Abstract
Objectives
Prosthetic joint infection (PJI) diagnosis is a major challenge in orthopaedics, and no reliable parameters have been established for accurate, preoperative predictions in the differential diagnosis of aseptic loosening or PJI. This study surveyed factors in synovial fluid (SF) for improving PJI diagnosis.
Methods
We enrolled 48 patients (including 39 PJI and nine aseptic loosening cases) who required knee/hip revision surgery between January 2016 and December 2017. The PJI diagnosis was established according to the Musculoskeletal Infection Society (MSIS) criteria. SF was used to survey factors by protein array and enzyme-linked immunosorbent assay to compare protein expression patterns in SF among three groups (aseptic loosening and first- and second-stage surgery). We compared routine clinical test data, such as C-reactive protein level and leucocyte number, with potential biomarker data to assess the diagnostic ability for PJI within the same patient groups.
Results
Cut-off values of 1473 pg/ml, 359 pg/ml, and 8.45 pg/ml were established for interleukin (IL)-16, IL-18, and cysteine-rich with EGF-like domains 2 (CRELD2), respectively. Receiver operating characteristic curve analysis showed that these factors exhibited an accuracy of 1 as predictors of PJI. These factors represent potential biomarkers for decisions associated with prosthesis reimplantation based on their ability to return to baseline values following the completion of debridement.
Conclusion
IL-16, IL-18, and CRELD2 were found to be potential biomarkers for PJI diagnosis, with SF tests outperforming blood tests in accuracy. These factors could be useful for assessing successful debridement based on their ability to return to baseline values following the completion of debridement.
Cite this article: M-F. Chen, C-H. Chang, L-Y. Yang, P-H. Hsieh, H-N. Shih, S. W. N. Ueng, Y. Chang. Synovial fluid interleukin-16, interleukin-18, and CRELD2 as novel biomarkers of prosthetic joint infections. Bone Joint Res 2019;8:179–188. DOI: 10.1302/2046-3758.84.BJR-2018-0291.R1.
Keywords: Prosthetic joint infection, Synovial fluid, Diagnosis, Interleukin-16, Biomarkers
Article focus
This study surveyed interleukin (IL)-16, IL-18, and cysteine-rich with EGF-like domains 2 (CRELD2) in synovial fluid (SF) for improving prosthetic joint infection (PJI) diagnosis and measured the efficacy of candidate factors not only as biomarkers for PJI diagnosis, but also for assessment of successful debridement.
Key messages
Using both clinical blood and synovial fluid specimens, we showed that SF IL-16, IL-18, and CRELD2 levels exhibited higher diagnostic accuracy for PJI than their levels in blood specimens.
Additionally, these factors might also represent potential biomarkers for decisions associated with prosthesis reimplantation based on their ability to return to baseline values after debridement is completed.
Strengths and limitations
Although establishment of such a scoring system requires continued efforts for marker isolation, our results identifying IL-16, IL-18, and CRELD2 as diagnostic markers of PJI and successful debridement represent a promising first step in this process.
The small number of samples (48) used in this study is a limitation.
Although the protein array approach has the advantage of being able to test the performance of many proteins at once, laboratory operational errors, as well as individual differences in patient SF samples, can affect the experimental results.
Introduction
Total joint arthroplasty is among the most frequently performed surgical procedures in orthopaedics.1 A prosthetic joint infection (PJI) is among the most severe complications of joint arthroplasty and remains the most common cause of total knee arthroplasty failure (25.2%) and the third most common indicator of hip arthroplasty revision (14.7%) in the United States.2,3 As the demand for joint arthroplasty is expected to increase substantially in the coming decades, the number of PJIs is also anticipated to escalate.4
Differentiating between PJI and aseptic loosening in hip or knee revision arthroplasty is a major challenge in PJI diagnosis.5,6 Most of the diagnostic criteria are based on combinations of various types of evidence, such as clinical findings, peripheral leucocyte counts, serum C-reactive protein (CRP) levels, microorganism culture data, and radiological interpretations, as well as evidence from other examinations, such as positron emission tomography.7,8 In 2011, a working group convened by the American Musculoskeletal Infection Society (MSIS) devised a new definition for PJI comprising a combination of clinical and laboratory diagnostic parameters.9 PJI was diagnosed by fulfilling one of the following three criteria: 1) sinus tract communicating with the prosthesis; 2) pathogen isolated from two or more samples obtained from the infected prosthetic joint; and 3) presence of purulence in the affected joint, along with elevated synovial white blood cell count and synovial polymorphonuclear neutrophil percentage (PMN%), combined with increased serum erythrocyte sedimentation rate (ESR) and serum CRP concentration.9,10 The PJI diagnostic criteria proposed by the MSIS are primarily based on the opinions or consensus of experts. To date, no reliable parameters have been established for providing accurate preoperative predictions in the differential diagnosis of aseptic loosening or PJI; therefore, PJI diagnoses are sometimes inaccurate, and improving these diagnoses remains a critical issue in orthopaedics.
