Abstract
Objective
The Surgical Apgar Score (SAS) is a simple 10-point scoring system that has been shown to be predictive of major postoperative complications and death after surgery. We evaluated the predictive ability of this score in a cohort of patients undergoing emergency abdominal surgery in a Caribbean tertiary hospital.
Methods
The SAS was calculated retrospectively from the anaesthesia records of all patients undergoing emergency abdominal surgery during a 12-month period. The postoperative surgical records of these patients were then examined for the presence of major complications and death. The association between the SAS and outcomes was tested using binary logistic regression, and the SAS discriminatory ability was determined from the receiver-operating curve (ROC) analysis.
Results
Of the 220 patients studied, 72 (33%) suffered an in-hospital major complication or death. The highest complication rate occurred in the low-scoring groups, with 68% of those scoring <4 being affected. Low-scoring patients (<4) had four times the risk of major complications when compared to higher-scoring groups (relative risk [RR], 4.21; 95% confidence interval [CI], 2.5–7.3; p<0.001). The odds ratio (OR) for major complications or death per unit increase in the SAS was 0.58 (95% CI, 0.47–0.72; p<0.001). The c-statistic of the SAS for predicting major complications or death was 0.71 (95% CI, 0.68–0.73; p<0.0001).
Conclusion
The SAS is a simple 10-point score that can be used in patients undergoing emergency surgery in a Caribbean setting to help identify those that are at a higher risk of postoperative complications. Due to its ease in calculation, it can be added to other commonly used criteria to help triage the postoperative patient.
Keywords: Anaesthesia, intensive care, risk, surgery
Introduction
There is a significant global volume of surgery worldwide with 187–200 million cases being done per year (1). However, despite this high volume, it has been estimated that each year, 1 million people die within 30 days after surgery (1, 2).
Scoring systems have been designed to allow an objective assessment of the surgical patient. With an objective assessment, the need for further care in an intensive care or high-dependency setting can be better predicted with a focus on reducing surgical morbidity (3, 4).
There are several systems used for the scoring of surgical patients: the American Society of Anesthesiology classification (5), Revised Cardiac Risk index (6), Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (7) score and the National Surgical Quality Improvement Programme (NSQIP) (8) score. All of the above scoring systems have limitations, which include an inter-observer variation, difficulty in calculation and the need for biochemical investigations.
The SAS relies on three variables that are easily obtained from the anaesthesia records (9). It is a 10-point score that incorporates the lowest heart rate, the lowest mean arterial pressure and the estimated blood loss. The SAS was initially validated in patients undergoing general and vascular surgery and was subsequently expanded to a majority of surgical subspecialties (10). Low SAS scores have been associated with an increased risk of death and major complications.
Emergency abdominal surgery is associated with a very high mortality rate, with values as high as 25% being reported in the United Kingdom (11). Given this high mortality rate in developed countries, the aim of this study was to investigate the ability of the SAS to predict major complications and death after emergency abdominal surgery, specifically in a resource-limited Caribbean setting.
Methods
The study was designed as a retrospective observational study. All patients who had undergone emergency abdominal surgery during the period from 1st January 2011 to 31st December 2012 had their notes analysed for post-surgery complications and their intraoperative SAS calculated.
Inclusion criteria were the following: subjects older than 18 years having emergency abdominal surgery of moderate to major severity (laparotomy, cholecystectomy, appendectomy) under general anaesthesia. Patients were excluded if their surgery was listed as orthopaedics, vascular, trauma, gynaecological, or any type that did not include abdominal surgery, or surgery not done under general anaesthesia. Patients were identified from a register in the operating room.
The SAS was determined from the patient’s intraoperative anaesthesia chart where blood pressures and heart rates were recorded every 5 minutes. The patients’ surgical notes were also analysed until the time of hospital discharge for the presence of major complications and death.
Major complications were classified according to the NSQIP (8, 12). They included acute renal failure, bleeding that required a red blood cells transfusion of 4 U or more within 72 hours after surgery, cardiac arrest requiring cardiopulmonary resuscitation, coma lasting 24 hours or longer, deep venous thrombosis, myocardial infarction, unplanned intubation, ventilator use for 48 hours or more, pneumonia, pulmonary embolism, stroke, wound disruption, deep or organ-space surgical site infection, sepsis, septic shock, systemic inflammatory response syndrome and vascular graft failure. Other complications were assessed on a case-by-case basis by the authors, and complications reaching a Clavien score of III or IV were counted as major complications (13).
