Abstract
This paper reviews the current status of the newer inverted internal limiting membrane flap technique for macular hole surgery. It gives an overview of the importance of patient selection and special considerations along with variations in technique. It discusses the pathophysiology and how the technique has been an important addition in the armamentarium of vitreoretinal surgeons to attain better anatomical as well as functional results in challenging situations.
Keywords: macular hole, inverted internal limiting membrane flap technique, optical coherence tomography
Introduction
Macular holes (MH) are considered one of the main vitreoretinal interface disorders responsible for poor central vision and consist of an anatomical defect in the fovea with interruption of neural retinal layers from the internal limiting membrane (ILM) to the retinal pigment epithelium (RPE).1 The prevalence of idiopathic MH in the general population varies from 0.2 to 3.3 per 1,000.2,3
They mostly occur in the sixth to seventh decade of life with female preponderance.3 MH (MH) were first described more than a century ago and were considered to be traumatic in origin. However, now, the role of the vitreous in the pathogenesis of MH is better understood.4 Sebag described the role of anomalous posterior vitreous detachment in the formation of MH, and felt that persistent vitreopapillary adhesion in the presence of centrifugal tangential traction induces macular hole formation.5
The major breakthrough in the management of MH was in 1991, when Kelly and Wendel, first described the role of pars plana vitrectomy (PPV) and removal of posterior hyaloid for MH, and reported an anatomic closure rate of 58%.6 Prior to this MH were considered to be untreatable. Over the years, numerous variations in technique and post-operative strategy have been introduced with the objective of improving anatomical and visual outcomes and patient comfort.
A breakthrough in imaging, which helped in our understanding of the pathophysiology of macular hole formation and pioneered various modifications in the surgical technique was the advent of optical coherence tomography (OCT). Based on the accurate assessment of macular hole on OCT, a classification was proposed by the International Vitreomacular Traction Study (IVTS) Group. The IVTS group classified MH as small (≤250 μm), medium (250–400 μm) and large (>400 μm). This classification was based on the horizontal linear width which was measured at the narrowest point of the hole.1 The base diameter of the macular hole on OCT is also an important prognostic factor. Freeman and coworkers,7 found that MH with a smaller diameter were associated with better functional results. The size of the external limiting membrane defect on SDOCT has found to be the strongest predictor of visual acuity after surgery for MH.8
Preoperative considerations, prognostication and closure patterns
As discussed previously, the dimension of the macular hole has been found to be inversely proportional to the surgical success rate.
According to one study, the hole closure pattern has been divided into two types. A type 1 closure is the preferred configuration with complete closure and no associated defect of the neurosensory retina. In type 2 closure, the rim of neurosensory retina at the edge of the MH is attached to the RPE layer but there is an associated foveal defect of the neurosensory retina.9 In fact, the type 2 closure would be considered as an anatomically open MH. Reduction in MH diameter, flattening of the edges of the hole and improvement in vision, made the authors label this as a partially successful outcome. However in these flat-open MH, visual gain is generally limited.
Imai et al,10 studied the OCT pattern of successfully repaired and categorized the closure patterns into three categories as U-type with a normal foveal contour, V-type with a steep foveal contour and W-type with a foveal neurosensory retinal defect. They found the postoperative visual recovery to correlated to these with best results in U and worst in W-type patterns. Kang et al,9 felt that type 1 closure would correlate with a U or V pattern, while a type 2 closure would correlate with a W-type pattern.
The hole closure pattern has been found to depend on the preoperative macular hole diameter. Therefore, it has been seen that larger MH seem to result more often in type 2 closure postoperatively, and smaller MH in type 1 closure.