Traditionally, several serum biomarkers, including ESR, CRP, interleukin (IL)-6, IL-1β, and procalcitonin, are applied for PJI diagnosis.11,12 IL-6 and IL-1β are secreted through stimulated immune cells, with both cytokines indicating the greatest decrease between first- and second-stage reimplantation procedures. This characteristic has the potential to be employed for monitoring treatment responses to PJIs; however, IL-6 and IL-1β criteria reportedly exhibit low sensitivity, making them susceptible to inaccurately ruling out infection before reimplantation.13 Serum procalcitonin has been applied for PJI diagnosis and reportedly exhibits high specificity (98%), but low sensitivity (33%), in the detection of PJIs.14,15 The shortcomings of these serum biomarkers of inflammation lie in their relatively low specificity or sensitivity levels, which might be influenced by age or sex and are frequently elevated in association with distant sites of infection or concurrent inflammatory conditions such as rheumatoid arthritis (RA).16 For example, in a group of patients who underwent total joint arthroplasty because of inflammatory arthropathies, such as RA, serum biomarkers, including ESR and CRP, might have remained high due to inflammatory status.17 Furthermore, the standard thresholds of ESR, CRP, IL-1, IL-6, and procalcitonin might vary considerably between clinical laboratories, with no consensus on a cut-off point for these values regarding PJI diagnosis.18
Compared with serum biomarkers, biomarkers in synovial fluid (SF) should provide higher sensitivity and specificity for PJI diagnosis. SF is readily obtained through preoperative or intraoperative aspiration. Its analysis can also provide valuable information regarding signalling pathways and the pathogenesis of PJIs. α-defensin is a naturally occurring antimicrobial peptide involved in the innate immune response and produced by neutrophils in response to pathogens. The presence of α-defensin in SF is reportedly associated with high sensitivity and specificity for PJI diagnosis;19,20 however, α-defensin sensitivity is unsatisfactory in the second-stage reimplantation phase of PJI treatment.12,19,21,22 Therefore, a survey of factors in SF capable of improving PJI diagnosis is urgently needed. The protein array method used in the study has the ability to multiplex screen the differential expression level of 155 proteins, which lends itself to a high-throughput diagnostic assay.
Patients and Methods
Patients and sampling
In this study, we enrolled 48 patients (24 female, 24 male) between January 2016 and December 2017 who required hip/knee revision surgery, including 39 PJI and nine aseptic loosening cases. PJI was defined by fulfilling one of the three MSIS criteria described above. All patients with aseptic loosening were scheduled to undergo revision arthroplasty. All patients with PJI were scheduled to undergo two-stage exchange arthroplasty.
Briefly, resection arthroplasty for PJI included radical debridement, prosthesis removal, and antibiotic-loaded cement-bone implantation and administration of systemic antimicrobial agents to control joint infection (first-stage surgery). The bone cements loaded with vancomycin (Vanco, Gentle Pharmaceutical Co., Yulin, Taiwan) and ceftazidime (Sintrix, China Chemical & Pharmaceutical Co. Ltd, Taichung, Taiwan), and those loaded with teicoplanin (Targocid, Sanofi-Aventis, Paris, France) and ceftazidime, yielded appropriate antibacterial results in our hospital.23 Delayed reimplantation of the prosthesis was performed after successful antimicrobial therapy, which was defined by the absence of signs of infection and return of ESR and serum CRP levels to normal (second-stage surgery).24 During the same enrolment period, nine patients (five hip, four knee) with aseptic loosening and who were scheduled for revision arthroplasty were enrolled as a control group. Specimens of joint fluid were collected by needle aspiration, immediately after opening the surgical wound, but before arthrotomy, to minimize contamination of blood. The patients were followed up every two weeks for three months before second-stage surgery. Patients with malignant tumours, and those who had received immunosuppressive agents, were excluded. The study was approved by the local institutional review board. Informed consent was obtained from all patients prior to study initiation.
SF specimens were delivered to the laboratory immediately after aspiration and centrifuged at 10 000 g to separate particulate and cellular material from each SF sample. The resulting supernatant was aliquoted and frozen at -80°C.
Protein array and enzyme-linked immunosorbent assay (ELISA)
SF was used to survey factors utilizing protein array panels containing 155 well-categorized monoclonal antibodies to compare SF-specific protein-expression patterns in three groups (aseptic loosening, first-stage surgery, and second-stage surgery (two patients in the aseptic loosening group and three patients in the PJI group)). Proteome Profiler Antibody arrays (Cat#ARY005B and Cat#ARY012; R&D Systems, Minneapolis, Minnesota) containing 155 spots were used for simultaneous evaluation of the expression levels of multiple factors, including cytokines, chemokines, and soluble receptors in SF. The signal-intensity levels of spots in the protein array were quantified using ImageJ software (National Institutes of Health, Bethesda, Maryland). Thereafter, candidate factors (i.e. IL-16, IL-18, CRELD2, E-selectin, lipocalin-2, and stromal-cell-derived factor 1 (SDF-1)) were selected for further measurement by ELISA (R&D Systems), with all ELISA measurements performed in triplicate. Thresholds for intra- and inter-assay coefficients of variation were set at < 15%, and the applicability of each candidate biomarker for PJI diagnosis was determined using receiver operating characteristic (ROC) curves.
Diagnostic and reimplantation biomarkers
For the treatment of PJI, a patient undergoes two-stage exchange arthroplasty for the removal of prosthesis, debridement, and implantation of an antibiotic-loaded polymethylmethacrylate (PMMA) spacer for joints with PJI (first-stage surgery). Three months after first-stage surgery, revision joint arthroplasty is performed after the infection is eradicated (second-stage surgery). Accurate confirmation of infection control is very important prior to performing the second-stage surgery. To evaluate the efficacy of the identified factors not only as diagnostic biomarkers, but also as prosthesis reimplantation biomarkers, we compared both blood- and SF-test values, obtained before and after surgical debridement, for the level of each potential biomarker (IL-16, IL-18, CRELD2, E-selectin, and lipocalin-2).