The SAS (see Table 1) was calculated as described previously by Gawande et al. (9). All variables for the score were determined from the intraoperative anaesthesia records. The variables extracted were the lowest heart rate, the lowest mean arterial pressure (MAP) and estimated blood loss. When not explicitly recorded, MAP was determined from the following formula: MAP=1/3 systolic BP+2/3 diastolic BP.
Table 1.
Determination of the Surgical Apgar Score using the three intraoperative variables
| Parameter | Points | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 1 | 2 | 3 | 4 | |
|
| |||||
| Estimated blood loss (mL) | >1000 | 601–1000 | 101–600 | ≤100 | - |
| Lowest heart Rate (beats min−1) | >85 | 76–85 | 66–75 | 56–65 | ≤55 |
| Lowest MAP (mmHg) | <40 | 40–54 | 55–69 | ≥70 | - |
MAP: mean arterial pressure
Approval to conduct this study was obtained from both the Ethics Committee of the University of the West Indies and the Ethical and Governance body of the San Fernando General Hospital, Trinidad, West Indies.
Statistical analysis
All data analyses were performed using the Statistical Packages for the Social Sciences (IBM SPSS Corp.; Armonk, NY, USA) version 20. Relative risk (RR) was used to explore the relationship between the various SAS levels (high, low and average). Binary logistic regression was used to test the association between the SAS and major complications or death, and the discriminatory ability of the model was measured by the c-statistic and receiver-operating curve (ROC) analysis. ROC curves were also used to determine the sensitivity and specificity of a threshold SAS for clinical use.
Results
A total of 318 patients were found to fit the inclusion criteria. However, we were unable to obtain a full dataset for 98 patients, which were then excluded from analysis. Two hundred and twenty complete records were available and analysed.
Patient characteristics are shown in Table 2. The median age of the cohort was 62 (18–94), with 99% of the procedures being performed open, and exploratory laparotomy being the most common type of the procedure performed (88.6%).
Table 2.
Characteristics of the patients studied. Results are presented as % (number) or median, unless stated otherwise
| Patient characteristics | |
|---|---|
|
| |
| No. of patients | 318 |
| No. of patients excluded | 98 |
| No. of patients included | 220 |
| Age (median, IQR) | 62 (18–4) |
| Male % (n) | 55 (109) |
| Major complications % (n) | 22 (48) |
| Minor or no complications % (n) | 78 (172) |
| Death % (n) | 11 (24) |
| Alive % (n) | 89 (196) |
| Surgery type % (n) | |
| Open | 99 (218) |
| Laparoscopic | 0.9 (2) |
| Exploratory laparotomy | 88.6 (195) |
| Appendectomy | 1.3 (3) |
| Cholecystectomy | 4.5 (10) |
| Other | 5.4 (12) |
| Postoperative ICU/HDU admission % (n) | 22 (49) |
| Surgical Apgar Score % (n) | |
| [0–2] | 1.1 (3) |
| [3–4] | 8.9 (25) |
| [5–6] | 47.7 (105) |
| [7–8] | 30.5 (67) |
| [9–10] | 9.1 (20) |
| Overall, median | 6 |
ICU: intensive care unit; HDU: high-dependency unit
Of the 220 patients studied, 33% (72 patients) suffered a major complication or death following emergency surgery. 67% (48) of these patients experienced a major complication, and 33% (24) died. The distribution of major complications or death by the SAS is shown in Figure 1. The median SAS was 6, and a lower SAS was associated with an increased risk of major complications or death (Figure 1).
Figure 1.
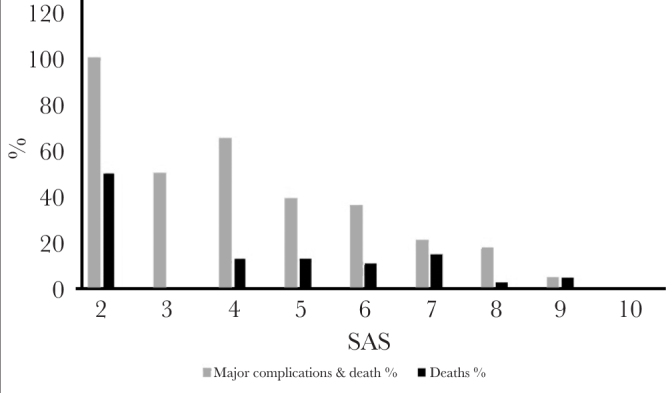
The trend of major complications and death for the various SAS. Note the decremental trend in major complications and death as the SAS increases
SAS: surgical Apgar Score
The RR of a major complication or death between the lowest-scoring patients with a SAS ≤4 (68% major complication or death rate) compared to the highest-scoring ones with a SAS ≥7 (16% major complication or death rate) was 4.21 (95% confidence interval [CI], 2.5–7.3; p<0.001).