Refractory MH and challenges posed by them
The current gold standard of management of macular hole is PPV, induction of posterior vitreous detachment, dye-assisted ILM peeling, fluid–air exchange followed by gas tamponade. Literature has shown macular hole closure rate of 85–90% after primary surgery.11,12
The occurrence of persistent MH varies between 8% and 44% and has been found to be positively related with preoperative determinants like the stage of the MH, its size, chronicity, the inability of patients to maintain a prone position in a postoperative recovery phase, and residual epiretinal membranes (ERMs)13
Another challenging category of MH to tackle are secondary MH. These are related to pathologic conditions like trauma, high myopia,14 macular schisis,15 macular telangiectasia16 and uveitis. These are situations where the retinal surgeon faces challenges both during the surgery and with postoperative outcomes. MH with retinal detachment (RD) is another challenging situation and more so if seen in high myopia. In the past, several surgical methods have been tried to attain improvement anatomically and visually in these eyes. Vitrectomy with ILM peeling is one of the most successful surgical procedures for treating these cases. Literature has shown that this technique achieved a moderately high percentage of retinal reattachment ranging from 42% to 93%, but a comparatively unimpressive macular hole closure varying from 10% to 70%.17–19 An open macular hole in patients with high myopia is a continued risk for recurrent RD. Due to these shortcomings in the available techniques for macular hole closure and in an effort to improve the type of closure along with functional outcome, several new innovative methods have been proposed.
Inverted flap technique
In 2010, an innovative technique termed the “Inverted ILM flap technique” was described by Michalewska et al20. It was recommended as an effective surgical procedure for treating large idiopathic full thickness MH and myopic MH. The authors reported that this technique increased the rate of complete MH closure to 98% for large idiopathic MHs (diameter exceeding 400 μm), whereas in conventional vitrectomy with ILM peeling, 88% closure rate was achieved.20 They also reported a 100% macular hole closure rate in myopic MH.21 This procedure is based on putting the ILM into the MH without completely removing the ILM. This procedure was found to lead to the further successful bridging of the hole and thereby early closure.
This technique facilitates improved anatomical and functional results in complicated cases. Further, it reduces the number of patients with “flat-open” postoperative MH, along with a favorable visual outcome.
Mechanism of inverted flap technique
Histopathological mechanism
The histopathological mechanism of MH closure is thought to be multifactorial. It has been proposed that ILM acts as a scaffold, which helps in expediting the proliferation of various cells including myofibroblasts, fibrocytes and RPE.22
The rationale for peeling the ILM along with any ERM is to relieve tractional forces acting on the fovea. ILM peeling is also said to enhance the extensibility of the retina and Muller cell gliosis, both of which help in MH closure.
Several mechanisms have also been proposed to explain the tissue repair that occurs with the inverted ILM flap technique. The inverted ILM, which contains Müller cell fragments, is said to induce glial cell proliferation, thereby filling the MH and supporting MH closure. It may also work as a scaffold for tissue proliferation, creating a microenvironment that enhances correct photoreceptor positioning and finally improving postoperative anatomic and functional outcome.22 This hypothesis is in agreement with histopathologic findings suggesting that a basement membrane is required for cell proliferation. Because ILM is a basement membrane, it allows glial cell proliferation and theoretically allows large MH to fill with tissue over time.23
OCT-based observations on the mechanism
After the use of inverted ILM flap technique, 14–16% of MH are initially covered only with a thin ILM flap, termed as “flap closure.”24,25 It was also found that this technique might herald the restoration of foveal architecture in due course.
In a recent study by Boninska and its associates, flap closure was observed on OCT images 1 week after surgery in 100% of cases. However, 1 month after surgery, flap closure remained in only in 30.8% eyes and in the rest of cases there was a restoration of the neurosensory retinal architecture. The predominant final type of the foveal contour they observed was U shape.25 It has been hypothesized there is a gradual restoration of normal contour of fovea with this new technique. This starts with the reconstruction of the ELM which precedes restoration of the foveal ellipsoid zone.
Surgical technique
In the original technique described by Michalewska et al,20 vitrectomy was performed followed by trypan blue ILM staining. ILM was peeled around two disc diameters around the MH. In this procedure, the ILM was not removed completely from the retina but was left attached at the edges of the macular hole. Peripheral ILM was trimmed with a vitreous cutter. Therefore, small remnants of the ILM remained surrounding the macular hole. The ILM was then manipulated gently over the macular hole from all sides until it became inverted, such that the surface which normally faced the vitreous body was now towards the RPE. Patients were advised prone position for 3–4 days. (Figures 1 and 2)
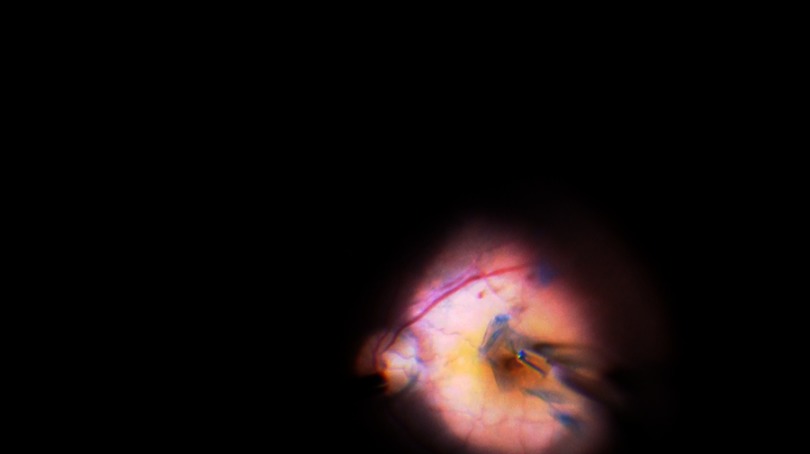
Figure 1.