Statistical analysis
Data are reported as the mean and standard error of the mean, and were analyzed using GraphPad Prism software (GraphPad Software, San Diego, California) and R software (R Foundation for Statistical Computing, Vienna, Austria). Changes in spot intensity were compared using two-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. p-values for ELISA measurements were determined by Mann–Whitney U test. p < 0.05 was considered to be a statistically significant difference. Results for the candidate biomarkers were assessed by ROC curve analysis to determine thresholds, with test sensitivity plotted against (1 - specificity) for each tested threshold. The area under the curve (AUC) was calculated using the Mann–Whitney U test, and Wilcoxon’s signed-rank test was used to compare the expression levels of each biomarker before and after debridement in patient specimens.
Results
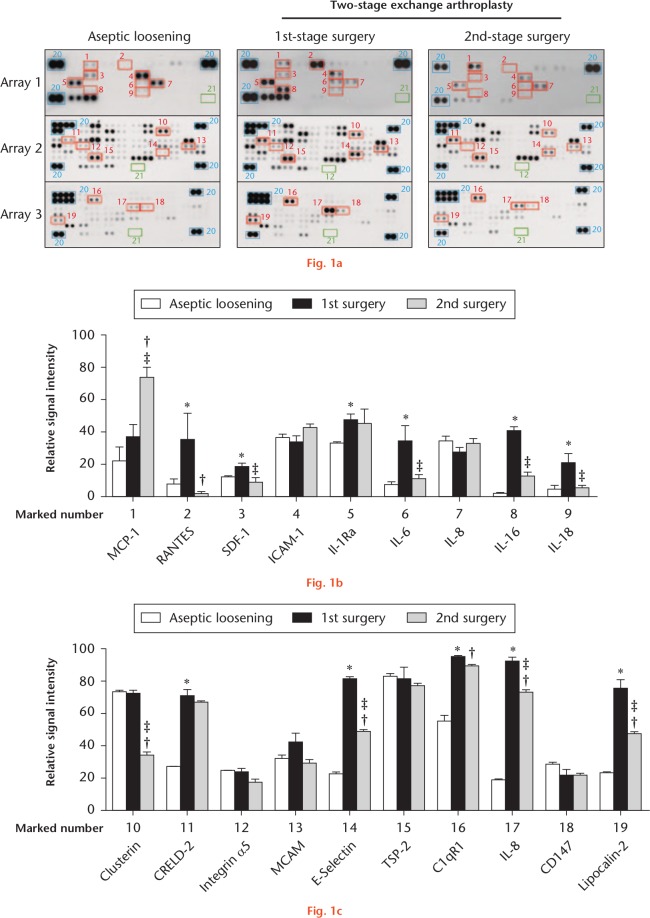
Protein microarray is a high-throughput method for tracking the expression of proteins on a large scale. The advantage is that a large number of proteins can be analyzed at one time, and the disadvantage is that the accuracy and labour cost are higher than those of the traditional ELISA method. Therefore, only five SF samples (two from aseptic and three PJI patients) were collected and analyzed by protein array assays. Each spot was measured according to intensity changes to identify potential biomarkers for PJI diagnosis (Fig. 1). SF samples from the PJI group were collected before and after first-stage surgery. Patient characteristics are summarized in Table I. All spot intensities were evaluated according to references and negative spots. We found that 19 of the 155 spots showed changes between different groups (Fig. 1a), with these spots selected for further intensity measurement (Fig. 1b). For spot-intensity analysis, we compared the expression levels of proteins associated with these spots between the three groups, resulting in 11 of the 19 proteins revealing elevated expression levels in the first-stage surgery (regulated on activation, normal T cell expressed and secreted (RANTES), SDF-1, interleukin-1 receptor antagonist (Il-1Ra), IL-6, IL-16, IL-18, CRELD2, E-selectin, C1qR1, IL-8, and lipocalin-2).
Fig. 1.
Different expression patterns shown in synovial fluid (SF) samples from different groups via protein array measurements: a) cytokine, chemokine, and soluble receptor expression in SF was measured for each group using protein array assays; b) and c) spots with high-intensity changes were measured by ImageJ software. Error bars represent the standard error of the mean (two patients in the aseptic loosening group and three patients in the PJI group). *p < 0.05, first-stage surgery versus aseptic loosening; †p < 0.05, second-stage surgery versus aseptic loosening; ‡p < 0.05, second-stage surgery versus first-stage surgery (two-way analysis of variance followed by Tukey’s post hoc test). MCP-1, monocyte chemoattractant protein 1; RANTES, regulated on activation, normal T cell expressed and secreted; SDF-1, stromal-cell-derived factor 1; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; Il-1Ra, interleukin-1 receptor antagonist; CRELD2, cysteine-rich with EGF-like domains 2; MCAM, melanoma cell adhesion molecule; TSP-2, thrombospondin-2.
Table I.