In a stepwise comparison between the groups (Table 3), using a SAS of 6–7 as a reference, the RR of major complications was 2.27 (95% CI, 1.52–3.51; p=0.001) in those scoring ≤4 and 0.44 (95% CI, 0.20–0.94; p=0.025) for groups scoring 8–10.
Table 3.
Showing the relative risk between the various groups. The asterisk indicates when that group is being used as the reference group. The average scoring [6–7] and high scoring [8–10] are the two reference groups used
| SAS | 0–4 | 6–7 | 8–10 |
|---|---|---|---|
|
| |||
| Relative risk of complications or death (95% CI) | 2.27 | 1(*) | 0.44 |
| (1.52–3.51) | 1(*) | (0.29–0.66) | |
| 2.69 | 2.22 | 1(*) | |
| (1.55–4.66) | (1.04–4.74) | 1(*) | |
SAS: Surgical Apgar Score
In assessing the goodness of fit of the logistic regression model with SAS as the continuous predictor and major complications or death as the outcome, the p-value for the Hosmer-Lemeshow test was 0.151 (>0.05), indicating a good fit of the regression model.
The odds ratio (OR) for major complications or death per unit increase in the SAS obtained from the regression model was 0.58 (95% CI, 0.47–0.72; p<0.001). Adjusting for age yielded an OR of 0.57 (95% CI, 0.45–0.71; p<0.001). From the regression model, the probability of major complications or death was as high as 70% for patients with a SAS of 2 and as low as 3% for those with a SAS of 10.
The ROC curve for the SAS (Figure 2) showed an area under curve (AUC) of 0.71 (95% CI, 0.68–0.73; p<0.001). This AUC indicates a moderate discriminatory ability of the SAS in the detection of major complications and death after emergency surgery.
Figure 2.
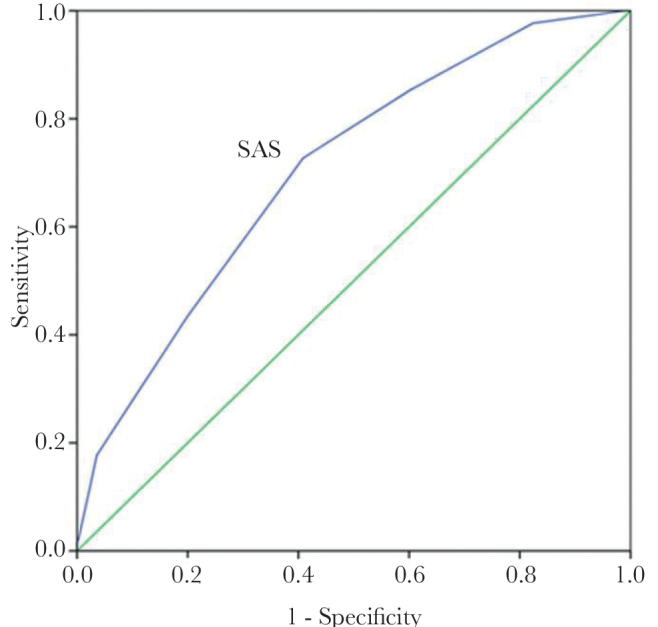
ROC curve for SAS. The ROC curve shows the AUC of 0.71 (95% CI, 0.68–0.73; p<0.001) for the SAS
ROC: receiver-operating curve; SAS: surgical Apgar Score; AUC: area under curve
From the ROC curve analysis, a SAS ≤6 had a 73% sensitivity and a 40% specificity for detecting major complications and death after emergency surgery.
Discussion
Since its inception, the SAS has been shown to be useful in predicting postoperative risk in many patient groups. In addition, the SAS has been cited as a simple and objective screening tool with the ability to identify high-risk patients similar to early warning systems (14).
The SAS has been used as a marker of major complications (9, 15–17), minor complications (17), death (9, 10) and intensive care unit admissions (16, 18). To date, the SAS has been used in almost all types of surgery: general surgery (colon and gastric), general surgery (vascular), neurosurgery, orthopaedic surgery and urology (10).
In our cohort, the major complication rate and death rate after emergency surgery were 33%, while the death rate alone was 11%. Similarly, in the first report by the UK Emergency Laparotomy Network, 30-day mortality after emergency laparotomy was found at approximately 15% for all patients (11). In a study done by Cihoric et al. (19) in Denmark focusing solely on emergency abdominal surgery, similar values were also obtained, with researchers noting a major complication rate of 32.7% and a death rate of 16.3%, with similar complication criteria being used as in our study.
