Abstract
White matter degradation has been proposed as one possible explanation for age-related cognitive decline. In the present study, we examined 2 main questions: 1) Do diffusion characteristics predict longitudinal change in cognition independently or synergistically with amyloid status? 2) Are the effects of diffusion characteristics on longitudinal cognitive change tract-specific or global in nature? Cognitive domains of executive function, episodic memory, and processing speed were measured annually (mean follow-up = 3.93 ± 1.25 years). Diffusion tensor imaging and Pittsburgh Compound-B positron emission tomography were performed at baseline in 265 clinically normal older adults (aged 63–90). Tract-specific diffusion was measured as the mean fractional anisotropy (FA) for 9 major white matter tracts. Global diffusion was measured as the mean FA across the 9 white matter tracts. Linear mixed models demonstrated independent, rather than synergistic, effects of global FA and amyloid status on cognitive decline. After controlling for amyloid status, lower global FA was associated with worse longitudinal performance in episodic memory and processing speed, but not executive function. After accounting for global FA, none of the individual tracts predicted a significant change in cognitive performance. These findings suggest that global, rather than tract-specific, diffusion characteristics predict longitudinal cognitive decline independently of amyloid status.
Keywords: aging; amyloid; cognition, MRI; white matter diffusion
How does white matter degradation relate to cognitive decline during aging? Numerous studies have demonstrated that reduced white matter microstructure is associated with poorer cognitive performance in clinically normal older adults (Davis et al. 2009; Kennedy and Raz 2009; Brickman et al. 2012; Madden et al. 2012; Gazes et al. 2016). However, there is little consensus in the literature regarding the cognitive domains impacted and whether the effects are global in nature or are related to specific white matter tracts (for review see Bennett and Madden 2014). Most of these studies have used cross-sectional designs; however, longitudinal studies are necessary for directly estimating the effects of white matter microstructure on cognition over time (Charlton et al. 2010; Lövdén et al. 2014; Ritchie et al. 2015; Köhncke et al. 2016; Fjell et al. 2017; Scott et al. 2017). In the present study, we investigated the relationship between white matter diffusion characteristics and longitudinal cognitive change in healthy older adults and in individuals with preclinical Alzheimer’s disease, as defined by elevated amyloid burden (Sperling et al. 2011; Dubois et al. 2016).
Microstructural alterations of white matter can be investigated in vivo using diffusion tensor imaging (DTI; Basser et al. 1994; Le Bihan 2003). DTI studies of healthy older adults consistently demonstrate widespread age-related changes in white matter diffusion characteristics, as evidenced by lower fractional anisotropy (FA) and higher diffusivity (Head et al. 2004; Salat et al. 2005; Gunning-Dixon et al. 2009; Barrick et al. 2010; Voineskos et al. 2012; Bennett and Madden 2014; Lövdén et al. 2014; Sexton et al. 2014; Cox et al. 2016; Rieckmann et al. 2016). Amyloid burden has also been related to white matter microstructure (Chao et al. 2013; Gold et al. 2014; Molinuevo et al. 2014; Rieckmann et al. 2016), possibly due to toxicity to glial cells that form the myelin sheath (Xu et al. 2001; Lee et al. 2004). Accelerated decline in white matter microstructure has been observed in select tracts in preclinical Alzheimer’s disease, including the fornix, corpus callosum, and parahippocampal cingulum (Chao et al. 2013; Gold et al. 2014; Molinuevo et al. 2014; Rieckmann et al. 2016), suggesting that amyloid pathology may have a predilection for particular white matter tracts early in the disease process.
Prior DTI studies have demonstrated that reduced white matter microstructure (i.e., decreased FA, increased diffusivity) is linked to poorer cognitive performance, and generally find stronger relationships between white matter microstructure and executive function and processing speed compared with episodic memory (Madden et al. 2012). However, there is substantial variability across studies. For instance, some studies demonstrate relationships with executive function (O’Sullivan et al. 2001; Grieve et al. 2007; Perry et al. 2009; Zahr et al. 2009; Ryan et al. 2011; Brickman et al. 2012; Cremers et al. 2016; Hedden et al. 2016; Fjell et al. 2017), whereas others do not (Burgmans et al. 2011; Laukka et al. 2013; Lövdén et al. 2014). Some studies demonstrate relationships with processing speed (Kennedy and Raz 2009; Penke et al. 2010; Kerchner et al. 2012; Salami et al. 2012; Laukka et al. 2013; Lövdén et al. 2014; Cremers et al. 2016; Hedden et al. 2016; Kuznetsova et al. 2016), whereas others fail to find such relationships (Charlton et al. 2008; Perry et al. 2009; Ritchie et al. 2015). Similarly, several studies find relationships with episodic memory (Persson et al. 2006; Kennedy and Raz 2009; Ziegler et al. 2010; Ryan et al. 2011; Lockhart et al. 2012; Voineskos et al. 2012; Bennett et al. 2015; Fjell et al. 2015; Ly et al. 2016), whereas others do not (Penke et al. 2010; Salami et al. 2012; Borghesani et al. 2013; Cremers et al. 2016). Some potential difficulties in interpreting these findings include inconsistencies across studies in the cognitive domains included for analysis, the specific tests used to assess the different cognitive domains, and the selection of white matter tracts examined.
A related debate in the literature concerns whether age-related cognitive decline is due to alterations within specific white matter tracts or to global white matter changes occurring across the whole brain (Bennett and Madden 2014). Tract-specific effects have been observed across several cognitive domains (Kennedy and Raz 2009; Madden et al. 2009; Zahr et al. 2009; Ryan et al. 2011; Voineskos et al. 2012; Lövdén et al. 2014; Bennett et al. 2015; Cremers et al. 2016; Lancaster et al. 2016; Ly et al. 2016), however, there are inconsistencies across studies regarding which tracts display significant relationships. In contrast, other researchers have found evidence in favor of a global effect (Grieve et al. 2007; Penke et al. 2010; Haász et al. 2013; Kuznetsova et al. 2016; Fjell et al. 2017), which is consistent with the observation that white matter tracts generally show high correlations with one another (Penke et al. 2010; Lövdén et al. 2014; Cox et al. 2016). Discrepancies across studies may relate to the selection of white matter tracts examined, the specific cognitive tests used, and whether studies separately control for global white matter when examining tract-specific effects.
One challenge is that the majority of studies examining the relationship between white matter diffusion characteristics and cognition have been cross-sectional in design. An advantage of longitudinal designs is that within-person change can be directly examined. The few longitudinal studies addressing this issue in clinically normal older adults have had relatively short follow-up periods (Charlton et al. 2010; Lövdén et al. 2014; Ritchie et al. 2015; Köhncke et al. 2016). Studies with longer and more frequent follow-up periods are essential to better assess the influence of diffusion characteristics on cognitive decline in aging.
The present study asked 2 main questions. Given the potential impact of amyloid pathology on white matter microstructure (Chao et al. 2013; Gold et al. 2014; Molinuevo et al. 2014; Rieckmann et al. 2016) and cognition (Hedden et al. 2013, 2016; Jagust 2016; Mormino et al. 2017), the first question was: Do diffusion characteristics predict longitudinal change in cognition independently or synergistically with amyloid status? The second question was: Are the effects of diffusion characteristics on longitudinal cognitive change tract-specific or global in nature? Here, we also examined whether tract-specific diffusion characteristics predict longitudinal cognitive change independently or synergistically with amyloid status. To address the question of tract-specificity, we examined a large set of tract-specific relationships to each cognitive domain while controlling for global white matter. This allowed us to examine the potential role of specific white matter tracts over and above a global measure of white matter. In the present study, we were motivated from a clinical standpoint to examine the utility of diffusion characteristics and amyloid pathology measured at baseline to predict subsequent cognitive decline. Cognition was measured annually for up to 7 years (mean follow-up = 3.93 ± 1.25 years). We used factor scores to assess the cognitive domains most often impacted by white matter diffusion characteristics, namely, executive function, episodic memory, and processing speed. Exploring these questions in a large, well-characterized elderly sample enabled us to thoroughly explore the impact of white matter microstructure on different cognitive domains within normal aging and preclinical Alzheimer’s disease.