Characteristics of patients undergoing revision joint arthroplasty
| Parameter | Aseptic patients (n = 9) | Prosthetic joint infections (n = 39) |
|---|---|---|
| Male:female patients, n (%) | 1:8 (11:89) | 23:16 (59:41) |
| Mean age at surgery (sem; range) | 61.3 (3.2; 42 to 77) | 64.3 (2.1; 29 to 81) |
| Knee:hip joint prosthesis, n (%) | 4:5 (44:56) | 25:14 (64:36) |
sem, standard error of mean
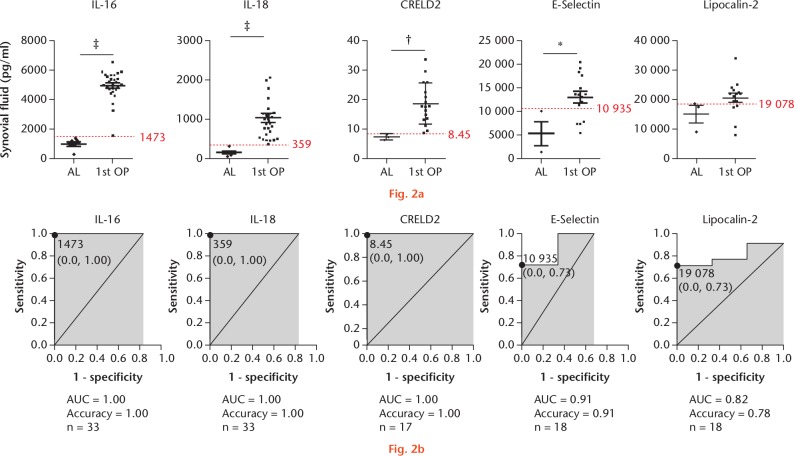
Previous studies have suggested that RANTES, IL-1Ra, IL-6, C1qR1, and IL-8 have low diagnostic accuracy for PJI;11,12,25 therefore, the mean concentration of the six remaining candidate factors (IL-16, IL-18, CRELD2, E-selectin, lipocalin-2, and SDF-1) were estimated (Table II; Fig. 2). Although the spot associated with SDF-1 showed a high intensity in the protein array, its concentration in SF was undetectable. We then compared differences in the SF-specific protein expression of each remaining factor between the aseptic group and the first-stage surgery group, revealing higher levels of each factor under infected conditions relative to those observed in aseptic conditions. Our data indicated SF IL-16, IL-18, and CRELD2 as highly promising potential predictors of PJI (Fig. 2a), with results indicating cut-off values of 1473 pg/ml, 359 pg/ml, and 8.45 pg/ml for these factors, respectively. Moreover, ROC analysis clearly demonstrated the excellent capabilities of SF IL-16, IL-18, and CRELD2 as predictors of PJI (Fig. 2b). A ROC curve was plotted for IL-16, IL-18, and CRELD2 and the areas under the curves were 1.00 (95% confidence interval (CI) 1.00 to 1.00); 1.00 (95% CI 1.00 to 1.00); and 0.82 (95% CI 0.627 to 1.017), respectively.
Table II.
Characteristics of test values in patients
| Parameter | Aseptic patients | Infected patients | |
|---|---|---|---|
| First-stage surgery | Second-stage surgery | ||
| Mean SF factors, pg/ml (sem; patients, n) | |||
| IL-16 | 983 (156; n = 6) | 4972 (187; n = 27) | 3107 (433; n = 11) |
| IL-18 | 174 (38; n = 6) | 1056 (116; n = 27) | 404 (65; n = 11) |
| CRELD2 | 7.3 (0.6; n = 3) | 18.7 (1.7; n = 17) | 12.2 (0.6; n = 10) |
| E-selection | 5440 (2563; n = 3) | 13 258 (1199; n = 15) | 10 257 (511; n = 9) |
| Lipocalin-2 | 15 124 (3044; n = 3) | 20 623 (1555; n = 15) | 18 925 (1658; n = 9) |
| Blood specimens, mean (sem; patients, n) | |||
| Plasma CRP, pg/ml | 2.0 (0.5; n = 9) | 52.4 (9.4; n = 39) | 15.4 (4.8; n = 29) |
| Leucocyte number, × 103 µl | 6.2 (0.3; n = 9) | 8.0 (0.6; n = 39) | 7.2 (0.4; n = 30) |
| Neutrophil percentage, % | 68.6 (1.8; n = 3) | 68.1 (2.2; n = 29) | 63.5 (2.1; n = 23) |
| Neutrophil number, × 103 µl | 4.7 (0.4; n = 3) | 6.1 (0.8; n = 26) | 4.8 (0.4; n = 23) |
| SF specimen, traditional factors, mean (sem; patients, n) | |||
| Leucocyte number, /µl | 571(287; n = 6) | 19 038 (3420; n = 29) | 3968 (2545; n = 18) |
| Neutrophil percentage, % | 34.5 (12.6; n = 6) | 78.2 (4.5; n = 29) | 39.5 (7.6; n = 18) |
| Neutrophil number, /µl | 300 (203; n = 6) | 18 144 (3297; n = 26) | 3498 (2365; n = 18) |
| Lymphocyte number, /µl | 191 (86; n = 6) | 891 (169; n = 26) | 136 (32; n = 18) |
| Monocyte number, /µl | 65 (36; n = 6) | 1663 (474; n = 24) | 346 (182; n = 16) |
SF, synovial fluid; sem, standard error of mean; IL, interleukin; CRELD2, cysteine-rich with EGF-like domains 2; CRP, C-reactive protein
Fig. 2.
Quantitation of prosthetic joint infection (PJI)-associated biomarkers shown in synovial fluid (SF) samples from different groups via enzyme-linked immunosorbent assay (ELISA) measurements. a) Five potential biomarkers were identified: interleukin (IL)-16, IL-18, cysteine-rich with EGF-like domains 2 (CRELD2), E-selection, and lipocalin-2. Patients undergoing first-stage surgery are depicted separately from the aseptic cohort. The dotted red line indicates the protein diagnostic threshold cut-off value. *p < 0.05; †p < 0.01; ‡p < 0.001. b) Receiver operating characteristic (ROC) curves for proteins showing area under the curve (AUC) values. AL, aseptic loosening; OP, operation.