Comparing the high-risk (SAS <4) and low-risk groups (SAS ≥7), the high-risk groups had a four times higher risk of complications (RR, 4.21; 95% CI, 2.5–7.3; p<0.001) than the lower-risk groups. While this risk is lower than most of the previously reported SAS data, with lower-scoring patients incurring as high as 16 times the risk of major complications or death compared to higher-scoring patients, our results are still clinically significant (9). Most of the SAS studies included primarily elective cases; however, subsequent works done in emergency surgery do report a reduced ability of the SAS to detect postoperative complications. A possible explanation for this includes the incidence of preoperative sepsis, which can be higher in patients presenting for emergency surgery. Although we did not screen for sepsis in our study, Cihoric et al. (19) reported a 56% incidence of preoperative sepsis in emergency patients compared to a 5% incidence in the initial SAS studies dealing with primarily elective patients.
The relationship between SAS and major complications was confirmed in the logistic regression with an OR of 0.57 (95% CI, 0.45–0.73; p<0.005). This OR represents the fold-change in odds (of death and major complications) per unit increase in the SAS, with the predicted probability of patients with a SAS of 2 being as high as 70%. This OR was similar to an adjusted OR obtained in the literature (17).
Using the ROC curves, the SAS was shown to have a moderate discriminatory ability in our cohort of patients with a c-statistic of 0.71 (95% CI, 0.68–0.73; p<0.001). Again, this compares well with the reported values in the various SAS trials, done in elective cases, which range from 0.69–0.73 (9, 20, 21). However, specifically, in emergency surgery, one trial reported a c-statistic of 0.63 and concluded that the SAS lacked discriminatory power in this high-risk cohort (19). As suggested by the authors in that trial, an institutional perioperative optimisation programme may have decreased the ability of the score (which relies solely on intraoperative data) to detect major complications. No optimisation was performed in our cohort, which consisted of mainly patients undergoing open exploratory laparotomy. With a similar patient population undergoing emergency laparotomy (78%), Ngarambe et al. (22) found a c-statistic of 0.75 for the detection of major complications.
There are several limitations to be noted in our retrospective analysis. Our final sample size for this retrospective analysis was only 220 patients. Despite this, the SAS was still found to be significantly associated with major complications. Coming from a single centre, our results may lack generalisability. However, our major complication and death rates are similar to many of the published data, and the SAS has been validated in many diverse countries (15). Thus, our paper adds to the growing global literature on the use of the SAS in various geographic regions.
Another source of error in this study is the lack of adjustment for confounding variables in the data analysis, with the OR of the logistic regression being presented as the unadjusted OR. Due to insufficient data, we were only able to adjust for age, which yielded a similar OR as the unadjusted ratio. It is worth noting that even after the risk adjustment done in previous studies, SAS was still shown to be a good marker of postoperative morbidity (9, 10, 15–17, 20, 21, 23). Additionally, the use of hand-written reports may have also decreased the reliability of the extracted variables (24).
Conclusion
In summary, the 10-point SAS score was found to be predictive of major complications and death after emergency abdominal surgery in a resource-limited Caribbean setting. Given the high-risk nature of emergency abdominal surgery, we suggest that the easy-to-calculate SAS be incorporated with other clinical data to risk stratify and triage emergency surgical patients.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of University of the West Indies Faculty of Medical Sciences (July 15th 2013).
