197–203. doi: 10.2215/CJN.10180917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han SS, Park JY, Kang S, et al. Dialysis Modality and mortality in the elderly: A meta-analysis. Clin J Am Soc Nephrol. 2015;10:983–93. doi: 10.2215/CJN.05160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chantrel F, de Cornelissen F, Deloumeaux J, et al. [Survival and mortality in ESRD patients]. Nephrol Ther. 2013;9(Suppl 1):S127–37. doi: 10.1016/S1769-7255(13)70042-7. [in French] [DOI] [PubMed] [Google Scholar]
- 7.Qi H, Casalena G, Shi S, et al. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes. 2017;66:763–78. doi: 10.2337/db16-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan S, Jena G, Tikoo K, Kumar V. Valproate attenuates the proteinuria, podocyte and renal injury by facilitating autophagy and inactivation of NF-kappaB/iNOS signaling in diabetic rat. Biochimie. 2015;110:1–16. doi: 10.1016/j.biochi.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: The beginning and end to diabetic nephropathy? Br J Pharmacol. 2014;171:1917–42. doi: 10.1111/bph.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato M, Natarajan R. Diabetic nephropathy – emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10:517–30. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang SC, Yiu WH, Lin M, Lai KN. Diabetic nephropathy and proximal tubular damage. J Ren Nutr. 2015;25:230–33. doi: 10.1053/j.jrn.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Grove KJ, Voziyan PA, Spraggins JM, et al. Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. J Lipid Res. 2014;55:1375–85. doi: 10.1194/jlr.M049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa D, Makino H. [Pathogenesis of diabetic nephropathy: The role of inflammation]. Nihon Rinsho. 2012;70(Suppl 5):389–92. [in Chinese] [PubMed] [Google Scholar]
- 14.Giroux V, Saidj S, Simon C, et al. Physical activity, energy expenditure and sedentary parameters in overfeeding studies – a systematic review. BMC Public Health. 2018;18:903. doi: 10.1186/s12889-018-5801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CW, Villani V, Buono R, et al. Fasting-mimicking diet promotes Ngn3-driven beta-cell regeneration to reverse diabetes. Cell. 2017;168:775–88e12. doi: 10.1016/j.cell.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dall TM, Yang W, Halder P, et al. The economic burden of elevated blood glucose levels in 2012: Diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2014;37:3172–79. doi: 10.2337/dc14-1036. [DOI] [PubMed] [Google Scholar]
- 18.Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13:435–44. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 19.Rutters F, Pilz S, Koopman AD, et al. The association between psychosocial stress and mortality is mediated by lifestyle and chronic diseases: The Hoorn Study. Soc Sci Med. 2014;118:166–72. doi: 10.1016/j.socscimed.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Schweier R, Romppel M. A web-based peer-modeling intervention aimed at lifestyle changes in patients with coronary heart disease and chronic back pain: sequential controlled trial. J Med Internet Res. 2014;16(7):e177. doi: 10.2196/jmir.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weston KS, Wisloff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br J Sports Med. 2014;48:1227–34. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 22.Gregorio PC, Favretto G, Sassaki GL, et al. Sevelamer reduces endothelial inflammatory response to advanced glycation end products. Clin Kidney J. 2018;11:89–98. doi: 10.1093/ckj/sfx074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franko B, Brault J, Jouve T, et al. Differential impact of glucose levels and advanced glycation end-products on tubular cell viability and pro-inflammatory/profibrotic functions. Biochem Biophys Res Commun. 2014;451:627–31. doi: 10.1016/j.bbrc.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 24.Elmhiri G, Mahmood DF, Niquet-Leridon C, et al. Formula-derived advanced glycation end products are involved in the development of long-term inflammation and oxidative stress in kidney of IUGR piglets. Mol Nutr Food Res. 2015;59:939–47. doi: 10.1002/mnfr.201400722. [DOI] [PubMed] [Google Scholar]
- 25.Giacco F, Du X, Carratu A, et al. GLP-1 cleavage product reverses persistent ROS generation after transient hyperglycemia by disrupting an ROS-generating feedback loop. Diabetes. 2015;64:3273–84. doi: 10.2337/db15-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin AH, El-Missiry MA, Othman AI. Melatonin ameliorates metabolic risk factors, modulates apoptotic proteins, and protects the rat heart against diabetes-induced apoptosis. Eur J Pharmacol. 2015;747:166–73. doi: 10.1016/j.ejphar.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Lizotte F, Denhez B, Guay A, et al. Persistent insulin resistance in podocytes caused by epigenetic changes of SHP-1 in diabetes. Diabetes. 2016;65:3705–17. doi: 10.2337/db16-0254. [DOI] [PubMed] [Google Scholar]
- 28.Sun LN, Liu XC, Chen XJ, et al. Curcumin attenuates high glucose-induced podocyte apoptosis by regulating functional connections between caveolin-1 phosphorylation and ROS. Acta Pharmacol Sin. 2016;37:645–55. doi: 10.1038/aps.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capitao M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016;117:2443–53. doi: 10.1002/jcb.25575. [DOI] [PubMed] [Google Scholar]
- 30.Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res. 2015;99:137–48. doi: 10.1016/j.phrs.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi A, Takabatake Y, Kimura T, et al. Autophagy inhibits the accumulation of advanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules. Diabetes. 2017;66:1359–72. doi: 10.2337/db16-0397. [DOI] [PubMed] [Google Scholar]
- 32.Decleves AE, Sharma K. Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nat Rev Nephrol. 2014;10:257–67. doi: 10.1038/nrneph.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EJ, Kang MK, Kim DY, et al. Chrysin inhibits advanced glycation end products-induced kidney fibrosis in renal mesangial cells and diabetic kidneys. Nutrients. 2018;10(7) doi: 10.3390/nu10070882. pii: E882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Y, Kim S, Lee SY, et al. Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol. 2014;25:2835–46. doi: 10.1681/ASN.2013101068. [DOI] [PMC free article] [PubMed] [Google Scholar]