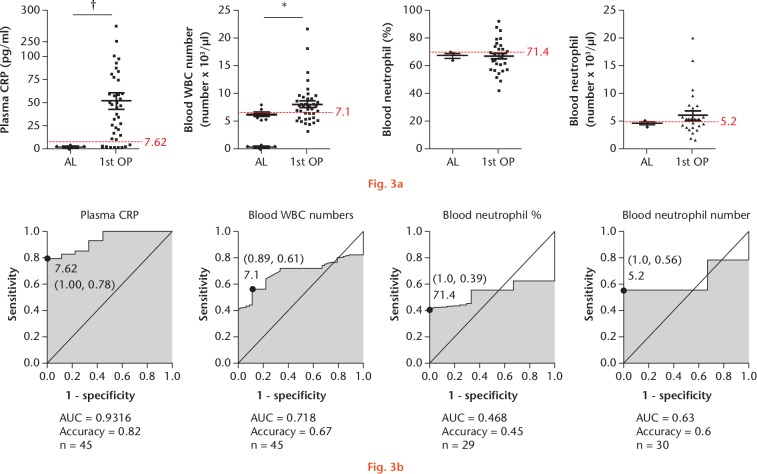
In recent years, differential leucocyte count and CRP concentration in both blood and SF specimens have been commonly used for PJI diagnosis. Therefore, we obtained clinical test data to evaluate differences in diagnostic capability between blood and SF specimens using the same groups of patients (Fig. 3). For test using blood specimens, we used cut-off values of 7.62 pg/ml, 7100 cells/µl, 71.4%, and 5200 cells/µl for plasma CRP, leucocyte count, blood neutrophil percentage, and blood neutrophil number, respectively (Fig. 3a), and determined that the diagnostic accuracy associated with each factor was 0.82, 0.67, 0.45, and 0.6, respectively (Fig. 3b). Plasma CRP showed a weak ability to distinguish between infection and non-infection groups (Fig. 3a), and we found that all blood-specific factors also showed low accuracy for PJI diagnosis. Moreover, ROC analysis confirmed that plasma CRP and blood-specific factors were poor predictors of PJI (Fig. 3b).
Fig. 3.
The relevant clinical laboratory findings identified in blood test in the aseptic and prosthetic joint infection (PJI) groups: a) factors in blood specimens, including C-reactive protein (CRP), leucocyte number, neutrophil percentage, and neutrophil number. Patients undergoing first-stage surgery are depicted separately from the aseptic cohort. The dotted red line indicates the protein diagnostic threshold cut-off value. *p < 0.05; †p < 0.01. b) Receiver operating characteristic (ROC) curves for proteins showing area under the curve (AUC) values. AL, aseptic loosening; OP, operation; WBC, white blood cell.
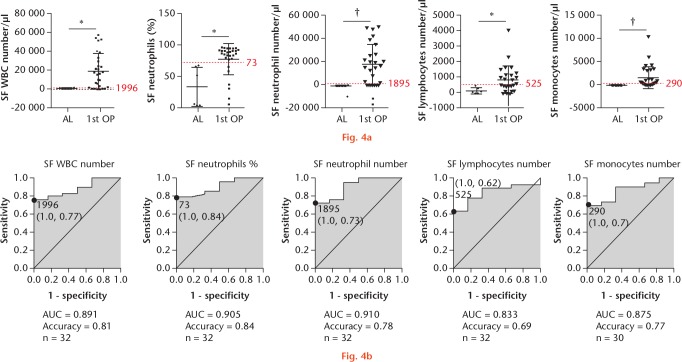
To determine the efficacy of SF specimens for PJI diagnosis (Fig. 4), we established cut-off values of 1996 cells/µl, 73%, 1895 cells/µl, 525 cells/µl, and 290 cells/µl for leucocyte number, neutrophil percentage, neutrophil number, lymphocyte number, and monocyte number, respectively (Fig. 4a).
Fig. 4.
The relevant clinical laboratory findings in the aseptic and prosthetic joint infection (PJI) groups identified in synovial fluid (SF) tests: a) factors in SF specimens, including leucocyte number, neutrophil percentage, lymphocyte percentage, monocyte percentage, neutrophil number, lymphocyte number, and monocyte number. Patients undergoing first-stage surgery are depicted separately from the aseptic cohort. The dotted red line indicates the protein diagnostic threshold cut-off value. *p < 0.05; ‡p < 0.001. b) Receiver operating characteristic (ROC) curves for proteins showing area under the curve (AUC) values. WBC, white blood cell; AL, aseptic loosening; OP, operation.
Our results showed that the diagnostic accuracy of the first four factors was 0.81, 0.84, 0.78, 0.69, and 0.77, respectively (Fig. 4b). These data revealed that SF tests returned results with higher diagnostic accuracy relative to blood tests.
