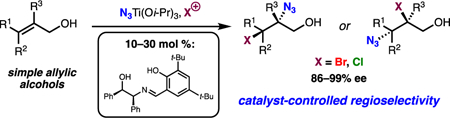
Abstract
Herein we report a highly regio- and stereoselective haloazidation of allylic alcohols. This enantioselective reaction uses readily available materials and can be performed on a variety of alkyl-substituted alkenes and can incorporate either bromine or chlorine as the electrophilic halogen component. Both halide and azido groups of the resulting products can be transformed into valuable building blocks with complete stereospecificity. The first example of an enantioselective 1,4-haloazidation of a conjugated diene is reported as well as its application to a concise synthesis of an aza-sugar.
Graphical Abstract
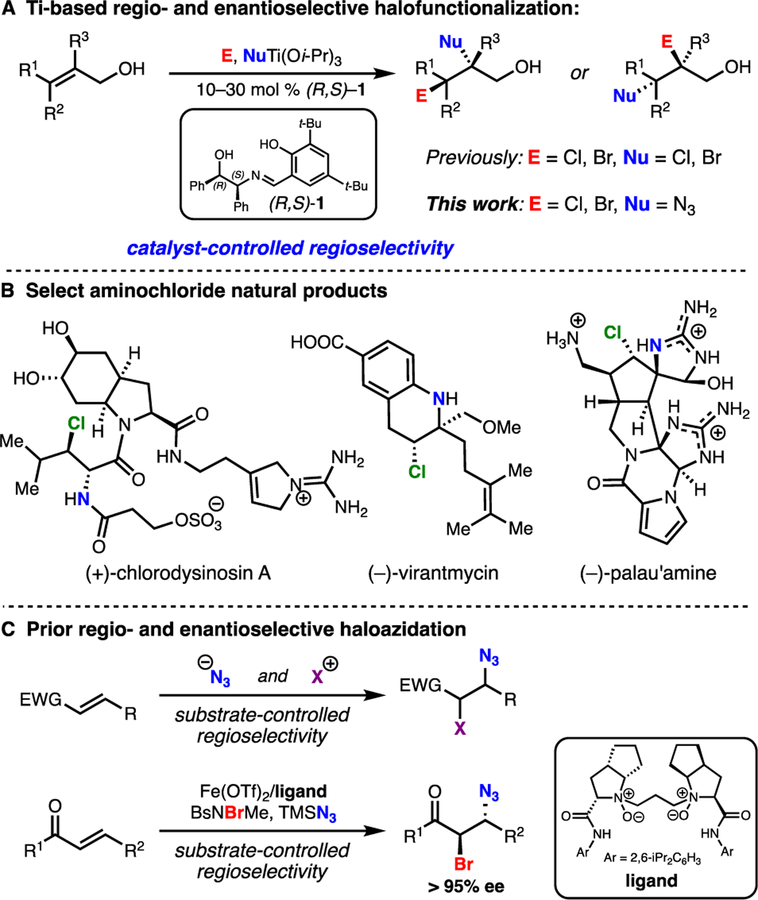
Vicinal difunctionalization of alkenes is a powerful way to rapidly build complexity into small molecules from readily available starting materials.1 However, adding two functional groups across alkenes with regio-, diastereo-, and enantiocontrol is a challenging task. Taking advantage of the inherent reactivity of olefins, halofunctionalization2,3 is among the most direct ways to react unactivated alkenes without resorting to π-bond activation by late-transition metals.1b,1c,1g Nevertheless, due to the highly reactive nature of most commonly employed halogen sources and the potential issue of configurational instability4 of haliranium ions, successful enantioselective halofunctionalizations have largely relied on the intramolecular capture of haliranium ion intermediates; this substantially limits their substrate scope and synthetic utility. Our group has recently developed an enantioselective titanium-mediated dihalogenation of allylic alcohols. Selectivity is achieved through the addition of substoichiometric amounts of simple chiral Schiff base (R,S)-1 that is available in one step from commercial materials (Figure 1A).5 This method has been used strategically to achieve enantioselective total syntheses of over 11 structurally diverse halogenated natural products.5h Due to the importance of nonracemic chiral amines in small molecules,6a we next sought to extend this system to C–N bond formation. For this, we envisioned a combination of halogen electrophiles with a titanium-bound nitrogen nucleophile to render this a more general platform for the selective 1,2-difunctionalization of allylic alcohols. Herein we describe the enantioselective haloazidation of allylic alcohols as an illustration of this process.