Shows the intraoperative image of the technique. The ILM flap is stained with brilliant blue dye and then being raised and inverted into the MH using ILM peeling forceps.
Abbreviation: ILM, internal limiting membrane; MH, macular hole.
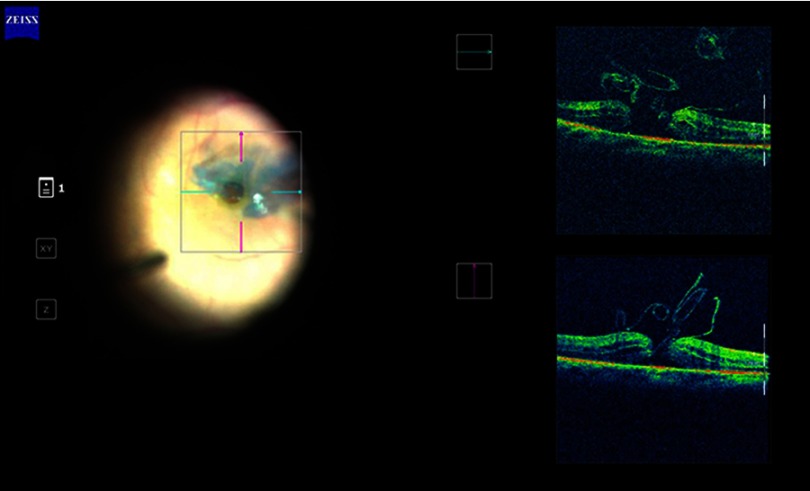
Figure 2.
Intraoperative SD OCT image of inverted ILM flap being manipulated into the MH. The ILM flap segments are seen clearly on the OCT scan.
Abbreviations: ILM, internal limiting membrane; MH, macular hole; OCT, optical coherence tomography.
Patient selection
Patient selection is extremely essential in order to reap the maximum benefits of the inverted ILM flap technique and maximize visual and anatomical outcome. With the advent of inverted ILM flap technique, several patients who previously would be refused surgery for fear of a poor anatomical and functional outcome are now being operated successfully. Some of the common indications are:
Large MH
Originally, this technique was tried in holes larger than 400 μ whereas other authors have successfully tried it for very large and extra-large MH. Yamashita and co-authors26 categorized extra-large MH as those with a diameter >550 μm. They compared results in these patients using conventional ILM peeling versus inverted ILM flap technique. In extra-large MHs, with the use of conventional ILM peeling technique, the closure rate was 88.4% (38/43) and 100% (41/41) by inverted ILM technique.