Informed Consent: Due to the retrospective design of the study, informed consent was waived by the ethics committee.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – K.S., S.H.; Design – K.S., S.H.; Supervision – K.S., S.H.; Data Collection and/or Processing – K.S.; Analysis and/or Interpretation – K.S., S.H.; Literature Search – K.S.; Writing Manuscript – K.S., S.H.; Critical Review – S.H.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–44. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Gawande AA, Regenbogen SE. Critical need for objective assessment of postsurgical patients. Anesthesiology. 2011;114:1269–70. doi: 10.1097/ALN.0b013e318219d76b. [DOI] [PubMed] [Google Scholar]
- 3.Hariharan S, Zbar A. Risk Scoring in Perioperative and Surgical Intensive Care Patients: A Review. Curr Surg. 2006;63:226–36. doi: 10.1016/j.cursur.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Sobol JB, Wunsch H. Triage of high-risk surgical patients for intensive care. Crit Care. 2011;15:217. doi: 10.1186/cc9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55:111–5. doi: 10.4103/0019-5049.79879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9. doi: 10.1161/01.CIR.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 7.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–60. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 8.Khuri SF, Daley J, Henderson W, Barbour G, Lowry P, Irvin G, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180:519–31. [PubMed] [Google Scholar]
- 9.Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204:201–8. doi: 10.1016/j.jamcollsurg.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds PQ, Sanders NW, Schildcrout JS, Mercaldo ND, St Jacques PJ. Expansion of the surgical Apgar score across all surgical subspecialties as a means to predict postoperative mortality. Anesthesiology. 2011;114:1305–12. doi: 10.1097/ALN.0b013e318219d734. [DOI] [PubMed] [Google Scholar]
- 11.Saunders DI, Murray D, Pichel AC, Varley S, Peden CJ. Variations in mortality after emergency laparotomy: the first report of the UK Emergency Laparotomy Network. Br J Anaesth. 2012;109:368–75. doi: 10.1093/bja/aes165. [DOI] [PubMed] [Google Scholar]
- 12.Fink AS, Campbell DA, Jr, Mentzer RM, Jr, Henderson WG, Daley J, Bannister J, et al. The National Surgical Quality Improvement Program in non-veterans administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236:344–54. doi: 10.1097/00000658-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 14.Augoustides JG, Patel P. Recent advances in perioperative medicine: highlights from the literature for the cardiothoracic and vascular anesthesiologist. J Cardiothorac Vasc Anesth. 2009;23:430–6. doi: 10.1053/j.jvca.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Haynes AB, Regenbogen SE, Weiser TG, Lipsitz SR, Dziekan G, Berry WR, et al. Surgical outcome measurement for a global patient population: validation of the Surgical Apgar Score in 8 countries. Surgery. 2011;149:519–24. doi: 10.1016/j.surg.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Thorn CC, Chan M, Sinha N, Harrison RA. Utility of the Surgical Apgar Score in a district general hospital. World J Surg. 2012;36:1066–73. doi: 10.1007/s00268-012-1495-2. [DOI] [PubMed] [Google Scholar]
- 17.Melis M, Pinna A, Okochi S, Masi A, Rosman AS, Neihaus D, et al. Validation of the Surgical Apgar Score in a veteran population undergoing general surgery. J Am Coll Surg. 2014;218:218–25. doi: 10.1016/j.jamcollsurg.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Sobol JB, Gershengorn HB, Wunsch H, Li G. The surgical Apgar score is strongly associated with intensive care unit admission after high-risk intraabdominal surgery. Anesth Analg. 2013;117:438–46. doi: 10.1213/ANE.0b013e31829180b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cihoric M, Toft Tengberg L, Bay-Nielsen M, Bang Foss N. Prediction of Outcome After Emergency High-Risk Intra-abdominal Surgery Using the Surgical Apgar Score. Anesth Analg. 2016;123:1516–21. doi: 10.1213/ANE.0000000000001501. [DOI] [PubMed] [Google Scholar]
- 20.Regenbogen SE, Bordeianou L, Hutter MM, Gawande AA. The intraoperative Surgical Apgar Score predicts postdischarge complications after colon and rectal resection. Surgery. 2010;148:559–66. doi: 10.1016/j.surg.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regenbogen SE, Ehrenfeld JM, Lipsitz SR, Greenberg CC, Hutter MM, Gawande AA. Utility of the surgical apgar score: validation in 4119 patients. Arch Surg. 2009;144:30–6. doi: 10.1001/archsurg.2008.504. discussion 7. [DOI] [PubMed] [Google Scholar]
- 22.Ngarambe C, Smart BJ, Nagarajan N, Rickard J. Validation of the Surgical Apgar Score After Laparotomy at a Tertiary Referral Hospital in Rwanda. World J Surg. 2017;41:1734–42. doi: 10.1007/s00268-017-3951-5. [DOI] [PubMed] [Google Scholar]
- 23.Regenbogen SE, Lancaster RT, Lipsitz SR, Greenberg CC, Hutter MM, Gawande AA. Does the Surgical Apgar Score measure intraoperative performance? Ann Surg. 2008;248:320–8. doi: 10.1097/SLA.0b013e318181c6b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanborn MDKV, Castro MDJ, Kuroda MPHM, Thys MDDM. Detection of Intraoperative Incidents by Electronic Scanning of Computerized Anesthesia RecordsComparison with Voluntary Reporting. Anesthesiology. 1996;85:977–87. doi: 10.1097/00000542-199611000-00004. [DOI] [PubMed] [Google Scholar]


