Abstract
Immunofluorescent confocal microscopy is a powerful tool for analysis of the morphology and development of vascular and lymphatic tissues. Schlemm’s canal (SC) is a large, lymphatic-like vessel in the anterior chamber of the eye, which is essential for aqueous humor drainage required to maintain intraocular pressure and is sensitive to defects in blood and lymphatic vascular signaling pathways. Here, we describe a method to stain and quantify SC area and morphology in enucleated mouse eyes, providing a tool for understanding its development and function in small animal genetic or disease models.
Keywords: Whole-mount, Immunostaining, Immunofluorescence, Confocal microscopy
1. Introduction
Schlemm’s canal (SC) is a large, lymphatic-like vessel in the anterior chamber of the eye and is essential for aqueous humor outflow [1–5]. Defects in SC structure and function lead to reduced aqueous humor outflow, elevated intraocular pressure (IOP) and glaucoma [2,5–8]. Imaging methods for evaluation of SC morphology and development are therefore valuable tools in the study of mouse models of ocular hypertension and glaucoma. While mice have a continuous SC morphologically similar to humans, both species exhibit marked variability in diameter along the length of the canal [9,6,3] making analysis using thin sections difficult and labor intensive. Recent studies have overcome this obstacle using whole-mount imaging of the enucleated eye, which allows 3-dimensional analysis of the full SC [2–4,6,7]. In mice this can be readily accomplished using confocal microscopy, as SC lies within 50 μm of the outer surface of the eye and a number of high-quality antibodies and fluorescent reporter mouse lines are available, which robustly label the SC endothelium. Here, we report a method to prepare and stain wholemounts of eye tissue from mice for confocal microscopy and SC analysis. We have successfully used this method to image the SC region in mice of all ages, from embryonic day 18.5 (before SC is present) until adulthood.
2. Materials
Prepare all solutions using ultrapure water and analytical grade reagents. Store all solutions at room temperature except for fixative and blocking buffer, which are stored at 4°C.
2.1: Fixation
Fixative: 2% formaldehyde in 0.1M phosphate buffer, pH 7.4. We prepare this solution as two 10X stocks, which can be quickly combined to prepare the fixative working solution. This working solution is prepared by adding 5 ml 1M sodium phosphate buffer and 5 ml 20% formaldehyde to 40 ml water, yielding 2% formaldehyde in 0.1M phosphate buffer. Working solution should be stored at 4°C and used within 2–3 days.
1M sodium phosphate pH 7.4: Dissolve 13.56 g NaH2PO4 and 54.94 g Na2HPO4 in water to a volume of 400 ml. Adjust pH if needed and set final volume to 500 ml.
20% formaldehyde: Add 20 g powdered paraformaldehyde (see note 1) to a glass beaker and add water to a volume of 90 ml. Place solution on a hot plate in a fume food and heat to 60° with stirring, gradually adding 1N NaOH until paraformaldehyde is dissolved. Do not add more than 1 ml of 1N NaOH or allow solution to heat above 70°. Allow solution to cool to room temperature and set volume to 100 ml with water. Aliquots of 20% formaldehyde should be stored at −20°C and thawed as needed by warming in a 60° water bath (see note 2).
Phosphate buffered saline (PBS), pH 7.4: 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4.
2.2. Tissue preparation and staining
Tris buffered saline (TBS), pH 7.4: 150 mM NaCl, 50 mM Tris-Cl.
TBST: TBS containing 0.05% Tween-20.
Permeabilization/blocking buffer: 5% donkey serum, 2.5% BSA, 0.5% Triton X100 in TBS. After preparation, this solution should be sterile filtered and stored as aliquots at −20°C. Thawed aliquots can be stored for several weeks at 4°C.
Rat anti-CD31/Pecam1 antibody
Goat anti-PROX1 antibody
Fluorescently labeled donkey anti-rat and anti-goat secondary antibodies.
3. Method
3.1: Tissue preparation
Sacrifice mice according to the protocol approved by your local animal care committee (see Note 3). To enucleate the eyes, use fine scissors and make an incision on the lateral side of the socket, cutting through the conjunctiva and surrounding tissue while being careful not to puncture the eye. After making this incision, enucleate the eye by pulling back the tissue surrounding the eye socket and firmly grasping the optic nerve with curved forceps.
Fix enucleated eyes by immersion in fixative (described above) and rotating overnight at 4°C.
After fixation, place the eye in a 10 cm tissue dish of TBS and, working under a dissecting microscope, use fine scissors (see Note 4) to remove the conjunctiva and any remaining connective tissue from the surface of the eye. Take care not to puncture the eye at this stage.