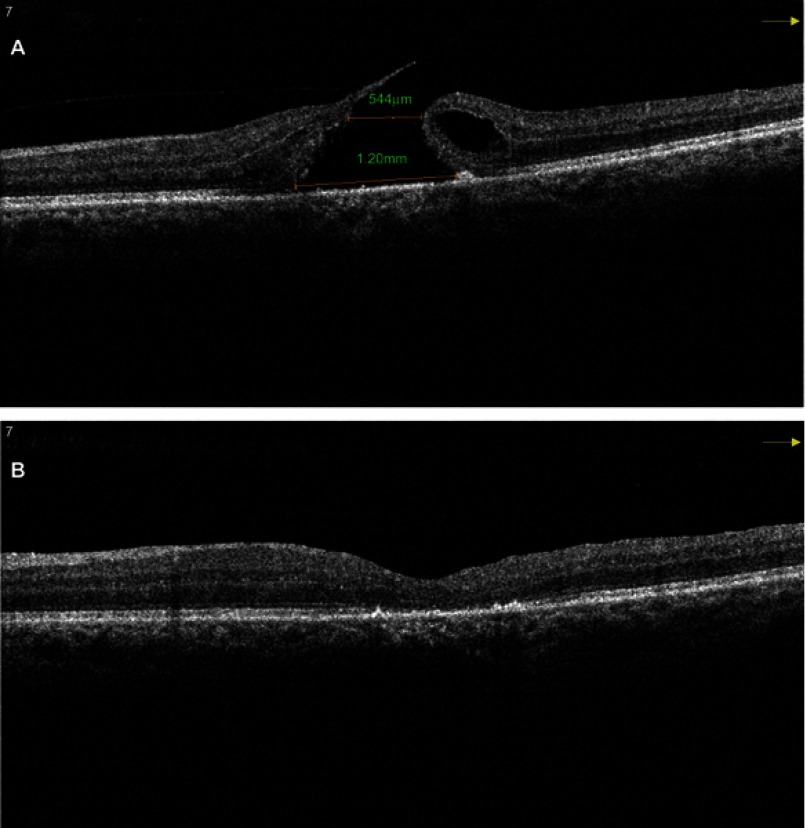
Okonkwo and associates27 studied extra-large MH which they defined as larger than 1,000 μm. In their study, the average symptom duration was 19 months. The average MH base diameter was 1,241 μm. All eyes achieved successful anatomical closure and there was no occurrence of a flat open type closure. A single arm meta-analysis which included eight studies wherein an inverted ILM flap technique was used to treat large MH (>400 μm) reported MH closure rate of 95% and VA improvement rate of 75%. They concluded that inverted ILM flap technique seemed to be a successful and safe method for treating large MH, with good closure rates and VA improvement.28 (Figure 3A and B show Pre and post-operative SD OCT images of a large MH with a U type closure after inverted ILM flap technique)
Figure 3.
(A) Pre-operative SD OCT of a large MH with a horizontal linear width of 544 μm and base diameter of 1,200 μm. (B) Post-operative SD OCT of the same patient shows a U-type successful closure after vitrectomy with the inverted ILM flap technique.
Abbreviations: SD OCT, sSpectral domain optical coherence tomography; MH, mMacular hole.
MH in high myopia
Myopic MH and their treatment pose a major surgical challenge. This is because the greater length of the posterior segment means that the residual neurosensory retina is unable to stretch and close the hole. This has been supported by various reports based on OCT studies which showed a lower correlation between anatomical and functional success rates with higher axial length.29 Another OCT-supported comparative study by Wu and Kung confirmed that the closure rate of myopic macular hole was much lower than in idiopathic cases ie 62.5% versus 94.1%.30
MH in high myopia that are closed with ILM peeling often have not only flat borders but also exposed pigment epithelium (these are flat-open MH) with limited visual acuity gain. This lead to several innovate techniques including suprachoroidal implants31 as well as macular buckling32 in order to close these challenging MH by changing the sector length.
Michalewska et al,21 expanded the indication of inverted ILM flap technique for MH caused by high myopia. The ILM flap does not alter the sector length but creates a scaffold to facilitate the neural tissue defect in these cases. They found encouraging results in the treatment of myopic MH with the inverted ILM flap technique. They reported that the rate of complete closure was 100% for high myopic MHs. They found that the foveal architectural repair continues for 12 months after the surgery. This technique allowed not only a high closure rate in myopic MHs, but also a functional improvement in visual acuity by a mean of 6 logMAR lines. It was observed that all MH closed without any evidence of a flat-open appearance. Hence, the inverted ILM flap technique improves not only the macular hole closure rate but also achieve good postoperative visual acuity in these cases.
Kuriyama et al,33 also used this procedure in Myopic MH. However, the surgical technique they used varied slightly from the original one in two ways. In their study, if ERM was present, it was not removed completely, but inverted along with the ILM. Secondly, the peripheral part of the ILM was not trimmed. The MH in their study were covered with inverted flaps that were larger than those described in the original technique.