Materials and Methods
Participants
The present sample consisted of 265 clinically normal, community-dwelling older adults from the Harvard Aging Brain Study (HABS), an ongoing longitudinal study. Study protocols were approved by the Partners Healthcare Institutional Review Board. At study entry, all participants had a global Clinical Dementia Rating (CDR) of zero (Morris 1993), less than 11 on the Geriatric Depression Scale (Yesavage et al. 1983), 25 or greater on the Mini-Mental State Examination (MMSE; Folstein et al. 1975), and performed within education-adjusted norms on Wechsler Memory Scale-Revised Logical Memory delayed recall (Wechsler 1987). Participants included in the present analyses were required to have both diffusion-weighted imaging and Pittsburgh Compound-B positron emission tomography (PiB-PET) data. Baseline demographic information is presented in Table 1 for the entire group and for dichotomized groups on the basis of amyloid status.
Table 1.
Baseline demographic information by amyloid status and mean diffusivity metrics
| Overall (n = 265) | Aβ− (n = 197) | Aβ+ (n = 68) | P-value | |
|---|---|---|---|---|
| Age in years, mean standard deviation (SD) | 73.72 (6.18) | 73.16 (6.19) | 75.32 (5.90) | 0.01 |
| Education in years, mean (SD) | 15.76 (3.08) | 15.69 (3.13) | 15.97 (2.94) | 0.51 |
| Females, n (%) | 155 (59) | 113 (57) | 42 (62) | 0.66 |
| PiB DVR FLR, mean (SD) | 1.15 (0.20) | 1.06 (0.05) | 1.44 (0.18) | <0.001 |
| Aβ+, n (%) | 26% | — | — | — |
| Global FA, mean (SD) | 0.561 (0.024) | 0.561 (0.023) | 0.561 (0.025) | 0.96 |
| ATR FA, mean (SD) | 0.568 (0.028) | 0.567 (0.028) | 0.570 (0.029) | 0.40 |
| CB FA, mean (SD) | 0.646 (0.032) | 0.646 (0.033) | 0.646 (0.032) | 0.91 |
| PHC FA, mean (SD) | 0.674 (0.045) | 0.673 (0.044) | 0.677 (0.046) | 0.59 |
| CST FA, mean (SD) | 0.651 (0.025) | 0.651 (0.025) | 0.653 (0.027) | 0.56 |
| IFOF FA, mean (SD) | 0.550 (0.029) | 0.550 (0.028) | 0.549 (0.031) | 0.91 |
| ILF FA, mean (SD) | 0.472 (0.024) | 0.470 (0.023) | 0.475 (0.027) | 0.19 |
| Fma FA, mean (SD) | 0.637 (0.030) | 0.639 (0.028) | 0.631 (0.034) | 0.11 |
| Fmi FA, mean (SD) | 0.532 (0.030) | 0.532 (0.031) | 0.531 (0.029) | 0.79 |
| SLF FA, mean (SD) | 0.523 (0.030) | 0.523 (0.029) | 0.521 (0.034) | 0.75 |
PiB DVR FLR, Pittsburgh Compound-B distribution volume ratio of frontal, lateral parietal and lateral temporal, and retrosplenial regions; FA, fractional anisotropy; ATR, anterior thalamic radiation; CB, cingulum bundle; PHC, parahippocampal cingulum; CST, corticospinal tract; IFOF, inferior frontal occipital fasciculus; ILF, inferior longitudinal fasciculus; Fma, forceps major; Fmi, forceps minor; SLF, superior longitudinal fasciculus.
Cognitive Measures
Participants underwent annual neuropsychological testing for up to 7 years. Because HABS is an ongoing study and enrollment was staggered, not all participants had the same number of follow-up visits. At the time of the present analyses, cognitive data were available for 265 participants at baseline, 262 at the first follow-up, 250 at the second follow-up, 241 at the third follow-up, 167 at the fourth follow-up, 101 at the fifth follow-up, and 18 at the sixth follow-up. The mean neuropsychological follow-up period was 3.93 years (SD = 1.25). We measured cognitive change with a battery of neuropsychological and behavioral tasks selected primarily to represent domains of executive function, episodic memory, and processing speed. Executive function was assessed by Wechsler Adult Intelligence Scale-III Letter-Number Sequencing (the number of trials correctly completed; Wechsler 1997), phonemic fluency (the sum of the words produced in response to the letters F, A, S, each over 60 s; Spreen and Benton 1977), and the Trail Making Test (time to complete Form B minus Form A; Reitan 1958). Episodic memory was assessed using the delayed recall score from the Wechsler Memory Scale-Revised Logical Memory subtest (Wechsler 1987), the free recall score from the Free and Cued Selective Reminding Test (Grober et al. 2000), and the delayed recall score from Six-Trial Selective Reminding test (Masur et al. 1990). Processing speed was assessed by Wechsler Adult Intelligence Scale-Revised Digit-Symbol Coding (number of items completed; Wechsler 1981) and Trail Making Test (time to complete Form A; Reitan 1958).
Confirmatory factor analyses were conducted using the lavaan R package (Rosseel 2012) and used to construct longitudinal factors for cognitive domains of executive function, episodic memory, and processing speed. The hypothesized factor structure was modeled after a previously reported cross-sectional analysis (Hedden et al. 2012) and adapted to include a set of tasks (listed above) with repeated administration across all annual neuropsychological visits (see Supplementary Material).
Magnetic Resonance Imaging Diffusion-weighted Imaging
Magnetic resonance imaging (MRI) was conducted at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital on a 3-Tesla Trio Tim scanner (Siemens Medical Systems, Erlangen, Germany) with a 12-channel phased-array head coil. Diffusion-weighted images were acquired with a standard sequence with 30 diffusion encoding gradient directions (repetition time [TR], 8040 ms, echo time [TE], 84 ms, inversion time [TI], 2100 ms, 2 × 2 × 2 mm voxels, 64 transverse slices, b-value, 700 s/mm2). Processing of diffusion-weighted data was performed in FSL v5.0.9 (The Oxford Centre for Functional MRI of the Brain Software Library). First, diffusion images were corrected for eddy current and motion distortions using FSL’s eddy tool (FMRIB Software Library; Andersson and Sotiropoulos 2016). The diffusion tensor model was then fitted at each voxel to extract FA. Next, following tract-based spatial statistics (TBSS) procedures (Smith et al. 2006), we created a subject-specific template in the atlas space of the Montreal Neurological Institute (MNI space; Montreal, Canada) using the most representative FA image from 272 clinically normal older adults (from the HABS cohort) by applying linear (FLIRT) and non-linear (FNIRT) registrations. The subject-specific template was then skeletonized and thresholded at 0.3 to exclude predominantly non-white matter voxels. At this stage, each participant’s FA image was non-linearly registered to the template (Avants et al. 2011) and the voxel with the highest FA value perpendicular to the skeleton was projected onto the mean skeleton using tbss_skeleton (Smith et al. 2006).