Using scissors, cut the optic nerve where it emerges from the globe.
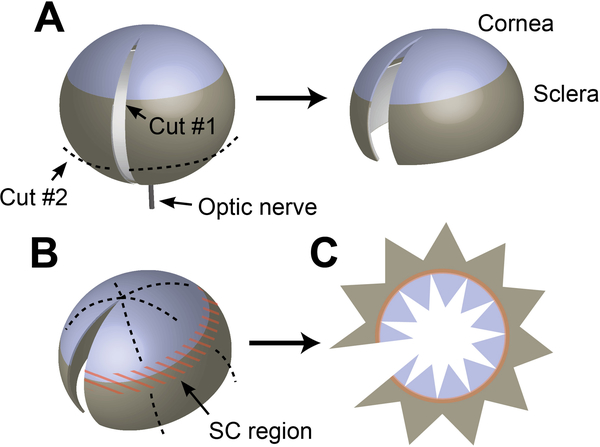
Grasp the eye with forceps by inserting one point into the hole where the optic nerve emerged from the sclera. With the orb thus gripped, use a sharp razor blade (see notes 5 and 6) to make an incision from the optic nerve to the center of the cornea. This incision should be made in one even, gentle stroke, applying little downwards pressure and instead relying on the sharp edge of the blade to make the cut. If the initial cut does not extend the full length of the needed opening, enlarge the incision with scissors (Figure 1 A).
Using scissors, remove the lower third of the sclera as shown by the dotted line in Figure 1 A, taking care not to damage the iridocorneal angle / limbal region where SC is located.
Using forceps, remove the crystalline lens and retina through the opening created in the previous steps (see Note 7).
Figure 1.
Preparing enucleated eyes for staining and mounting. (A) After removing the conjunctiva and other connective tissue from the surface of the globe, remove the optic nerve by cutting flush with the surface of the eye with scissors. While grasping the eye through the hole left by the optic nerve, a razor blade is used to make an incision from the optic nerve to the center of the cornea. A second cut (dashed line) is then made to remove the lower third of the sclera. (B) After staining, scissors are used to make a series of cuts in the sclera and cornea in preparation for flat mounting—taking care to avoid cutting into the iridocorneal angle/limbal region (orange). (C) After cutting, the eye is opened and laid flat on a glass slide in preparation for mounting.
3.2: Staining
Transfer the prepared eyes to 2ml round-bottom eppendorf tubes completely filled with permeabilization/blocking buffer. Rotate on an end-over-end or nutating shaker for 6 hours at room temperature or overnight at 4°C.
Prepare primary antibody dilutions in fresh permeabilization/blocking buffer. Mature SC is strongly labeled by antibodies against CD31 (Pecam1) and PROX1 [3,4,7], and we prefer a double-labeling method using both of these antibodies. 100 μl of antibody working solution is sufficient to stain an adult mouse eye in a 2 ml round bottom Eppendorf tube.
Aspirate blocking buffer and replace with primary antibody diluted in fresh permeabilization/blocking buffer. Place tubes upright in an Eppendorf tube rack and rotate overnight at 4°C on a bellydancer or nutating shaker.
Aspirate antibody solution and wash 6x in TBST. For each wash, completely fill the Eppendorf tube with TBST and rotate at room temperature for 1 hour. Repeat by aspirating and replacing the solution with fresh TBST.
Prepare dilutions of appropriate fluorescently labeled secondary antibodies in permeabilization/blocking buffer. Aspirate TBST after the final wash and replace with 100 μl of secondary antibody solution. Place tubes upright in Eppendorf tube rack, wrap in foil to protect from light, and rotate overnight as in step 2.3.
Wash as in step 2.4.
Mount. Place a clean microscope slide under a dissecting microscope and place a large drop of TBS in the center. Using forceps, transfer the tissue into this drop so that it is submerged and easily visible under the microscope. Use fine scissors to trim the eye as shown in Figure 1 B, making a series of cuts through the sclera towards the limbal region. A similar series of cuts should then be made from the center of the cornea. Still working within the drop of TBS, unroll the eye and flatten it against the slide, with the outer surface facing upwards. Additional cuts in the cornea and sclera can be made as required to ensure that the tissue lies flat as shown in Figure 1 C. When making cuts, take care to avoid cutting through and damaging the limbal region where SC is located, marked in orange in Figure 1. Use tissue paper to slowly aspirate TBS from the surface of the slide, encouraging the tissue to lie flat and stopping as needed to make additional cuts and straighten the tissue with forceps. Once TBS is removed and tissue is fully flattened, gently place a drop of mounting media (see Note 8) onto the slide and cover with an appropriate cover glass.