Previous studies have found the anatomical and functional results to be worse in cases with macular retinoschisis. In their study, two eyes with macular retinoschisis had good final results. Thus, suggesting that the inverted ILM flap technique is successful even for MH surrounded by macular retinoschisis.
MH with RD
Patients with MH associated RD especially in high myopia present a challenging situation. Vitrectomy with membrane peeling was showed high anatomical reattachment rate, but the hole closure rate was low, usually around 50%. An open MH not only compromises central vision but also runs the risk of recurrent RD.
Chen et al,34 described a technique by inserting the inverted perifoveal ILM about 1.5-disc diameter in size into the hole to facilitate hole closure. They performed this technique in 20 cases and were able to attain a 100% closure rate in highly myopic eyes with MH-associated RD.
In another study, the same authors found that during the ILM insertion procedure, extended manipulation was sometimes required because the ILM tissue tends to fold back. In addition, part of the parafoveal ILM tissue may sometimes be torn away during ERM removal, leaving insufficient perifoveal ILM tissues for proper insertion. So they modified the inverted ILM flap technique by adding another piece of free ILM flap into the hole to address the above-mentioned problems. They found that this additional step made the plugging of the ILM tissue faster and the resultant ILM plug much more secure and described it as “Double ILM insertion” technique.35
A recent meta-analysis included four studies and 98 eyes. They studied the difference in best corrected visual acuity and macular hole closure rate, retinal reattachment in patients with a macular hole with RD. They found a significant difference in macular hole closure rate, and retinal reattachment in patients who underwent vitrectomy with inverted flap technique as compared to ILM peeling only.36
Traumatic MH
Traumatic MH (TMHs) tend to be large, irregular and have variable predictability. Being rare there are no large series on surgical intervention on TMH. They often have RPE and choroidal changes due to concussive forces, which would also limit the visual gain, even after successful anatomical closure. Few isolated reports, including one by our group, Astir et al,37 have found inverted flap technique a safe and effective surgical maneuver for such cases.
Patients with positioning issues
Patients who have undergone surgical procedures which preclude them from prone positioning present a challenging situation. Michalewska et al,38 reported a case who could not position post tracheostomy. The macular hole, in this case, closed eventually using the inverted flap technique with silicone oil tamponade. Takai et al,39 reported a case who could not position in view of a transdermal bladder catheter, this patient was unable to maintain the prone position, so the temporal inverted ILM flap technique was proposed. This technique apparently expedited bridge formation between the walls of the MH just beneath the ILM flap, which obviated the need for postoperative prone positioning.
Special considerations and variations
In this section, we would be taking up technical aspects of the inverted ILM technique as well as special considerations on how to execute the optimum peel. Several variations have been proposed and these along with their basis would be discussed. Other techniques proposed for refractory MH are also touched upon.
Temporal ILM flap
This was a modification by Michalewska et al,24 in which the ILM is peeled from the temporal side of the fovea only. A 2-disc-diameter area of ILM was removed from the temporal side of the fovea and inverted to cover the macular hole. Authors found no significant difference in initial and final visual acuities between conventionally inverted ILM flap and temporal ILM flap techniques. In both groups, photoreceptors and the external limiting membrane layer discontinuity reduced with time. Successive postoperative imaging revealed that patients with the dissociated optic nerve fiber layer appearance were less common in the temporal ILM flap technique. Their study results suggest that the temporal inverted ILM flap technique is as effective yet safer as the classic inverted ILM flap technique for the repair of large MH.
Cabbage leaf inverted ILM flap
This technique has been proposed by authors particularly in cases of chronic, large, full-thickness MH. In this procedure, multiple ILM flaps were inverted over each other covering the hole looking like cabbage leaves.40
Inverted ILM flap without extra manipulation
There is a controversy whether the inverted ILM flap should be tucked into the macular hole or should just cover it. In the original study by Michalewska et al,20 the ILM flap was tucked inside the macular hole, but subsequent studies have described only covering of the macular hole with the ILM flap. Surgical manipulation to tuck the ILM flap into the hole could potentially damage the retinal pigmentary epithelium at the base of the macular hole and compromise visual results. Chung and co-workers41 described a case in which no extra surgical manipulation was used to cover the macular hole with the ILM flap. They concluded that in case no extra surgical maneuver was used to cover the MH with the ILM flap, a longer period of observation was warranted as delayed MH closure was noted in these eyes without adversely affecting the final visual outcome.