Figure 1.
Selective Haloazidation and Aminochloride Natural Products
Since the first preparation of iodine azide by Hantsch6b in 1900, alkene haloazidation has seen use in synthetic chemistry.7 Given the utility of organic azides8 and alkyl halides,9 the haloazide motif serves as an obvious precursor to valuable chiral amines and potentially to a handful of aminochlorinated natural products (Figure 1B).10 For electron-deficient alkenes, conjugate 1,4-addition of azide followed by trapping with electrophilic halogen sources provides substrate-controlled regioselective access to α-halo-β-azides (Figure 1C). Using this strategy to control regioselectivity, Feng11 recently reported a highly enantioselective Lewis acid-catalyzed haloazidation of enones as the first and only enantioselective example of such a reaction. The haloazidation of electron-neutral or rich olefins typically uses preformed or in situ generated haloazide reagents, however, enantioselective variants are still unknown, presumably because these highly reactive and unstable reagents render the control of selectivity challenging. In addition, free radical7b,7d,7r,7s and ionic addition pathways have been proposed under different conditions, further complicating the design of a system for asymmetric catalysis. We thus set out to determine if our titanium–Schiff base combination would be amenable to such a selective alkene functionalization.
To apply our titanium-based system to selective alkene haloazidation, we posited that a titanium azide species would be Lewis acidic enough to activate an electrophilic halogen source (bracketed intermediate, Table 1) for intramolecular transfer of Br+ or Cl+ to form a transient bromonium or chloronium ion. Azide could then add to the transient halonium to deliver a haloazide product. In accord with the seminal work of Sharpless and others on the ring opening of epoxides by Ti(N3)2(Oi-Pr)2 and TiN3(Oi-Pr)3,12 we observed formation of solid TiN3(Oi-Pr)3 by IR (2076 cm–1) upon combining stoichiometric amounts of TMSN3 and Ti(Oi-Pr)4. A mild exotherm was observed in combining these two reagents (see Supporting Information). We obtained calorimetry/thermogravimetric analysis data on TiN3(Oi-Pr)3 and observed an onset temperature of decomposition of 230 ˚C (see Supporting Information). The resulting TiN3(Oi-Pr)3 can then be dissolved in hexanes and directly employed in enantioselective haloazidations. With N-bromosuccinimide (NBS) as the halogen source, the bromoazidation is highly selective for a variety of allylic alcohols. Cinnamyl alcohol (Table 1, entry 1), geminal disubstituted alkenes (entries 2, 3 and 4), 1,2-disubstituted allylic alkenes (entries 5 and 6), linear trisubstituted alkene (entry 7) and cyclic alkenes (entry 8) are all suitable substrates, and the resulting vicinal bromo azides can be obtained in good yields, excellent enantioselectivities, and good to moderate constitutional isomer ratios (cr). C-3 disubstitution, including tetrasubstitution, is not tolerated. Evidence for a similar reaction pathway with our dihalogenation is provided by identical observed regioselectivities.5c With the exception of cinnamyl alcohols (entry 1) and trans-disubstituted allylic alcohols (entry 5), C-2 azide products are obtained as the major isomer. High chemoselectivity for allylic alcohol haloazidition is demonstrated on a doubly unsaturated substrate (entry 4). When substituting NBS with t-BuOCl, the corresponding vicinal chloroazides are produced with similarly high selectivities. A collection of allylic alcohols is well tolerated (entries 9 to 13), although higher Schiff base loadings are necessary, likely due to the more reactive nature of t-BuOCl and intermediate chloronium ions. Density functional theory calculations (M06–2X/6–31g(d), pcm = n-hexane) comparing N-1 with N-3 azide addition show an energetic preference for the distal N-3 serving as the nucleophile (Figure 2).
Table 1.