Mounted slides should be dried while protected from light and stored at 4°C until imaging.
3.3: Imaging and analysis
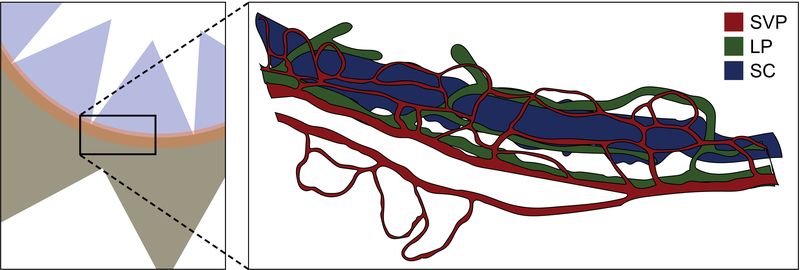
Image slides using a confocal microscope equipped with appropriate laser lines and filters for secondary antibody fluorophores selected in step 11 and a 20x objective (see Note 9). When scanning through the iridocorneal angle region in the Z-axis, the CD31-positive superficial vascular plexus [3] and PROX1/CD31-positive limbal lymphatic vasculature [2,5] will be readily apparent (Figure 2). SC resides 15–20 μm below these vessels, and can be identified by its large diameter as well as its PROX1/CD31 double staining (see Note 10). Scanning deeper will reveal the vasculature of the iris (although this may not be visible if using eyes from pigmented mice).
Figure 2.
When imaging the iridocorneal region using a confocal microscope, the superficial vascular (SVP, red) and lymphatic (LP, green) plexuses will be easily visible near the surface of the eye. Schlemm’s canal (SC, blue) resides 10–15 μm below them.
3.4: Measurement of SC area
With the confocal pinhole set to 1 Airy unit and a step size of 1.67 μm, capture 3–5 10-image SC Z-stacks at intervals around the circumference of the iridocorneal angle.
Using ImageJ Fiji software [10], generate maximum intensity Z-projections of each image and measure the SC area obtained in each field. Average these measurements to obtain a single value for each eye.
3.5. Quantification of focal defects and convolutions
To quantify the presence of focal defects and convolutions which do not have a significant impact on total SC area, large area scans encompassing the entire SC may be used. Using a 10 or 20× objective with a fully opened pinhole, focus on the SC and use the large area scan function (see Note 11) of a confocal microscope equipped with a computer controlled stage to capture an image including the entire circumference of the iridocorneal angle and the canal.
Use large area scan images to quantify focal defects (i.e. narrowings or gaps) in canal morphology. These can be easily counted manually using Fiji software.
Acknowledgement
This work was supported by NIH grants R01HL124120 and R01 EY025799 to SEQ.
Footnotes
Wear a mask and minimize dust formation when weighing paraformaldehyde.
Solution must be completely clear before use. If precipitate is observed in thawed 20% formaldehyde aliquots after warming for 30 minutes, a small amount of 1N NaOH may be used to aid redissolving.
In our lab, mice are euthanized by cervical dislocation following anesthesia by ketamine/xylazine.
We find extra fine Bonn scissors or Vannas scissors with straight blades work well throughout this protocol. Curved scissors may be used, but we find it more difficult to make straight cuts for flat mounting the tissue.
We prefer double-edged razor blades of the type used in a vibrotome for making this cut, although a standard razor blade will work if very sharp. Scalpel blades can be used, but their curvature and shorter length can make it more difficult to get a straight cut.
If the eye has been punctured during dissection or preparation, there may not be enough fluid pressure within the globe to provide support for the sclera and allow a clean cut. In this case, it may be easier to use scissors—inserting the tip into the existing puncture and cutting in both directions.
Although antibody can be trapped behind the iris and create fluorescent background, we typically do not remove the iris as it is very easy to damage the trabecular meshwork and SC inner wall while doing so. Using confocal microscopy, any background from this area usually presents little problem, especially when staining eyes from pigmented mice. If background is a problem, especially when using albino mice, the iris may be removed with care.
We prefer non-hardening mounting media such as Shandon Immu-mount. If slides are allowed to dry for 2–3 hours after mounting, evaporation helps to pull the cover slip down tightly, flattening the tissue. After this drying period, cover glasses are sealed with clear nail polish and slides can be stored at 4°C.