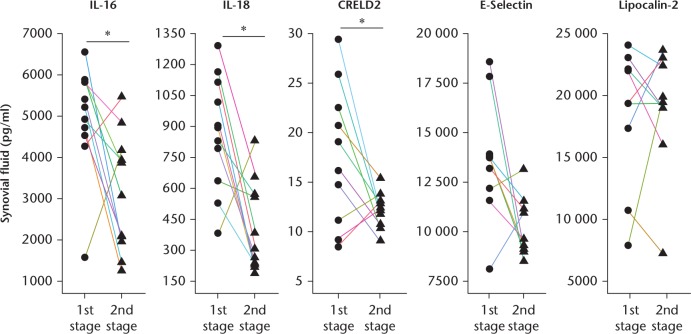
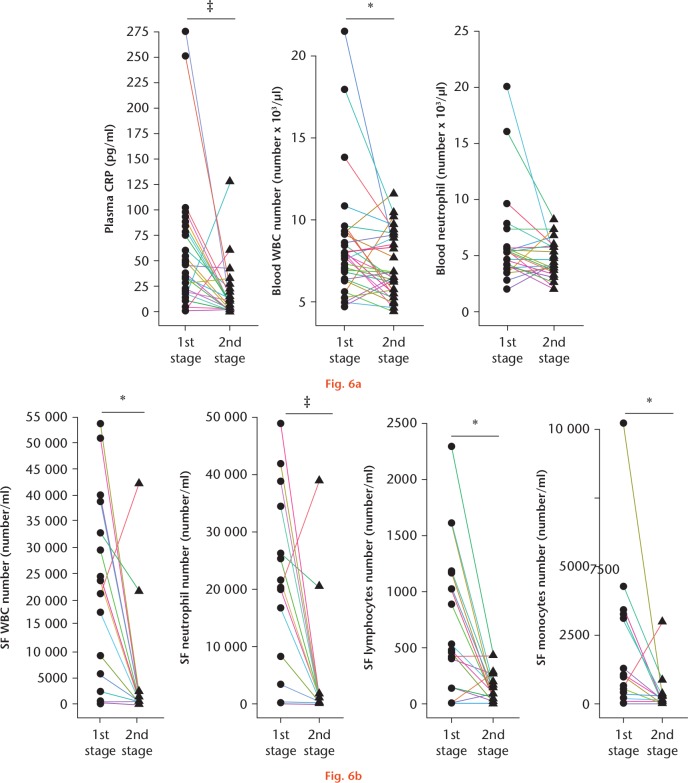
Furthermore, our results showed that IL-16, IL-18, and CRELD2 levels returned to their respective baseline values following the completion of debridement, suggesting them as potential biomarkers for determining the timing of prosthesis reimplantation (Fig. 5). Moreover, we compared test values from clinical laboratory findings derived before and after debridement, and found that plasma CRP concentration and blood leucocyte number also returned to their respective baseline levels after debridement completion (Fig. 6a). Furthermore, we verified that total leucocyte number, neutrophil number, lymphocyte number, and monocyte number in SF also returned to their respective baseline values after debridement completion (Fig. 6b). The test values of patients undergoing revision of joint arthroplasty are shown in Table II. These data indicated that IL-16, IL-18, and CRELD2 not only represent efficacious biomarkers for PJI diagnosis, but are also capable of determining successful debridement.
Fig. 5.
Comparison of biomarker expression levels before and after debridement in patient synovial fluid (SF). Factors in SF specimens, including interleukin (IL)-16, IL-18, cysteine-rich with EGF-like domains 2 (CRELD2), E-selection, and lipocalin-2. Each SF value represents levels in patients before (first-stage surgery) and three months after (second-stage surgery) joint debridement. *p < 0.05.
Fig. 6.
Comparison of biomarker expression levels in clinical laboratory findings before and after debridement in patient blood and synovial fluid (SF). a) Factors in blood specimens, including C-reactive protein (CRP), leucocyte number, and neutrophil number. b) Factors in SF specimens, including leucocyte number, neutrophil number, lymphocyte number, and monocyte number. Each value represents levels in patients before (first-stage surgery) and three months after (second-stage surgery) joint debridement. WBC, white blood cell. *p < 0.05; ‡p < 0.001.
Discussion
Although many serum biomarkers have been proposed for PJI diagnosis, those related to inflammation exhibit low specificity and sensitivity levels, can potentially be influenced by age or sex, and are frequently elevated as a result of concurrent inflammation or infection status.16 Inflammatory processes are induced by joint infection; therefore, the levels of many inflammatory factors will be elevated in SF obtained from PJI patients relative to specimens obtained from aseptic loosening patients. Protein array analysis is an effective tool for rapid detection of proteomic variations in clinical samples. Following identification of candidate factors by protein array, ELISA allows quantification of differences observed between aseptic loosening and PJI patients. Here, we used these methods to identify three factors (IL-16, IL-18, and CRELD2) that are potentially useful for increasing the accuracy of PJI diagnosis.
Based on previous studies of RANTES, Il-1Ra, IL-6, and IL-8,11,12,25-27 we chose to evaluate the diagnostic efficacy of SDF-1, IL-16, IL-18, CRELD2, E-selectin, and lipocalin-2. IL-16 is synthesized by several cells, including T cells, eosinophils, dendritic cells, fibroblasts, epithelial cells, and neuronal cells,28,29 and exhibits diagnostic and prognostic values for patients with diseases, such as gastric cancer,30 acute myocardial infarction,31 allergic conjunctival disorders,32 preeclampsia,33 Sezary syndrome,34 and RA.35 In the present study, we confirmed that elevated SF IL-16 levels represent a biomarker for PJI diagnosis.
IL-18 is a cytokine that belongs to the IL-1 superfamily and is produced by macrophages and other cells to induce cell-mediated immunity following microbial infection. IL-18 is reportedly a biomarker enabling differential diagnosis between adult-onset Still’s disease and sepsis,36 as well as between systemic lupus erythematosus patients with active renal disease and irreversible organ damage.37 Additionally, SF IL-18 level in RA patients is an accurate indicator of disease activity.38 In the present study, we demonstrated the efficacy of IL-18 for PJI diagnosis.
A previous report showed that CRELD2 plays a crucial role in regulating bone morphogenetic protein-9-induced osteogenic differentiation,39 and another study demonstrated urinary CRELD2 as a urinary endoplasmic reticulum (ER)-stress biomarker with potential utility in early diagnosis, risk stratification, treatment-response monitoring, and directing ER-targeted therapies in precision nephrology.40 In the present study, we demonstrated that SF CRELD2 levels represent potential biomarkers for PJI diagnosis.