Haloazidation Substrate Scope
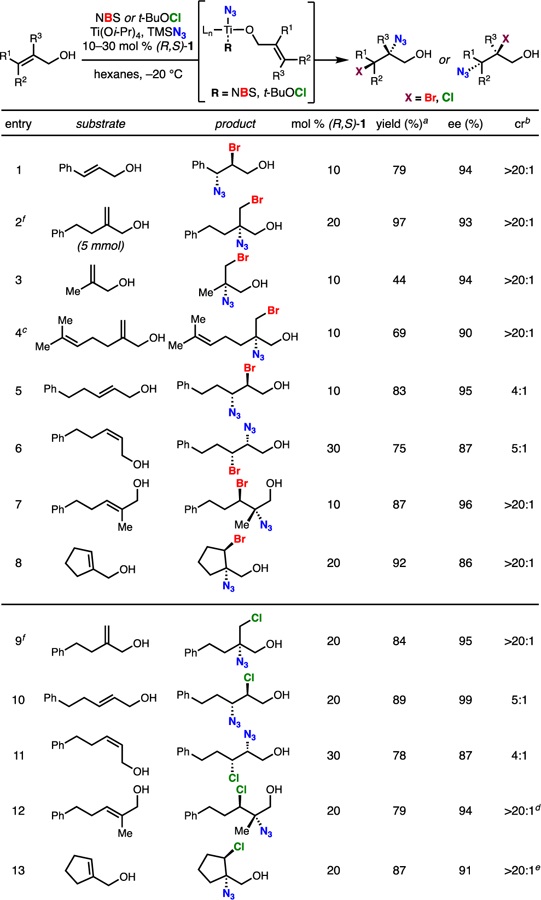 |
Conditions unless otherwise noted: 1.0 mmol allylic alcohol, 1.3 equiv of NBS or 2.0 equiv of t-BuOCl, 1.2 equiv Ti(Oi-Pr)4, 1.1 equiv TMSN3, 10–30 mol % (R,S)-1, hexanes, –20 °C, 12–18 h;
reported isolated yields are for the sum of constitutional isomers;
cr = constitutional isomer ratio;
reaction run at 0 °C;
10:1 ratio of product to dichlorinated alkene;
5:1 ratio of product to dichlorinated alkene;
See Supporting Information for X-ray structures of ferrocene triazole derivatives.
Figure 2.
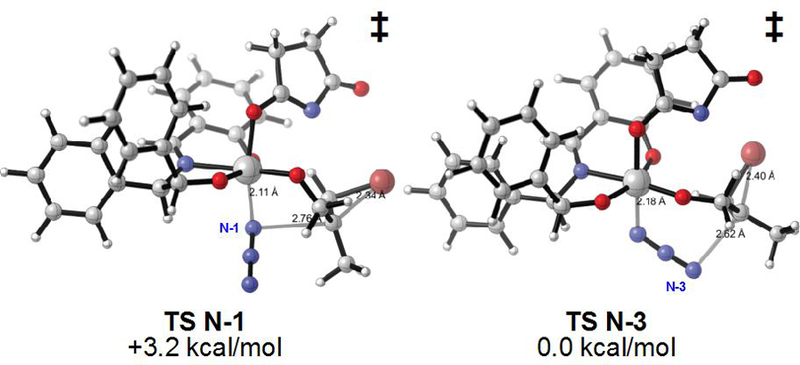
Computed isomeric transition state structures for N-1 versus N-3 nucleophilic addition; M06–2X/6–31g(d) level of theory (PCM = n-hexane); relative uncorrected electronic energies.