Casini et al,42 presented a comparative study in which no extra surgical manipulation was used to cover the MH with the ILM flap. However, extreme care was taken during fluid-air exchange to keep the MH covered, regardless of which way the air pushed the flap. They found that surgical manipulation to tuck the ILM in the hole, the ILM massage and the manual covering of the hole were not necessary during the inverted ILM technique for large MH. The authors found that the modified technique using an inverted flap without manipulation of the graft seems to be safe and easy to perform, and it seems that it could reduce the risks of iatrogenic damage to the RPE in patients with large FTMH.
Other techniques for failed MH
Autologous blood or plasma
Chakrabarti et al,43 described a macular plug consisting of autologous gluconated blood plug (AGBL) for closing MH without need for prone positioning or any tamponade. They found this to be a safe procedure especially in patients unable to maintain prone positioning. In their series of 26 patients with large MH, 100% closure of the hole was achieved with a utilization of the inverted ILM flap and AGBL to form a macular plug
Lai et al,44 studied a modified technique of combining an inverted ILM flap and layering it with autologous blood for closing MH in high myopes with MH associated RD.
Neurosensory retinal flap
Grewal and Mahmoud45 described a new technique involving the use of an autologous neurosensory retinal free flap for closure of refractory myopic MH. This technique involves using an autologous neurosensory retinal free flap and positioning it over the MH to provide a scaffold and plug for hole closure.
Conclusion
To conclude the inverted flap technique is a promising addition to our armamentarium for treating MH. It has proved its efficacy in patients with challenging MH including large holes, highly myopic eyes and MH with RD. Not only does the inverted ILM flap technique increases the hole closure rate in these eyes, but it also allows a better and more natural type of foveal contour at closure and better visual results. Careful patient selection and further refinements in techniques in the future would enable vitreoretinal surgeons to successfully tackle such challenging situations.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi: 10.1016/j.ophtha.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 2.Casuso LA, Scott IU, Flynn HW Jr, et al. Long-term follow-up of unoperated macular holes. Ophthalmology. 2001;108:1150–1155. doi: 10.1016/S0161-6420(01)00581-4 [DOI] [PubMed] [Google Scholar]
- 3.Ezra E. Idiopathic full thickness macular hole: natural history and pathogenesis. Br J Ophthalmol. 2001;85(1):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson M. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537–567. [PMC free article] [PubMed] [Google Scholar]
- 5.Sebag J. Anomalous PVD – a unifying concept in vitreoretinal diseases. Graefes Arch Clin Exp Ophthalmol. 2004;242(8):690–698. doi: 10.1007/s00417-004-0980-1 [DOI] [PubMed] [Google Scholar]
- 6.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654–659. doi: 10.1001/archopht.1991.01080050068031 [DOI] [PubMed] [Google Scholar]
- 7.Freeman WR, Azen SP, Kim JW, el-Haig W, Mishell DR 3rd, Bailey I. Vitrectomy for the treatment of full-thickness stage 3 or 4 MH. Results of a multicentered randomized clinical trial. The vitrectomy for treatment of macular hole study group. macular hole study group. Arch Ophthalmol. 1997;115(1):11–21. [DOI] [PubMed] [Google Scholar]
- 8.Houly JR, Veloso CE, Passos E, Nehemy MB. Quantitative analysis of external lilmiting membrane, ellipsoid zone and interdigitation zone defects in patients with macular holes. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1297–1306. doi: 10.1007/s00417-017-3820-9 [DOI] [PubMed] [Google Scholar]
- 9.Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003;87(8):1015–1019. doi: 10.1136/bjo.87.8.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai M, Iijima H, Gotoh T, et al. Optical coherence tomography of successfully repaired idiopathic macular holes. Am J Ophthalmol. 1999;128(5):621–627. doi: 10.1016/S0002-9394(99)00200-7 [DOI] [PubMed] [Google Scholar]