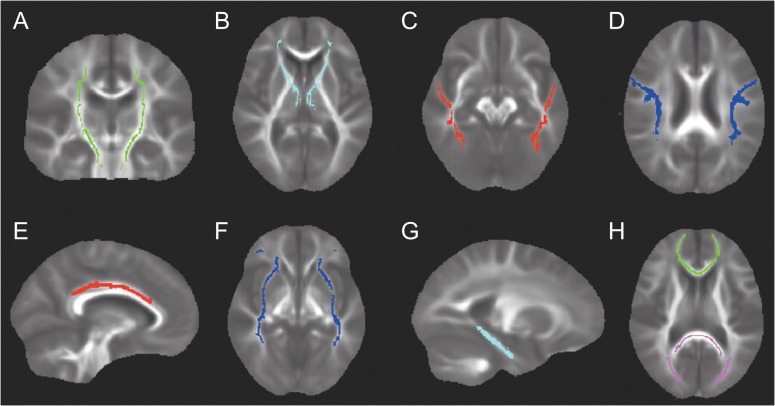
As depicted in Figure 1, we examined 9 white matter tracts, including the anterior thalamic radiation (ATR), corticospinal tract (CST), cingulum bundle (CB), parahippocampal cingulum (PHC), forceps major (Fma), forceps minor (Fmi), inferior frontal occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), and superior longitudinal fasciculus (SLF). We selected the tracts most commonly examined in prior studies relating white matter diffusion characteristics to cognition (e.g., Kennedy and Raz 2009; Madden et al. 2012; Cremers et al. 2016; Gazes et al. 2016) and amyloid (Chao et al. 2013; Molinuevo et al. 2014; Racine et al. 2014; Rieckmann et al. 2016). Although the fornix has received considerable attention in aging and Alzheimer’s disease, it was not included in the present analyses due to its close proximity to cerebrospinal fluid, making it highly susceptible to partial volume effects.
Figure 1.
A depiction of the 9 white matter tract regions of interest (ROIs) examined in the present study. (A) corticospinal tract (B) anterior thalamic radiation (C) inferior longitudinal fasciculus (D) superior longitudinal fasciculus (E) cingulum bundle (F) inferior frontal occipital fasciculus (G) parahippocampal cingulum, and (H) forceps minor is depicted in green and forceps major is depicted in pink. ROIs are displayed on the mean FA of the baseline sample (n = 265).
Regions of interest (ROIs) were defined for each tract using the Johns Hopkins University (JHU) probabilistic white matter atlas (Hua et al. 2008). Minor edits of the JHU ROIs were performed manually to 1) correct for misregistration between the ROIs and the subject-specific white matter skeleton and 2) correct for overlap between ROIs, so that tracts were distinct from one another (i.e., non-overlapping). Because the ILF and IFOF overlapped extensively with the uncinate fasciculus, the uncinate fasciculus was not included in the present analyses. For the SLF ROI, we excluded the temporal portion of the tract because it had a relatively low correlation with the rest of the tract (r = 0.56). Mean FA from the skeleton was extracted from each ROI. Since, we had no a priori hypotheses regarding laterality, mean FA for the left and right ROIs were averaged together to reduce the number of comparisons. To compute a measure of global white matter microstructure, all ROIs were combined to form a single mask. Mean FA from the skeleton was extracted from this aggregate mask and represented the global measure of FA.
Because one of the goals of the study was to investigate whether specific tracts predict cognitive change over and above a global measure of white matter, we created 9 modified global masks that contained all tracts except for the tract under investigation. This enabled us to conservatively examine the effects of specific white matter tracts while controlling for the remaining 8 tracts. Mean FA was extracted from the skeleton of each of these modified global masks.
Pittsburgh Compound-B Positron Emission Tomography
Participants underwent baseline neocortical amyloid imaging with PiB-PET. The acquisition protocol has been described in detail previously (Hedden et al. 2012). Briefly, PiB-PET images were acquired with an 8.5–15.0 mCi bolus injection and immediately followed by a 60-minute dynamic acquisition. PET data preprocessing was performed using SPM12 (WellcomeTrust Centre for Neuroimaging). A summary distribution volume ratio (DVR) was calculated for each participant by averaging the median PiB uptake value across voxels in frontal, lateral parietal and temporal, and retrosplenial cortices (the FLR region). The cerebellar cortex served as the reference region. A previously described Gaussian mixture modeling approach was used to classify participants as amyloid positive or amyloid negative (DVR cutoff value = 1.2; Mormino, Betensky, Hedden, Schultz, Ward et al. 2014). This approach resulted in 197 individuals classified as amyloid negative and 68 individuals classified as amyloid positive. We also examined amyloid burden as a continuous variable to ensure that the selection of a cutoff value did not dictate the results. The significant results reported below were present both when the cutoff value was used and when amyloid was treated as a continuous variable.
Statistical Analyses
Statistical analyses were performed using R, version 3.2.4. Linear mixed models were used to examine the associations between baseline diffusion characteristics and amyloid status on longitudinal cognitive change (nlme package). Random effects of intercept and slope for each participant were modeled using maximum likelihood estimation. Time was operationalized as years from baseline for each participant. All models included the following covariates: age at baseline, sex, years of education, and motion in the scanner. To facilitate comparisons, continuous predictor variables were z-transformed prior to model entry. The primary analyses used FA as a measure of white matter microstructure. For completeness, we report analyses using other diffusivity metrics (mean, radial, and axial diffusivity) in the Supplementary Material (Supplementary Tables 1–3). Supplemental analyses also examined the relationships between diffusion characteristics and baseline cognition for comparison to prior cross-sectional studies (Supplementary Tables 4–7). When practice effects were explicitly modeled in the main analyses, the estimates for the effects of interest were essentially unchanged (Vivot et al. 2016); for simplicity, we report the results without the inclusion of terms for practice effects. We used one-tailed significance values because we had directional a priori predictions that reduced white matter microstructure and increased amyloid burden would negatively impact cognitive decline (Hedden et al. 2013, 2016; Bennett and Madden 2014). Multiple comparisons were corrected as described below.
Do Diffusion Characteristics Predict Longitudinal Cognitive Change Independently or Synergistically with Amyloid Status?
To address whether diffusion characteristics predict longitudinal cognitive change independently (Model 1) or synergistically (Model 2) with amyloid status, we examined the following models for each cognitive domain (i.e., executive function, episodic memory, processing speed):
Model 1: Cognition ~ global FA × time + amyloid status × time + covariates × time
Model 2: Cognition ~ global FA × amyloid status × time + covariates × time
Cognition = executive function, episodic memory, or processing speed
Covariates = age at baseline, sex, years of education, and motion in the scanner
Time = time from baseline (years)
Note that the above models include all lower order effects.
To correct for multiple comparisons, a Bonferonni correction was applied to account for testing 3 different cognitive domains (P = 0.05/3 = 0.017).
Are the Effects of Diffusion Characteristics on Longitudinal Cognitive Change Tract-specific or Global in Nature?
The second set of statistical analyses examined whether the effects of diffusion characteristics on cognitive change are tract-specific or global in nature. Because any tract-specific effects observed in these analyses may simply represent a proxy of the global effect (Penke et al. 2010; Bennett and Madden 2014), we controlled for global FA in each of these models (using the modified measure of global FA that does not include the specific tract under investigation). Within this context, we examined whether tract-specific diffusion characteristics predict cognitive change independently (Model 3) or synergistically (Model 4) with amyloid status with the following models:
Model 3: Cognition ~ tract-specific FA × time + modified global FA × time + amyloid status × time + covariates × time
Model 4: Cognition ~ tract-specific FA × amyloid status × time + modified global FA × amyloid status × time + covariates × time
Cognition = executive function, episodic memory, or processing speed
Covariates = age at baseline, sex, years of education, and motion in the scanner
Time = time from baseline (years)
Note that the above models include all lower order effects.