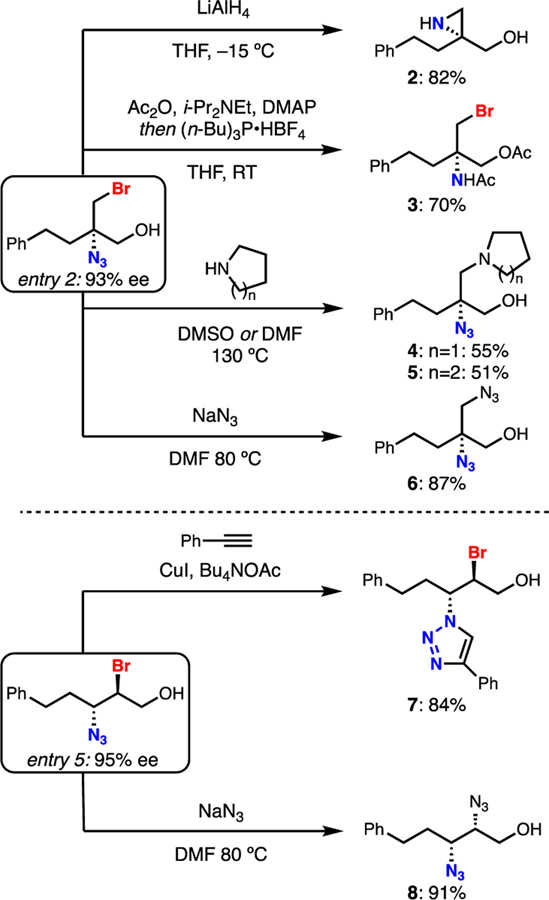
In order to further demonstrate the utility and stereointegrity of the product haloazides, several derivatizations were investigated. All derivatizations occur with complete stereospecifity (Scheme 1). LiAlH4 is known to reduce haloazides to the corresponding aminohalides which spontaneously collapse to aziridines,7g and this proceeded smoothly in 82% yield to give hydroxy N-H aziridine 2.13 Selective reduction of the azide to the primary amine without aziridine formation was also achieved. It was found that a combination of Ac2O, tri-n-butylphosphonium tetrafluoroborate,14 and base was capable of reducing the azide and trapping the incipient iminophosphorane to provide diacyl bromoaminoalcohol 3 in 70% yield. Bromides can be selectively substituted by pyrrolidine, piperidine, or sodium azide to provide 4, 5, and 6/8, respectively, demonstrating efficiency in simple nucleophilic displacements.15 In addition, an azide-alkyne cycloaddition16 proceeds under mild conditions in the presence of a copper catalyst without interference of the secondary alkyl bromide to produce triazole 7 in 84% yield.
Scheme 1.
Bromoazide Derivatizations
Lastly, in an interesting display of regiochemistry, 2,4-hexadien-1-ol is functionalized in a 1,4-manner, giving rise to selective formation of unsaturated 1,4-bromoazide 9 (Scheme 2). This method represents the first example of an enantioselective intermolecular 1,4-halofunctionalization of a conjugated diene17 and sets two remote stereocenters. Here it is likely that the N-3 nitrogen of the azide serves as the nucleophilic atom, however we propose that this pathway involves diene 1,2-addition followed by [3,3] allylic azide rearrangement (Scheme 2, top).18 Such rearrangements are known to have a first order rate constant of at least 4.9×105 sec-1.18a Seeing potential for such products in the construction of highly substituted chiral pyrrolidines we targeted aza-sugar 11 (Scheme 2); such monosaccharide mimics have served as therapeutic agents in a variety of diseases including diabetes and viral infection as glycosidase inhibitors.19 Following TBS protection of alcohol 9, the internal alkene undergoes a stereoselective Sharpless dihydroxylation to give diol 10. Without the (DHQ)2PHAL ligand, a 1:1 mixture of diastereomers was observed. The diol is subsequently protected as the acetonide, and the azide can then be be reduced by LiAlH4 to give the primary amine. After global deprotection with HCl, clean cyclization is achieved under basic conditions at elevated temperatures to provide target 11. This application paves the way for the synthesis of other enantioenriched pyrrolidines utilizing this haloazidation technology.
Scheme 2.