A higher-power objective can be used, however an objective with sufficient working distance is essential as SC is located approximately 30 μm from the surface of the eye and will be even further from the surface of the cover slip. We have found that a 20× Plan Apochromat objective with a numeric aperture of 0.75 and a working distance of 1 mm provides good results.
While PROX1 is expressed by the SC endothelium, the level of expression is markedly lower than that observed in the limbal lymphatic capillaries. Using the staining level on these true lymphatics to set laser power and other microscope exposure parameters often results in low or absent PROX1 staining observed in SC until the signal intensity is increased.
The NIS-Elements software on our Nikon A1R confocal microscope allows us to capture a large ‘free-shape” image, rather than a complete square. This and similar imaging modes are useful for whole-mount imaging as they allow focus adjustments between each tile.
References
- 1.Gong H, Tripathi RC, Tripathi BJ (1996) Morphology of the aqueous outflow pathway. Microsc Res Tech 33 (4):336–367. doi: [DOI] [PubMed] [Google Scholar]
- 2.Aspelund A, Tammela T, Antila S, Nurmi H, Lepp xE, nen V-M, Zarkada G, Stanczuk L, Francois M, xE, kinen T, Saharinen P, Immonen I, Alitalo K (2014) The Schlemm’s canal is a VEGF-C/VEGFR-3–responsive lymphatic-like vessel. J Clin Invest 124 (9):3975–3986. doi: 10.1172/JCI75395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kizhatil K, Ryan M, Marchant JK, Henrich S, John SWM (2014) Schlemm’s Canal Is a Unique Vessel with a Combination of Blood Vascular and Lymphatic Phenotypes that Forms by a Novel Developmental Process. PLoS Biol 12 (7):e1001912. doi: 10.1371/journal.pbio.1001912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park D-Y, Lee J, Park I, Choi D, Lee S, Song S, Hwang Y, Hong KY, Nakaoka Y, Makinen T, Kim P, Alitalo K, Hong Y-K, Koh GY (2014) Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J Clin Invest 124 (9):3960–3974. doi: 10.1172/JCI75392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, Sung H-K, Onay T, Chen H, Yamaguchi S, Economides AN, Flenniken A, Gale NW, Hong Y-K, Fawzi A, Liu X, Kume T, Quaggin SE (2014) A lymphatic defect causes ocular hypertension and glaucoma in mice. J Clin Invest 124 (10). doi: 10.1172/JCI77162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souma T, Tompson SW, Thomson BR, Siggs OM, Kizhatil K, Yamaguchi S, Feng L, Limviphuvadh V, Whisenhunt KN, Maurer-Stroh S, Yanovitch TL, Kalaydjieva L, Azmanov DN, Finzi S, Mauri L, Javadiyan S, Souzeau E, Zhou T, Hewitt AW, Kloss B, Burdon KP, Mackey DA, Allen KF, Ruddle JB, Lim S-H, Rozen S, Tran-Viet K-N, Liu X, John S, Wiggs JL, Pasutto F, Craig JE, Jin J, Quaggin SE, Young TL Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J Clin Invest 126 (7):2575–2587. doi: 10.1172/JCI85830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson BR, Souma T, Tompson SW, Onay T, Kizhatil K, Siggs OM, Feng L, Whisenhunt KN, Yanovitch TL, Kalaydjieva L, Azmanov DN, Finzi S, Tanna CE, Hewitt AW, Mackey DA, Bradfield YS, Souzeau E, Javadiyan S, Wiggs JL, Pasutto F, Liu X, John SWM, Craig JE, Jin J, Young TL, Quaggin SE (2017) Angiopoietin-1 is required for Schlemm’s canal development in mice and humans. J Clin Invest 127 (12):4421–4436. doi: 10.1172/JCI95545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Park D-Y, Bae H, Park DY, Kim D, Lee C-k, Song S, Chung T-Y, Lim DH, Kubota Y, Hong Y-K, He Y, Augustin HG, Oliver G, Koh GY (2017) Impaired angiopoietin/Tie2 signaling compromises Schlemm’s canal integrity and induces glaucoma. J Clin Invest 127 (10):38773896. doi: 10.1172/JCI94668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashton N (1951) Anatomical study of Schlemm’s canal and aqueous veins by means of neoprene casts. Part I. Aqueous veins. Br J Ophthalmol 35 (5):291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Meth 9 (7):676–682. doi:http://www.nature.com/nmeth/journal/v9/n7/abs/nmeth.2019.html#supplementaryinformation [DOI] [PMC free article] [PubMed] [Google Scholar]