- 11.Brooks HL Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000;107(10):1939–1948. doi: 10.1016/S0161-6420(00)00331-6 [DOI] [PubMed] [Google Scholar]
- 12.Christmas NJ, Smiddy WE, Flynn HW Jr. Reopening of MH after initially successful repair. Ophthalmology. 1998;105(10):1835–1838. doi: 10.1016/S0161-6420(98)91025-9 [DOI] [PubMed] [Google Scholar]
- 13.Tam ALC, Yan P, Gan NY, Lam WC. The current surgical management of large, recurrent or persistent macular holes. Retina. 2018;38(7):1263–1275. [DOI] [PubMed] [Google Scholar]
- 14.Alkabes M, Pichi F, Nucci P, et al. Anatomical and visual outcomes in high myopic macular hole (HM-MH) without retinal detachment: a review. Graefes Arch Clin Exp Ophthalmol. 2014;252(2):191–199. doi: 10.1007/s00417-013-2540-z [DOI] [PubMed] [Google Scholar]
- 15.Shukla D, Naresh KB, Rajendran A, Kim R. Macular hole secondary to X-linked retinoschisis. Eye (Lond). 2006;20:1459–1461. doi: 10.1038/sj.eye.6702338 [DOI] [PubMed] [Google Scholar]
- 16.Shukla D. Evolution and management of macular hole secondary to type 2 idiopathic macular telangiectasia. Eye (Lond). 2011;25(4):532–533. doi: 10.1038/eye.2010.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ripandelli G, Coppe AM, Fedeli R, Parisi V, D’Amico DJ, Stirpe M. Evaluation of primary surgical procedures for retinal detachment with macular hole in highly myopic eyes: a comparison of vitrectomy versus posterior episcleral buckling surgery. Ophthalmology. 2001;108(12):2258–2264. doi: 10.1016/S0161-6420(01)00861-2 [DOI] [PubMed] [Google Scholar]
- 18.Lim LS, Tsai A, Wong D, et al. Prognostic factor analysis of vitrectomy for retinal detachment associated with myopic MH. Ophthalmology. 2014;121(1):305–310. doi: 10.1016/j.ophtha.2013.08.033 [DOI] [PubMed] [Google Scholar]
- 19.Seike C, Kusaka S, Sakagami K, Ohashi Y. Reopening of MH in highly myopic eyes with retinal detachments. Retina. 1997;17(1):2–6. doi: 10.1097/00006982-199701000-00001 [DOI] [PubMed] [Google Scholar]
- 20.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large MH. Ophthalmology. 2010;117(10):2018–2025. doi: 10.1016/j.ophtha.2010.02.011 [DOI] [PubMed] [Google Scholar]
- 21.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J. Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina. 2014;34(4):664–666. doi: 10.1097/IAE.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 22.Spiteri Cornish K, Lois N, Scott N, et al. Vitectomy with internal limiting membrane (ILM) peeling versus vitrectomy with no peeling for idiopathic full thickness macular hole (FTMH). Cochrane Database Syst Rev. 2013;5(6):Cd009306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh J, Yang SM, Choi YM, Kim SW, Huh K. Glial proliferation after vitrectomy for a macular hole: a spectral domain optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):477–484. doi: 10.1007/s00417-012-2058-9 [DOI] [PubMed] [Google Scholar]
- 24.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, et al. Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: a comparative study. Retina. 2015;35(9):1844–1850. doi: 10.1097/IAE.0000000000000555 [DOI] [PubMed] [Google Scholar]
- 25.Bonnska K, Nawrocki J, Michalewska Z. Mechanism of flap closure after the inverted internal limiting membrane flap technique. Retina. 2018;38(11):2184–2189. doi: 10.1097/IAE.0000000000001861 [DOI] [PubMed] [Google Scholar]
- 26.Yamashita T, Sakamoto T, Terasaki H, et al. Best surgical technique and outcomes for large MH: retrospective multicentre study in Japan. Acta Ophthalmol. 2018. doi: 10.1111/aos.13795-] [DOI] [PubMed] [Google Scholar]
- 27.Okonkwo ON, Hassan AO, Oderinlo O. Inverted internal limiting membrane flap technique for extra large MH. Cur Tre Opthol. 2018;1(1):07–13. [Google Scholar]
- 28.Gu C, Qiu Q. Inverted internal limiting membrane flap technique for large MH: a systematic review and single-arm meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2018;256(6):1041–1049. doi: 10.1007/s00417-018-3956-2 [DOI] [PubMed] [Google Scholar]