To correct for multiple comparisons in these analyses, a Bonferonni correction was applied to account for the 9 white matter tracts examined within each of the cognitive domains (P = 0.05/9, corresponding to P = 0.006).
To better understand the global effect in relation to cognitive change, follow-up analyses examined whether each of the 9 modified global FA measures predicted change in cognitive performance (controlling for amyloid status). These analyses allowed us to address whether any individual tract was having an undue influence on the global measure of FA in relation to cognitive decline. To examine this question, global FA was replaced with each of the modified measures of global FA in Model 1. Separate models were run for each modified measure of global FA (Supplementary Table 8).
Results
Cross-Sectional Relationships Between Imaging Markers
As summarized in Table 1, global and tract-specific measures of FA at baseline did not differ between amyloid positive and amyloid negative individuals. A partial correlation (controlling for age and sex) between global FA and amyloid burden (used here as a continuous variable) was not significant (r = 0.03, P = 0.61). The correlation remained non-significant when age and sex were removed as covariates (r = −0.03, P = 0.64). As shown in Table 2, FA of the 9 white matter tracts significantly correlated with the modified measure of global FA (that excluded the examined tract; correlations ranged from 0.49 to 0.86) and with one another (correlations ranged from 0.23 to 0.77). Baseline cognitive performance was significantly correlated across the 3 cognitive domains. The correlation between executive function and episodic memory was 0.39 (P < 0.001), the correlation between executive function and processing speed was 0.51 (P < 0.001), and the correlation between episodic memory and processing speed was 0.35 (P < 0.001).
Table 2.
Correlations between the Modified Global FA and FA of specific tracts
| Modified global | ATR | CB | PHC | CST | IFOF | ILF | Fma | Fmi | SLF | |
|---|---|---|---|---|---|---|---|---|---|---|
| ATR | 0.70 | — | ||||||||
| CB | 0.76 | 0.50 | — | |||||||
| PHC | 0.49 | 0.38 | 0.42 | — | ||||||
| CST | 0.66 | 0.66 | 0.44 | 0.49 | — | |||||
| IFOF | 0.86 | 0.68 | 0.73 | 0.38 | 0.54 | — | ||||
| ILF | 0.76 | 0.52 | 0.62 | 0.51 | 0.49 | 0.70 | — | |||
| Fma | 0.65 | 0.40 | 0.60 | 0.23 | 0.33 | 0.66 | 0.62 | — | ||
| Fmi | 0.75 | 0.54 | 0.69 | 0.34 | 0.43 | 0.77 | 0.62 | 0.63 | — | |
| SLF | 0.84 | 0.63 | 0.68 | 0.45 | 0.69 | 0.77 | 0.69 | 0.57 | 0.66 | — |
All tracts were significantly associated with the modified global FA and one another, all P values < 0.001. The modified measure of global FA was used in these analyses and refers to the sum of all tracts excluding the tract examined. FA, fractional anisotropy; ATR, anterior thalamic radiation; CB, cingulum bundle; PHC, parahippocampal cingulum; CST, corticospinal tract; IFOF, inferior frontal occipital fasciculus; ILF, inferior longitudinal fasciculus; Fma, forceps major; Fmi, forceps minor; SLF, superior longitudinal fasciculus.
Do Diffusion Characteristics Predict Longitudinal Cognitive Change Independently or Synergistically with Amyloid Status?
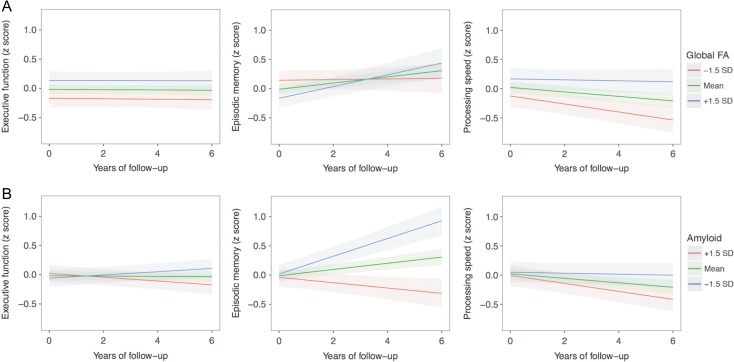
As depicted in Figure 2, global FA at baseline predicted a significant longitudinal change in episodic memory (β = 0.032, SE = 0.011, t = 2.87, P = 0.002) and processing speed (β = 0.022, SE = 0.009, t = 2.45, P = 0.007), but not in executive function (β = 0.00, SE = 0.008, t = 0.039, P = 0.485). In the case of episodic memory, higher global FA was associated with improvement over time, likely indicating a practice effect, whereas lower global FA was associated with stable performance over time. With respect to processing speed, lower global FA was associated with the steepest decline in performance over time. Removing amyloid status from these models did not alter the impact of global FA on longitudinal cognitive change. To better understand the non-significant relationship between global FA and executive function, post hoc analyses examined the relationship with the individual tests that comprise the executive function factor score. These analyses also yielded non-significant results (all P > 0.51), suggesting that the null effect was present in all 3 tests. In terms of amyloid status, amyloid positivity was associated with worse longitudinal performance in episodic memory (β = −0.158, SE = 0.023, t = −6.75, P < 0.001) and executive function (β = −0.043, SE = 0.017, t = −2.61, P = 0.005). The association between amyloid status and processing speed did not survive multiple comparison correction (β = −0.036, SE = 0.019, t = −1.94, P = 0.027). The three-way interaction between global FA, amyloid status, and time did not predict a change in any of the cognitive domains (all P > 0.232). With respect to age, older age significantly predicted worse performance across all 3 cognitive domains, including episodic memory (β = −0.032, SE = 0.011, t = −2.94, P = 0.002), processing speed (β = −0.034, SE = 0.009, t = −3.84, P < 0.001), and executive function (β = −0.025, SE = 0.008, t = −3.20, P < 0.001). These findings suggest that there remains a substantial age-associated influence on cognitive decline that is not explained by global FA or amyloid status.
Figure 2.
Longitudinal effects of global FA and amyloid status on cognitive change (controlling for one another). (A) Longitudinal effects of global FA on cognitive change, controlling for amyloid status. Global FA at baseline was associated with longitudinal change in episodic memory (P = 0.002) and processing speed (P = 0.007), but not in executive function (P = 0.485). (B) Longitudinal effects of amyloid status on cognitive change, controlling for global FA. Amyloid status at baseline was associated with longitudinal change in executive function (P = 0.005) and episodic memory (P < 0.001). The association between amyloid status and processing speed did not survive multiple comparison correction (P = 0.027). For ease of comparison with global FA, amyloid is depicted as a continuous variable. Shading represents 95% confidence intervals. The green lines represent mean global FA (top row) or mean amyloid burden (bottom row) in the sample. The red and blue lines represent 1.5 standard deviations above/below the mean.
Are the Effects of Diffusion Characteristics on Longitudinal Cognitive Change Tract-specific or Global in Nature?