Diene 1,4-Bromoazidation and Application to the Synthesis of Aza-Sugars
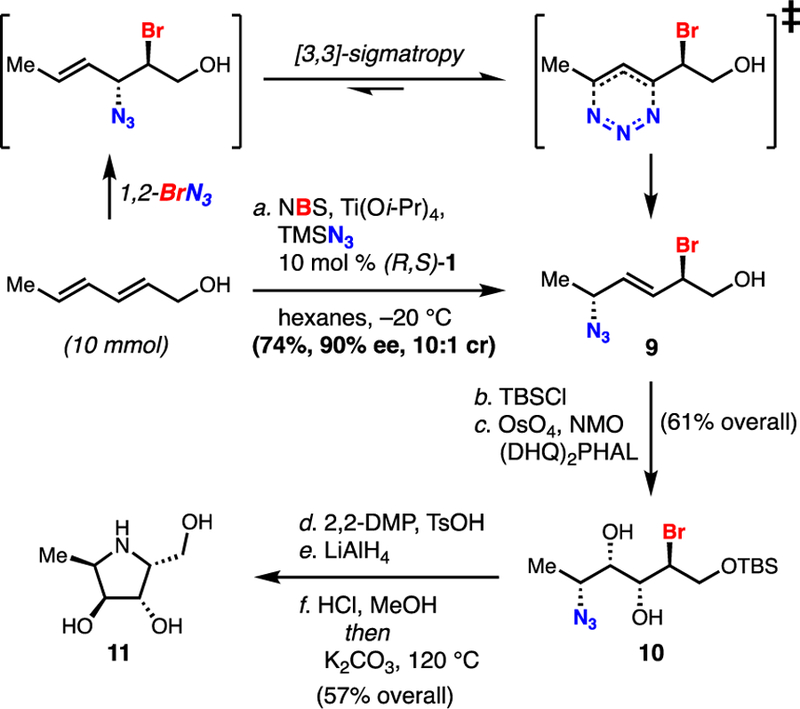
Conditions: a. 10.0 mmol allylic alcohol, 1.3 equiv of NBS, 1.2 equiv Ti(Oi-Pr)4, 1.1 equiv TMSN3, 10 mol % (R,S)-1, hexanes, –20 °C, 74%, 90% ee; b. TBSCl (2 equiv), Et3N (1.5 equiv), DMAP (0.5 equiv), DCM, RT, 90%; c. OsO4 (2 mol %), (DHQ)2PHAL (6 mol %), NMO (2 equiv), acetone/pH 7 buffer, 0 °C to RT, 68%; d. TsOH·H2O (10 mol %), 2,2-dimethoxypropane/acetone, RT, 92%; e. LiAlH4 (3 equiv), THF, –78 °C to RT, 72%; f. 6N HCl, MeOH, H2O, then K2CO3 (14 equiv), 120 °C (µw), 6 h, 87%.
In summary, we have developed a highly practical method for the catalytic enantioselective haloazidation of electronically-unbiased alkenes with catalyst-controlled regio- and enantioselectivity. We have also used this system in the first example of an enantioselective 1,4-haloazidation of a 1,3-conjugated diene. The obtained products have been demonstrated as precursors to chiral nitrogen-containing small molecules including an aza-sugar. The continuing development of our titanium-based catalytic system in other difunctionalizations of π-systems is ongoing in our lab.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to Dr. A. Oliver (University of Notre Dame) for X-ray crystallographic analysis, Dr. S. Lynch (Stanford University) for assistance with NMR spectroscopy, and Prof. Stefan Bräse (Karlsruher Institut für Technologie) for helpful discussion.
Funding Sources
This work was supported by Stanford University and the National Institutes of Health (R01 GM114061).