CRP concentration and leucocyte number in both blood and SF specimens are common clinical markers used for laboratory examination in many hospitals. Here, we demonstrate that the diagnostic accuracy of IL-16, IL-18, and CRELD2 levels outperformed clinical laboratory findings in both blood and SF.
This study was not without limitations, including the small number of samples (n = 48) used. Furthermore, although the protein array approach has the advantage of being able to test the performance of many proteins at once, laboratory operational errors, as well as individual differences in patient SF samples, can affect the experimental results.
To our knowledge, this was the first study to use the protein array method to survey SF biomarkers, Based on the findings of this study and future diagnostic trends, we believe that single-molecule-based diagnosis is inadequate. A scoring system using multiple factors for PJI diagnosis would be optimal. Although the establishment of such a scoring system requires continued efforts to perform marker evaluation, the identification of IL-16, IL-18, and CRELD2 as diagnostic markers for PJI and successful debridement in this study represents a promising first step in the process.
Acknowledgments
We acknowledge the statistical analyses provided by the Biostatistics Unit, Clinical Trial Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan.
Footnotes
Author contributions: M-F. Chen: Designed the study, Acquired, analyzed, and interpreted data, Wrote and edited the manuscript.
C-H. Chang: Designed the study, Acquired, analyzed, and interpreted data.
L-Y. Yang: Analyzed and interpreted the data.
P-H. Hsieh: Designed the study, Acquired the data.
H-N. Shih: Designed the study, Acquired the data.
S. W. N. Ueng: Designed the study, Acquired the data.
Y. Chang: Designed the study, Acquired, analyzed, and interpreted data, Wrote and edited the manuscript.
Ethical review statement: The study protocol was approved by our institutional review board, and was compliant with accepted ethical standards at Chang Gung Memorial Hospital. Written informed consent was obtained from all patients prior to their participation in the study (Protocol Title: The optimization of diagnosis of prosthetic joint infection; Protocol Number: CRRPG3E0041; IRB Number: 105-1046C). This study was carried out in accordance with the ethical standards in the 1964 Declaration of Helsinki.
Follow us @BoneJointRes
Supplementary Material
Figure showing surgical procedures and synovial fluid collection timepoints for revision arthroplasty and two-stage exchange arthroplasty patients.
Funding statement
During the study period, the authors received grant funding support from the Ministry of Science and Technology, Taiwan (MOST 105- 2321-B-182A-004-MY3, MOST 106-2320-B-182A-017- and MOST 107-2314-B-182A-036 -MY3), from Chang Gung Memorial Hospital (CRRPG3E0041, CRRPG3E0042, CMRPG3F2011, CMRPG3F2012, CMRPG3F2013, and CRRPG3H0051), and from the Ministry of Health and Welfare of Taiwan (MOHW107-TDU-B-212-123005). The funders did not participate in study design, data collection, data interpretation and analysis, decision to publish, or preparation of the manuscript.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg [Am] 2004;86-A:963-974. [DOI] [PubMed] [Google Scholar]
- 2. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 2010;468:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg [Am] 2009;91-A:128-133. [DOI] [PubMed] [Google Scholar]
- 4. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 2012;27(8 Suppl):61-65.e1. [DOI] [PubMed] [Google Scholar]
- 5. Barrack RL, Aggarwal A, Burnett RS, et al. The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty 2007;22(6 Suppl 2):94-99. [DOI] [PubMed] [Google Scholar]
- 6. Saleh A, Guirguis A, Klika AK, et al. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty 2014;29:2181-2186. [DOI] [PubMed] [Google Scholar]
- 7. Peel TN, Buising KL, Choong PF. Diagnosis and management of prosthetic joint infection. Curr Opin Infect Dis 2012;25:670-676. [DOI] [PubMed] [Google Scholar]
- 8. Tarabichi M, Shohat N, Goswami K, Parvizi J. Can next generation sequencing play a role in detecting pathogens in synovial fluid? Bone Joint J 2018;100-B:127-133. [DOI] [PubMed] [Google Scholar]
- 9. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011;469:2992-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parvizi J. New definition for periprosthetic joint infection. Am J Orthop (Belle Mead NJ) 2011;40:614-615. [PubMed] [Google Scholar]
- 11. Mateos J, Lourido L, Fernández-Puente P, et al. Differential protein profiling of synovial fluid from rheumatoid arthritis and osteoarthritis patients using LC-MALDI TOF/TOF. J Proteomics 2012;75:2869-2878. [DOI] [PubMed] [Google Scholar]
- 12. Deirmengian C, Kardos K, Kilmartin P, et al. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res 2014;472:3254-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frangiamore SJ, Siqueira MB, Saleh A, et al. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res 2016;474:1630-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Worthington T, Dunlop D, Casey A, et al. Serum procalcitonin, interleukin-6, soluble intercellular adhesin molecule-1 and IgG to short-chain exocellular lipoteichoic acid as predictors of infection in total joint prosthesis revision. Br J Biomed Sci 2010;67:71-76. [DOI] [PubMed] [Google Scholar]