As summarized in Table 3, after statistically controlling for global FA (using the modified measures of global FA) and amyloid status, as well as correcting for multiple comparisons, none of the specific tracts predicted significant decline in any of the 3 cognitive domains (executive function: all P > 0.010; episodic memory: all P > 0.059; processing speed: all P > 0.018). Removing amyloid status from these models did not significantly alter the impact of tract-specific FA on cognitive change. The interaction between FA for each tract and amyloid status with time did not significantly predict a change in any of the cognitive domains after controlling for the interaction between the modified measure of global FA and amyloid status with time and correcting for multiple comparisons (all P > 0.091).
Table 3.
Summary of linear mixed models examining tract-specific FA on cognitive change (controlling for modified global FA)
| Region | Estimate | Standard error | t-value | P-value |
|---|---|---|---|---|
| Executive function | ||||
| ATR | 0.010 | 0.010 | 0.982 | 0.163 |
| CB | 0.026 | 0.011 | 2.326 | 0.010 |
| PHC | −0.006 | 0.009 | −0.715 | 0.737 |
| CST | −0.003 | 0.010 | −0.270 | 0.894 |
| Fma | 0.005 | 0.011 | 0.466 | 0.321 |
| Fmi | 0.007 | 0.012 | 0.587 | 0.279 |
| IFOF | 0.013 | 0.015 | 0.871 | 0.192 |
| ILF | −0.023 | 0.011 | −2.089 | 0.518 |
| SLF | −0.012 | 0.014 | −0.912 | 0.681 |
| Episodic memory | ||||
| ATR | 0.009 | 0.014 | 0.603 | 0.273 |
| CB | 0.025 | 0.016 | 1.563 | 0.059 |
| PHC | −0.008 | 0.012 | −0.608 | 0.772 |
| CST | −0.006 | 0.014 | −0.450 | 0.826 |
| Fma | 0.005 | 0.015 | 0.346 | 0.365 |
| Fmi | 0.023 | 0.017 | 1.359 | 0.087 |
| IFOF | −0.013 | 0.021 | −0.620 | 0.768 |
| ILF | −0.006 | 0.016 | −0.406 | 0.842 |
| SLF | 0.012 | 0.019 | 0.636 | 0.263 |
| Processing speed | ||||
| ATR | 0.021 | 0.012 | 1.820 | 0.035 |
| CB | 0.007 | 0.013 | 0.501 | 0.308 |
| PHC | −0.005 | 0.010 | −0.517 | 0.803 |
| CST | −0.011 | 0.012 | −0.919 | 0.679 |
| Fma | 0.015 | 0.012 | 1.173 | 0.121 |
| Fmi | 0.012 | 0.014 | 0.901 | 0.184 |
| IFOF | 0.036 | 0.017 | 2.109 | 0.018 |
| ILF | 0.004 | 0.013 | 0.310 | 0.379 |
| SLF | −0.036 | 0.015 | −2.320 | 0.510 |
Each row summarizes the results from a separate linear mixed model that examines the contribution of each tract over and above a global measure of white matter microstructure to longitudinal cognitive change. All models control for age, sex, education, motion in the scanner, modified global FA, amyloid status, and their interactions with time. The modified global FA does not include the specific tract under investigation. After accounting for modified global FA and multiple comparisons (P < 0.006), none of the individual tracts predicted a significant change in cognitive performance. FA, fractional anisotropy; ATR, anterior thalamic radiation; CB, cingulum bundle; PHC, parahippocampal cingulum; CST, coticospinal tract; IFOF, inferior frontal occipital fasciculus; ILF, inferior longitudinal fasciculus; Fma, forceps major; Fmi, forceps minor; SLF, superior longitudinal fasciculus.
To further confirm that no one tract was having an undue influence on the relationship between global FA and cognitive decline, follow-up analyses examined whether each of the 9 modified measures of global FA predicted a change in cognition. As summarized in Supplementary Table 8, these analyses demonstrated the same pattern of findings as described above. That is, all 9 modified measures of global FA significantly predicted a change in episodic memory (all P < 0.004) and processing speed (all P < 0.013), but not in executive function (all P > 0.417).
Discussion
The present study addressed 2 main questions: 1) Do white matter diffusion characteristics predict longitudinal cognitive change independently or synergistically with amyloid status? 2) Are the effects of diffusion characteristics on longitudinal cognitive change tract-specific or global in nature? Here, we also examined whether tract-specific diffusion characteristics predict longitudinal cognitive change independently or synergistically with amyloid status. With respect to the first question, we found that global white matter diffusion characteristics predicted a significant change in cognition independent of amyloid status. Specifically, lower global FA was associated with worse episodic memory and processing speed over time, but not executive function. In terms of the second question, analyses provided support for global, rather than tract-specific, effects of white matter diffusion characteristics in relation to longitudinal cognition.
Our results add to a body of literature demonstrating a relationship between white matter diffusion characteristics and cognition (Kennedy and Raz 2009; Vernooij et al. 2009; Bendlin et al. 2011; Madden et al. 2012; Booth et al. 2013; Borghesani et al. 2013; Jacobs et al. 2013; Bennett and Madden 2014), and further suggest that this relationship is independent of amyloid status. Longitudinal analyses demonstrated that global FA measured at baseline was associated with change in episodic memory and processing speed, but not in executive function. In the case of episodic memory, higher global FA was associated with improvement over time, whereas lower global FA was associated with stable performance over time, likely reflecting a diminished practice effect. A diminished practice effect may be a meaningful marker of an underlying neuropathological process in clinically normal individuals (Mormino, Betensky, Hedden, Schultz, Amariglio, et al. 2014; Hassenstab et al. 2015; Mormino et al. 2016). With respect to processing speed, lower global FA predicted the steepest decline in performance over time. The annual rate of cognitive decline attributable to global FA was approximately 0.03 and 0.02 standard deviations per year for episodic memory and processing speed, respectively. In comparison to the impact of age on cognitive decline, global FA had a comparable effect on episodic memory and a 1.5 times smaller effect on processing speed (based on comparisons of the t-values). Reduced white matter microstructure may have a small impact on cognitive decline, however this effect is nontrivial considering that it can be observed in clinically normal individuals and represents only one of many age-related neurobiological changes associated with cognitive decline.
Contrary to prior reports (Madden et al. 2012) and our cross-sectional data (see Supplementary Material), we did not observe a significant association between global (or tract-specific) FA and longitudinal change in executive function. It is not entirely clear why we did not observe such a relationship. Insufficient variability in the executive function factor score over time is an unlikely explanation, given that significant relationships were observed with amyloid status and with age. It is also unlikely that the measure of executive function was not sensitive to variability in diffusion characteristics, given that a significant relationship was observed in the cross-sectional analyses (see Supplementary Material). Nonetheless, our observation of no significant relationship between diffusion characteristics and decline in executive function is more likely to be due to measurement issues rather than to biology. It remains possible that the executive function tests used here lack the sensitivity for detecting relationships between diffusion characteristics and longitudinal change. Perhaps more challenging executive function measures would have demonstrated a longitudinal relationship with diffusion characteristics. Additional longitudinal studies are necessary to better understand the relationship between white matter diffusion characteristics and cognitive decline in healthy aging and in preclinical Alzheimer’s disease.
In our relatively large sample, amyloid burden was not associated with measures of global or tract-specific white matter microstructure, suggesting that these 2 processes may result from separable neuropathological processes in clinically normal individuals. These findings are consistent with recent studies examining the cross-sectional relationships between microstructural alterations and amyloid pathology (Kantarci et al. 2014; Rieckmann et al. 2016), but not with others, which have shown relationships between amyloid and specific tracts, such as the fornix, parahippocampal cingulum, and corpus callosum (Chao et al. 2013; Gold et al. 2014; Molinuevo et al. 2014; Racine et al. 2014). It is possible that an association is more likely to be observed when following individuals over time (Rieckmann et al. 2016) or in later stages of the disease process (Nowrangi et al. 2013). Future studies with longitudinal imaging data will serve to further clarify this relationship.