- 29.Suda K, Hangai M, Yoshimura N. Axial length and outcomes of macular hole surgery assessed by spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;151(1):118–127. doi: 10.1016/j.ajo.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 30.Wu TT, Kung YH. Comparison of anatomical and visual outcomes of macular hole surgery in patients with high myopia vs. non-high myopia: a case-control study using optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):327–331. doi: 10.1007/s00417-011-1821-7 [DOI] [PubMed] [Google Scholar]
- 31.El Rayes EN. Supra choroidal buckling in managing myopic vitreoretinal interface disorders: 1-year data. Retina. 2014;34(1):129–135. doi: 10.1097/IAE.0b013e31828fcb77 [DOI] [PubMed] [Google Scholar]
- 32.Alkabes M, Mateo C. Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):863–877. doi: 10.1007/s00417-018-3947-3 [DOI] [PubMed] [Google Scholar]
- 33.Kuriyama S, Hayashi H, Jingami Y, Kuramoto N, Akita J, Matsumoto M. Efficacy of inverted internal limiting membrane flap technique for the treatment of macular hole in high myopia. Am J Ophthalmol. 2013;156(1):125–131. doi: 10.1016/j.ajo.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 34.Chen SN, Yang CM. Inverted internal limiting membrane insertion for macular hole-associated retinal detachment in high myopia. Am J Ophthalmol. 2016;162:99–106. doi: 10.1016/j.ajo.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 35.Chen SN, Yang CM. Double internal limiting membrane insertion for macular hole associated retinal detachment. J Ophthamol. 2017; doi: 10.1155/2017/3236516 Epub 2017 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan J, Zhang LL, Lu YJ, Han MY, Yu AH, Cai XJ. Vitrectomy with internal limiting membrane peeling versus inverted internal limiting membrane flap technique for macular hole-induced retinal detachment:a systematic review of literature and meta-analysis. BMC Ophthalmol. 2017;17(1):219. doi: 10.1186/s12886-017-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Astir S, Shroff D, Gupta C, Shroff C. Inverted flap technique for a large traumatic macular hole with choroidal rupture and subretinal hemorrhage. Oman J Ophthalmol. 2018;11(1):68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michalewska Z, Nawrocki J. Macular hole surgery in a patient who cannot maintain facedown positioning. Case Rep Ophthalmol. 2013;4(1):1–6. doi: 10.1159/000343701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takai Y, Tanito M, Sugihara K, Kodama T, Ohira A. Temporal internal limiting membrane flap technique for a macular hole patient unable to maintain postoperative prone positioning. Retin Cases Brief Rep. 2016;10(4):323–326. doi: 10.1097/ICB.0000000000000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aurora A, Seth A, Sanduja N. Cabbage leaf inverted flap ILM peeling for macular hole: a novel technique. Ophthalmic Surg Lasers Imaging Retina. 2017;48(10):830–832. doi: 10.3928/23258160-20170928-08 [DOI] [PubMed] [Google Scholar]
- 41.Chung CY, Wong DS, Li KK. Is it necessary to cover the macular hole with the inverted internal limiting membrane flap in macular hole surgery? A case report. BMC Ophthalmol. 2015;15:115. doi: 10.1186/s12886-015-0104-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casini G, Mura M, Figus M, et al. Inverted internal limiting membrane flap technique for macular hole surgery without extra manipulation of the flap. Retina. 2017;37(11):2138–2144. doi: 10.1097/IAE.0000000000001470 [DOI] [PubMed] [Google Scholar]
- 43.Chakrabarti M, Benjamin P, Chakrabarti K, Chakrabarti A. Closing MH with “macular plug” without gas tamponade and postoperative posturing. Retina. 2017;37(3):451–459. doi: 10.1097/IAE.0000000000001206 [DOI] [PubMed] [Google Scholar]
- 44.Lai CC, Chen YP, Wang NK, et al. Vitrectomy with internal limiting membrane repositioning and autologous blood for macular hole retinal detachment in highly myopic eyes. Ophthalmology. 2015;122(9):1889–1898. doi: 10.1016/j.ophtha.2015.05.040 [DOI] [PubMed] [Google Scholar]
- 45.Grewal DS, Mahmoud TH. Autologous neurosensory retinal free flap for closure of refractory myopic MH. JAMA Ophthalmol. 2016;134(2):229. doi: 10.1001/jamaophthalmol.2015.5237 [DOI] [PubMed] [Google Scholar]