- 15. Bottner F, Wegner A, Winkelmann W, et al. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg [Br] 2007;89-B:94-99. [DOI] [PubMed] [Google Scholar]
- 16. Osei-Bimpong A, Meek JH, Lewis SM. ESR or CRP? A comparison of their clinical utility. Hematology 2007;12:353-357. [DOI] [PubMed] [Google Scholar]
- 17. Fink B, Makowiak C, Fuerst M, et al. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J Bone Joint Surg [Br] 2008;90-B:874-878. [DOI] [PubMed] [Google Scholar]
- 18. Parvizi J, Della Valle CJ. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg 2010;18:771-772. [DOI] [PubMed] [Google Scholar]
- 19. Frangiamore SJ, Gajewski ND, Saleh A, et al. α-defensin accuracy to diagnose periprosthetic joint infection-best available test? J Arthroplasty 2016;31:456-460. [DOI] [PubMed] [Google Scholar]
- 20. Sigmund IK, Holinka J, Gamper J, et al. Qualitative α-defensin test (Synovasure) for the diagnosis of periprosthetic infection in revision total joint arthroplasty. Bone Joint J 2017;99-B:66-72. [DOI] [PubMed] [Google Scholar]
- 21. Xie K, Qu X, Yan M. Procalcitonin and alpha-defensin for diagnosis of periprosthetic joint infections. J Arthroplasty 2017;32:1387-1394. [DOI] [PubMed] [Google Scholar]
- 22. Suen K, Keeka M, Ailabouni R, Tran P. Synovasure ‘quick test’ is not as accurate as the laboratory-based α-defensin immunoassay: a systematic review and meta-analysis. Bone Joint J 2018;100-B:66-72. [DOI] [PubMed] [Google Scholar]
- 23. Hsu YH, Hu CC, Hsieh PH, et al. Vancomycin and ceftazidime in bone cement as a potentially effective treatment for knee periprosthetic joint infection. J Bone Joint Surg [Am] 2017;99-A:223-231. [DOI] [PubMed] [Google Scholar]
- 24. Wheat LJ, Freifeld AG, Kleiman MB, et al. Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007;45:807-825. [DOI] [PubMed] [Google Scholar]
- 25. Beekhuizen M, Gierman LM, van Spil WE, et al. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthritis Cartilage 2013;21:918-922. [DOI] [PubMed] [Google Scholar]
- 26. Cuéllar VG, Cuéllar JM, Kirsch T, Strauss EJ. Correlation of synovial fluid biomarkers with cartilage pathology and associated outcomes in knee arthroscopy. Arthroscopy 2016;32:475-485. [DOI] [PubMed] [Google Scholar]
- 27. Neven B, Marvillet I, Terrada C, et al. Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum 2010;62:258-267. [DOI] [PubMed] [Google Scholar]
- 28. Ren F, Zhan X, Martens G, et al. Pro-IL-16 regulation in activated murine CD4+ lymphocytes. J Immunol 2005;174:2738-2745. [DOI] [PubMed] [Google Scholar]
- 29. Kaser A, Dunzendorfer S, Offner FA, et al. A role for IL-16 in the cross-talk between dendritic cells and T cells. J Immunol 1999;163:3232-3238. [PubMed] [Google Scholar]
- 30. Yang H, Han Y, Wu L, Wu C. Diagnostic and prognostic value of serum interleukin‑16 in patients with gastric cancer. Mol Med Rep 2017;16:9143-9148. [DOI] [PubMed] [Google Scholar]
- 31. Schernthaner C, Paar V, Wernly B, et al. Elevated plasma levels of interleukin-16 in patients with acute myocardial infarction. Medicine (Baltimore) 2017;96:e8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shoji J, Aso H, Inada N. Clinical usefulness of simultaneous measurement of the tear levels of CCL17, CCL24, and IL-16 for the biomarkers of allergic conjunctival disorders. Curr Eye Res 2017;42:677-684. [DOI] [PubMed] [Google Scholar]
- 33. Rădulescu C, Bacârea A, Huțanu A, Gabor R, Dobreanu M. Placental growth factor, soluble fms-like tyrosine kinase 1, soluble endoglin, IL-6, and IL-16 as biomarkers in preeclampsia. Mediators Inflamm 2016;2016:3027363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richmond J, Tuzova M, Parks A, et al. Interleukin-16 as a marker of Sézary syndrome onset and stage. J Clin Immunol 2011;31:39-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murota A, Suzuki K, Kassai Y, et al. Serum proteomic analysis identifies interleukin 16 as a biomarker for clinical response during early treatment of rheumatoid arthritis. Cytokine 2016;78:87-93. [DOI] [PubMed] [Google Scholar]
- 36. Priori R, Colafrancesco S, Alessandri C, et al. Interleukin 18: a biomarker for differential diagnosis between adult-onset Still’s disease and sepsis. J Rheumatol 2014;41:1118-1123. [DOI] [PubMed] [Google Scholar]
- 37. Mende R, Vincent FB, Kandane-Rathnayake R, et al. Analysis of serum interleukin (IL)-1β and IL-18 in systemic lupus erythematosus. Front Immunol 2018;9:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Petrovic-Rackov L, Pejnovic N. Clinical significance of IL-18, IL-15, IL-12 and TNF-alpha measurement in rheumatoid arthritis. Clin Rheumatol 2006;25:448-452. [DOI] [PubMed] [Google Scholar]
- 39. Zhang J, Weng Y, Liu X, et al. Endoplasmic reticulum (ER) stress inducible factor cysteine-rich with EGF-like domains 2 (Creld2) is an important mediator of BMP9-regulated osteogenic differentiation of mesenchymal stem cells. PLoS One 2013;8:e73086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim Y, Park SJ, Manson SR, et al. Elevated urinary CRELD2 is associated with endoplasmic reticulum stress-mediated kidney disease. JCI Insight 2017;2:92896. [DOI] [PMC free article] [PubMed] [Google Scholar]