With respect to the relationship between amyloid status and cognition, our longitudinal analyses demonstrated significant associations with episodic memory and executive function. These findings may appear at odds with prior suggestions that amyloid status is preferentially associated with memory performance (e.g., Pike et al. 2007; Hedden et al. 2012; Sperling et al. 2013). However, amyloid’s association with episodic memory was approximately 2.5 times larger than its association with executive function (based on a comparison of the t-values). These results are broadly consistent with a meta-analysis that found the largest association between amyloid and episodic memory, but also observed a significant association with amyloid for global function and executive function (Hedden et al. 2013). These results are also consistent with several longitudinal studies suggesting that amyloid negatively impacts multiple cognitive domains (e.g., Storandt et al. 2009; Resnick et al. 2010; Doraiswamy et al. 2012; Insel et al. 2016; Petersen et al. 2016). Furthermore, our longitudinal results indicated that the association of amyloid status with episodic memory was also approximately 2.5 times the size of the independent association between white matter microstructure and episodic memory (based on a comparison of the t-values). These results suggest that both white matter microstructure and amyloid status are predictive of subsequent change in episodic memory, but to differing degrees. Hence, the co-occurrence of reduced white matter microstructure and elevated amyloid burden may accelerate memory decline in clinically normal individuals, thereby shortening the preclinical phase of Alzheimer’s disease.
The second question addressed whether age-related cognitive decline is due to alterations within specific white matter tracts or to global white matter changes occurring across the brain. Because advanced aging is associated with widespread alterations in white matter diffusion characteristics (Head et al. 2004; Salat et al. 2005; Barrick et al. 2010; Voineskos et al. 2012; Lövdén et al. 2014; Sexton et al. 2014; Cox et al. 2016; Rieckmann et al. 2016), findings of specific white matter tracts on cognitive decline may result from sampling this general effect (Bennett and Madden 2014). To overcome this issue, some studies have used factor analytic techniques, voxel-wise analysis, or have controlled for global white matter in tract-specific models. Results from these studies are mixed, with some demonstrating tract-specific effects (Lövdén et al. 2014; Bennett et al. 2015; Cremers et al. 2016) and others showing global effects on cognition (Penke et al. 2010; Haász et al. 2013; Ritchie et al. 2015; Kuznetsova et al. 2016). Our findings are consistent with the latter. After controlling for global white matter microstructure, we did not find a significant relationship between any of the individual white matter tracts (as measured by FA) and cognitive change. Further support for a global effect comes from the finding that each of the 9 modified measures of global FA significantly predicted change in domains of episodic memory and processing speed, suggesting that iteratively removing each tract from the measure of global FA did not impact the relationship with cognitive decline. In supplemental analyses, we also examined tract-specific effects without covarying the modified measure of global FA. While a few significant tract-specific relationships emerged, these relationships were not consistent with those reported in the literature nor did they reveal a clear pattern. An exception was the relationship between the cingulum bundle and episodic memory (Table 9 in Supplemental Material). Taken together, the findings reported here provide support for a relationship between global, rather than tract-specific FA, and change in episodic memory and processing speed.
The discrepancy between our findings and prior studies demonstrating tract-specific effects may be due to methodological differences. For instance, not all studies reporting tract-specific effects controlled for global white matter in statistical models (e.g., Kennedy and Raz 2009; Perry et al. 2009; Ryan et al. 2011). Another possible reason for discrepancies across studies may relate to the age range examined. In the present study, we focused on older adults between the ages of 63 and 90, whereas a prior study showing tract-specific effects over and above a global measure included a broader age range of participants (Cremers et al. 2016). The inclusion of younger participants may increase the variation in both cognition and diffusion measures to enable detection of what may be relatively small relationships. Additional differences across studies include the specific cognitive tests used and the white matter tracts selected for analysis. For example, Bennett and colleagues (2015) reported a relationship between fornix microstructure and pattern separation, neither of which was examined in the present study. While tasks targeting specific cognitive processes may allow detection of relationships with tracts connected to brain regions specific to those processes, such results may not be generalizable to broader categories of cognition.
When examining the other diffusivity metrics (mean, radial, and axial diffusivity), we consistently found a relationship between the ATR and longitudinal decline in processing speed (see Supplemental Material). This association was significant for mean and axial diffusivity, and trend-level for radial diffusivity. Based on previous studies, we would not have predicted an association between the ATR and processing speed over and above a global measure of white matter (Penke et al. 2010; Lövdén et al. 2014), and thus this finding warrants replication. Overall, the present findings provide weak support for tract-specific effects on cognitive decline and appear to be more consistent with the idea that widespread white matter alterations are linked to change in cognitive performance across multiple cognitive domains.
The finding that global, rather than tract-specific, white matter relates to cognitive decline suggests that the cognitive domains examined in the present study likely depend upon the coordination of multiple brain regions. Thus, the present findings are in line with “disconnection” theories of cognitive aging (O’Sullivan et al. 2001; Bartzokis et al. 2004; Andrews-Hanna et al. 2007; Bennett and Madden 2014) and suggest that reduced white matter microstructure across the brain may disrupt communication between key cortical regions, thereby contributing to cognitive decline. One inconsistency with this interpretation is that we did not observe a significant relationship between global FA and longitudinal change in executive function, although we did observe a relationship with cross-sectional measures of executive function (see Supplementary Material). The global nature of the diffusion measure suggests that any cognitive domain involving multiple cortical regions, including executive function, should be affected by degradation in this measure. As mentioned above, our observation of no relationship with decline in executive function may be due to measurement issues.
Strengths of this study include 1) the longitudinal design, with annual testing for up to 7 years; 2) the assessment of multiple cognitive domains; 3) the relatively large sample size (n = 265), and 4) the large number of white matter tracts examined. There were also several limitations. First, we used the average FA value across each of the white matter tracts examined, which may have obscured regionally specific effects. It is possible that examining more fine-grained segments of each tract would allow for improved specificity; however, this would come at the cost of increased multiple comparisons. A second limitation is that our sample did not include individuals with cognitive impairment at baseline. It may be that relationships between specific white matter tracts and cognition are more likely to be found with the wider range of performance observed in individuals with mild cognitive impairment or dementia. Third, although the fornix plays an important role in episodic memory (Aggleton et al. 2016), we did not include this tract in our analyses because of its close proximity to cerebrospinal fluid, making it highly susceptible to partial volume effects. Fourth, in this study we applied TBSS, which projects the maximal FA value onto the skeleton. While this approach minimizes partial volume effects, one disadvantage of this approach is that it is insensitive to the degradation of white matter in the periphery of the tracts. Finally, participants included in the present study are primarily highly educated volunteers and may not be representative of the general population.
To summarize, the present study provides additional support for the involvement of global white matter diffusion characteristics in age-related cognitive decline that is separable from amyloid status. These results highlight the need for a better understanding of the ways in which white matter microstructural alterations can be prevented or ameliorated in both normal aging and in preclinical Alzheimer’s disease. To the extent that independent yet comorbid neuropathological changes impact longitudinal cognition, the potential that white matter diffusion characteristics can be modified through cardiovascular and lifestyle interventions makes it a potentially important target for mitigating cognitive decline.
Supplementary Material
Notes
We thank Elizabeth Mormino for assistance with the factor analysis. We gratefully acknowledge the participants in the Harvard Aging Brain Study for their dedication to research. Conflict of Interest: None declared.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (P01 AG036694 to R.A.S. and K.A.J, K24 AG035007 to R.A.S., R01 AG053509 and K01 AG040197 to T.H., R01 AG034556 to R.L.B., and P50 AG005134), and a Canadian Institutes of Health Research Postdoctoral Fellowship Award to J.S.R. This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant numbers S10RR023401, S10RR019307, S10RR019254, and S10RR023043.
References
- Aggleton JP, Pralus A, Nelson AJD, Hornberger M. 2016. Thalamic pathology and memory loss in early Alzheimer’s disease: moving the focus from the medial temporal lobe to Papez circuit. Brain. 139:1877–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN. 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. 2007. Disruption of large-scale brain systems in advanced aging. Neuron. 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 54:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS. 2010. NeuroImage White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 51:565–577. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. 2004. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 25:843–851. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. 1994. MR diffusion tensor spectroscopy and imaging. Biophys J. 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Erik K, Thiel BW, Rowley HA, Lazar M, Andrew L, Johnson SC. 2011. White matter in aging and cognition: a cross-sectional study of microstructure in adults aged eighteen to eighty-three. Dev Neuropsychol. 35:257–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Huffman DJ, Stark CE. 2015. Limbic tract integrity contributes to pattern separation performance across the lifespan. Cereb Cortex. 25:2988–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ. 2014. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience. 276:187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T, Bastin ME, Penke L, Maniega SM, Murray C, Royle NA, Gow AJ, Corley J, Henderson RD, Hernández MDCV, et al. 2013. Brain white matter tract integrity and cognitive abilities in community-dwelling older people: the Lothian Birth Cohort, 1936. Neuropsychology. 27:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Madhyastha TM, Aylward EH, Reiter MA, Swarny BR, Schaie KW, Willis SL. 2013. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia. 51:1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, Wasserman BT, Williams LM, Zimmerman ME. 2012. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiol Aging. 33:1699–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmans S, Gronenschild EHBM, Fandakova Y, Shing YL, van Boxtel MPJ, Vuurman EFPM, Uylings HBM, Jolles J, Raz N. 2011. Age differences in speed of processing are partially mediated by differences in axonal integrity. Neuroimage. 55:1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, DeCarli C, Kriger S, Truran D, Zhang Y, Laxamana J, Villeneuve S, Jagust WJ, Sanossian N, Mack WJ, et al. 2013. Associations between white matter hyperintensities and β amyloid on integrity of projection, association, and limbic fiber tracts measured with diffusion tensor MRI. PLoS One. 8:e65175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. 2008. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiol Aging. 29:1547–1555. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Schiavone F, Barrick TR, Morris RG, Markus HS. 2010. Diffusion tensor imaging detects age related white matter change over a 2 year follow-up which is associated with working memory decline. J Neurol Neurosurg Psychiatry. 81:13–19. [DOI] [PubMed] [Google Scholar]
- Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, Wardlaw JM, Gale CR, Bastin ME, Deary IJ. 2016. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat Commun. 7:13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers LGM, de Groot M, Hofman A, Krestin GP, van der Lugt A, Niessen WJ, Vernooij MW, Ikram MA. 2016. Altered tract-specific white matter microstructure is related to poorer cognitive performance: the Rotterdam Study. Neurobiol Aging. 39:108–117. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. 2009. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 46:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD, Grundman M, Sabbagh MN, Sadowsky CH, Fleisher AS, et al. 2012. Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study. Neurology. 79:1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, et al. 2016. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 12:292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Sneve MH, Grydeland H, Storsve AB, Walhovd KB. 2017. The disconnected brain and executive function decline in aging. Cereb Cortex. 27:2303–2317. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Sneve MH, Storsve AB, Grydeland H, Yendiki A, Walhovd KB. 2015. Brain events underlying episodic memory changes in aging: a longitudinal investigation of structural and functional connectivity. Cereb Cortex. 26:1272–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12:189–198. [DOI] [PubMed] [Google Scholar]
- Gazes Y, Bowman FDB, Razlighi QR, O’Shea D, Stern Y, Habeck C. 2016. White matter tract covariance patterns predict age-declining cognitive abilities. Neuroimage. 125:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Zhu Z, Brown CA, Andersen AH, LaDu MJ, Tai L, Jicha GA, Kryscio RJ, Estus S, Nelson PT, et al. 2014. White matter integrity is associated with cerebrospinal fluid markers of Alzheimer’s disease in normal adults. Neurobiol Aging. 35:2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. 2007. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. Am J Neuroradiol. 28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Grober E, Lipton RB, Hall C, Crystal H. 2000. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 54:827–832. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. 2009. Aging of cerebral whtie matter: a review of MRI findings. Int J Geriatr Psychiatry. 24:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassenstab J, Ruvolo D, Jasielec M, Xiong C, Grant E, Morris JC. 2015. Absence of practice effects in preclinical Alzheimer’s disease. Neuropsychology. 29:940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haász J, Westlye ET, Fjær S, Espeseth T, Lundervold A, Lundervold AJ. 2013. General fluid-type intelligence is related to indices of white matter structure in middle-aged and old adults. Neuroimage. 83:372–383. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony J, Williams L, Akbudak E, Conturo T, McAvoy M, Morris JC, Snyder AZ. 2004. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 14:410–423. [DOI] [PubMed] [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM. 2012. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 32:16233–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA. 2013. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 80:1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Schultz AP, Rieckmann A, Mormino EC, Johnson KA, Sperling RA, Buckner RL. 2016. Multiple brain markers are linked to age-related variation in cognition. Cereb Cortex. 26:1388–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. 2008. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PS, Mattsson N, Mackin RS, Schöll M, Nosheny RL, Tosun D, Donohue MC, Aisen PS, Jagust WJ, Weiner MW. 2016. Accelerating rates of cognitive decline and imaging markers associated with β-amyloid pathology. Neurology. 86:1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Leritz EC, Williams VJ, Van Boxtel MPJ, Van Der Elst W, Jolles J, Verhey FRJ, Mcglinchey RE, Milberg WP, Salat DH. 2013. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum Brain Mapp. 34:77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. 2016. Is amyloid-β harmful to the brain? Insights from human imaging studies. Brain. 139:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Schwarz CG, Reid RI, Przybelski SA, Lesnick TG, Zuk SM, Senjem ML, Gunter JL, Lowe V, Machulda MM, et al. 2014. White matter integrity determined with diffusion tensor imaging in older adults without dementia: influence of amyloid load and neurodegeneration. JAMA Neurol. 71:1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. 2009. Aging white natter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 47:916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Racine CA, Hale S, Wilheim R, Laluz V, Miller BL, Kramer JH. 2012. Cognitive pocessing speed in older adults: relationship with white matter integrity. PLoS One. 7:e50425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova KA, Maniega SM, Ritchie SJ, Cox SR, Storkey AJ, Starr JM, Wardlaw JM, Deary IJ, Bastin ME. 2016. Brain white matter structure and information processing speed in healthy older age. Brain Struct Funct. 221:3223–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhncke Y, Laukka EJ, Brehmer Y, Kalpouzos G, Li TQ, Fratiglioni L, Bäckman L, Lövdén M. 2016. Three-year changes in leisure activities are associated with concurrent changes in white matter microstructure and perceptual speed in individuals aged 80 years and older. Neurobiol Aging. 41:173–186. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Seidenberg M, Smith JC, Nielson KA, Woodard JL, Durgerian S, Rao SM. 2016. Diffusion tensor imaging predictors of episodic memory decline in healthy elders at genetic risk for Alzheimer’s disease. J Int Neuropsychol Soc. 22:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukka EJ, Lövdén M, Kalpouzos G, Li T-Q, Jonsson T, Wahlund L-O, Fratiglioni L, Bäckman L. 2013. Associations between white matter microstructure and cognitive performance in old and very old age. PLoS One. 8:e81419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. 2003. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 4:469–480. [DOI] [PubMed] [Google Scholar]
- Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. 2004. Amyloid-β peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 164:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SN, Mayda AB, Roach AE, Fletcher E, Carmichael O, Maillard P, Schwarz CG, Yonelinas AP, Ranganath C, DeCarli C. 2012. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front Hum Neurosci. 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M, Adluru N, Destiche DJ, Lu SY, Oh JM, Hoscheidt SM, Alexander AL, Okonkwo OC, Rowley HA, Sager MA, et al. 2016. Fornix microstructure and memory performance is associated with altered neural connectivity during episodic recognition. J Int Neuropsychol Soc. 22:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Köhncke Y, Laukka EJ, Kalpouzos G, Salami A, Li TQ, Fratiglioni L, Bäckman L. 2014. Changes in perceptual speed and white matter microstructure in the corticospinal tract are associated in very old age. Neuroimage. 102:520–530. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N-K, Song AW. 2012. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 1822:386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. 2009. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 21:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur DM, Fuld PA, Blau AD, Crystal H, Aronson MK. 1990. Predicting development of dementia in the elderly with the Selective Reminding Test. J Clin Exp Neuropsychol. 12:529–538. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Ripolles P, Simó M, Lladó A, Olives J, Balasa M, Antonell A, Rodriguez-Fornells A, Rami L. 2014. White matter changes in preclinical Alzheimer’s disease: a magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid β protein 42 levels. Neurobiol Aging. 35:2671–2680. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, Johnson KA, Sperling RA. 2014. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 71:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, Rentz DM, Johnson KA, Sperling RA. 2014. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 82:1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Papp KV, Rentz DM, Donohue MC, Amariglio RE, Quiroz YT, Chhatwal J, Marshall GA, Donovan N, Jackson J, et al. 2017. Early and late change on the preclinical Alzheimer’s cognitive composite in clinically normal older individuals with elevated β-amyloid. Alzheimer’s Dement. 13:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Papp KV, Rentz DM, Schultz AP, LaPoint M, Amariglio RE, Hanseeuw B, Marshall GA, Hedden T, Johnson KA, et al. 2016. Heterogeneity in suspected Non–Alzheimer disease pathophysiology among clinically normal older individuals. JAMA Neurol. 2129:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- Nowrangi MA, Lyketsos CG, Leoutsakos J-MS, Oishi K, Albert M, Mori S, Mielke MM. 2013. Longitudinal, region-specific course of diffusion tensor imaging measures in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Dement. 9:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. 2001. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 57:632–638. [DOI] [PubMed] [Google Scholar]
- Penke L, Maniega SM, Murray C, Gow AJ, Valdes Hernandez MC, Clayden JD, Starr JM, Wardlaw JM, Bastin ME, Deary IJ. 2010. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci. 30:7569–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry ME, McDonald CR, Hagler DJ, Gharapetian L, Kuperman JM, Koyama AK, Dale AM, McEvoy LK. 2009. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia.. 47:2835–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. 2006. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 16:907–915. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Wiste HJ, Weigand SD, Rocca WA, Roberts RO, Mielke MM, Lowe VJ, Knopman DS, Pankratz VS, Machulda MM, et al. 2016. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 73:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. 2007. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 130:2837–2844. [DOI] [PubMed] [Google Scholar]
- Racine AM, Adluru N, Alexander AL, Christian BT, Okonkwo OC, Oh J, Cleary CA, Birdsill A, Hillmer AT, Murali D, et al. 2014. Associations between white matter microstructure and amyloid burden in preclinical Alzheimer’s disease: a multimodal imaging investigation. NeuroImage Clin. 4:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. 1958. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 8:271–276. [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, Dannals RF, Mathis CA, Klunk WE, Ferrucci L, et al. 2010. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11 C]PiB. Neurology. 74:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Van Dijk KRA, Sperling RA, Johnson KA, Buckner RL, Hedden T. 2016. Accelerated decline in white matter integrity in clinically normal individuals at risk for Alzheimer’s disease. Neurobiol Aging. 42:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Bastin ME, Tucker-Drob EM, Muñoz Maniega S, Engelhardt LE, Cox SR, Royle NA, Gow AJ, Corley J, Pattie A, et al. 2015. Coupled changes in brain white matter microstructure and fluid intelligence in later life. J Neurosci. 35:8672–8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. 2012. Lavaan: An R Package for Structural Equation Modeling and More. Version 0.5–12 (BETA). Ghent, Belgium: Ghent University. [Google Scholar]
- Ryan L, Walther K, Bendlin BB, Lue L-F, Walker DG, Glisky EL. 2011. Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage. 54:1565–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Eriksson J, Nilsson LG, Nyberg L. 2012. Age-related white matter microstructural differences partly mediate age-related decline in processing speed but not cognition. Biochim Biophys Acta, Mol Basis Dis. 1822:408–415. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, Van Der Kouwe AJW, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Diana Rosas H, et al. 2005. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 26:1215–1227. [DOI] [PubMed] [Google Scholar]
- Scott JA, Tosun D, Braskie MN, Maillard P, Thompson PM, Weiner M, DeCarli C, Carmichael OT. 2017. Independent value added by diffusion MRI for prediction of cognitive function in older adults. NeuroImage Clin. 14:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Walhovd K, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, Fjell AM. 2014. Accelerated changes in white matter microstructure during aging: A longitudinal diffusion tensor imaging study. J Neurosci. 34:15425–15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, et al. 2011. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, Sadowsky CH, Carpenter A, Davis MD, Lu M, et al. 2013. Amyloid deposition detected with florbetapir F 18 (18 F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging. 34:822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Benton AL. 1977. Neurosensory Center Comprehensive Examination for Aphasia. Neuropsychology Laboratory, Department of Psychology, University of Victoria.
- Storandt M, Mintun MA, Head D, Morris JC. 2009. Cognitive decline and brain volume loss as signatures of cerebral amyloid-β peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Aβ deposition. Arch Neurol. 66:1476–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MMB. 2009. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 66:545–553. [DOI] [PubMed] [Google Scholar]
- Vivot A, Power MC, Glymour MM, Mayeda ER, Benitez A, Spiro A, Manly JJ, Proust-Lima C, Dufouil C, Gross AL. 2016. Jump, hop, or skip: modeling practice effects in studies of determinants of cognitive change in older adults. Am J Epidemiol. 183:302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH. 2012. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol Aging. 33:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1981. WAIS-R manual: Wechsler adult intelligence scale-revised New York, NY: The Psychological Corporation. [Google Scholar]
- Wechsler D. 1987. WMS-R: Wechsler memory scale-revised. San Antonio, TX: The Psychological Corporation.
- Wechsler D. 1997. Wechsler Adult Intelligence Scale III: Administration and Scoring Manual. New York, NY: The San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Xu J, Chen S, Ahmed SH, Chen H, Ku G, Goldberg MP, Hsu CY. 2001. Amyloid-beta peptides are cytotoxic to oligodendrocytes. J Neurosci. 21:RC118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. 1983. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 17:37–49. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Rohl T, Pfefferbaum A, Sullivan EV. 2009. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. NeuroImage. 44:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. 2010. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 31:1912–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.