Footnotes
ASSOCIATED CONTENT
Experimental procedures, characterizations, spectral data, and CIF files. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1) (a).Kolb HC; VanNieuwenhze MS; Sharpless KB Catalytic asymmetric dihydroxylation. Chem. Rev 1994, 94, 2483. [Google Scholar]; (b) Jensen KH; Sigman MS Mechanistic approaches to palladium-catalyzed alkene difunctionalization reactions. Org. Biomol. Chem 2008, 6, 4083. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) McDonald RI; Liu GS; Stahl SS Palladium(ii)-catalyzed alkene functionalization via nucleopalladation: Stereochemical pathways and enantioselective catalytic applications. Chem. Rev 2011, 111, 2981. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chemler SR; Bovino MT V catalytic aminohalogenation of alkenes and alkynes. ACS Catal 2013, 3, 1076. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Romero RM; Woeste TH; Muniz K Vicinal difunctionalization of alkenes with iodine(iii) reagents and catalysts. Chem. - Asian J 2014, 9, 972. [DOI] [PubMed] [Google Scholar]; (f) Wu K; Liang YJ; Jiao N Azidation in the difunctionalization of olefins. Molecules 2016, 21. [DOI] [PMC free article] [PubMed]; (g) Yin G; Mu X; Liu G Palladium(ii)-catalyzed oxidative difunctionalization of alkenes: Bond forming at a high-valent palladium center. Acc. Chem. Res 2016, 49, 2413. [DOI] [PubMed] [Google Scholar]; (h) Lin J; Song RJ; Hu M; Li JH Recent advances in the intermolecular oxidative difunctionalization of alkenes. Chem. Rec 2018, doi: 10.1002/tcr.201800053. [DOI] [PubMed] [Google Scholar]; (i) Sauer GS; Lin S An electrocatalytic approach to the radical difunctionalization of alkenes. ACS Catal 2018, 8, 5175. [Google Scholar]; (j) Wang F; Chen P; Liu G Copper-Catalyzed Radical Relay for Asymmetric Radical Transformations. Acc. Chem. Res 2018, 51, 2036. [DOI] [PubMed] [Google Scholar]
- (2) (a).Sakakura A; Ukai A; Ishihara K Enantioselective halocyclization of polyprenoids induced by nucleophilic phosphoramidites. Nature 2007, 445, 900. [DOI] [PubMed] [Google Scholar]; (b) Cai Y; Liu X; Hui Y; Jiang J; Wang W; Chen W; Lin L; Feng X Catalytic asymmetric bromoamination of chalcones: Highly efficient synthesis of chiral α-bromo-β-amino ketone derivatives. Angew. Chem. Int. Ed 2010, 49, 6160. [DOI] [PubMed] [Google Scholar]; (c) Murai K; Matsushita T; Nakamura A; Fukushima S; Shimura M; Fujioka H Asymmetric bromolactonization catalyzed by a c3-symmetric chiral trisimidazoline. Angew. Chem. Int. Ed 2010, 49, 9174. [DOI] [PubMed] [Google Scholar]; (d) Veitch GE; Jacobsen EN Tertiary aminourea-catalyzed enantioselective iodolactonization. Angew. Chem. Int. Ed 2010, 49, 7332. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Whitehead DC; Yousefi R; Jaganathan A; Borhan B An organocatalytic asymmetric chlorolactonization. J. Am. Chem. Soc 2010, 132, 3298. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhou L; Tan CK; Jiang X; Chen F; Yeung Y-Y Asymmetric bromolactonization using amino-thiocarbamate catalyst. J. Am. Chem. Soc 2010, 132, 15474. [DOI] [PubMed] [Google Scholar]
- (3) (a).Denmark SE; Kuester WE; Burk MT Catalytic, asymmetric halofunctionalization of alkenes--a critical perspective. Angew. Chem. Int. Ed 2012, 51, 10938. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mendoza A; Fananas FJ; Rodriguez F Asymmetric halocyclizations of unsaturated compounds: An overview and recent developments. Curr. Org. Synth 2013, 10, 384. [Google Scholar]; (c) Murai K; Fujioka H Recent progress in organocatalytic asymmetric halocyclization. Heterocycles 2013, 87, 763. [Google Scholar]; (d) Tan CK; Yeung YY Recent advances in stereoselective bromofunctionalization of alkenes using n-bromoamide reagents. Chem. Commun 2013, 49, 7985. [DOI] [PubMed] [Google Scholar]; (e) Cheng YA; Yu WZ; Yeung YY Recent advances in asymmetric intra- and intermolecular halofunctionalizations of alkenes. Org. Biomol. Chem 2014, 12, 2333. [DOI] [PubMed] [Google Scholar]; (f) Tan CK; Yu WZ; Yeung YY Stereoselective bromofunctionalization of alkenes. Chirality 2014, 26, 328. [DOI] [PubMed] [Google Scholar]; (g) Zheng SQ; Schienebeck CM; Zhang W; Wang HY; Tang WP Cinchona alkaloids as organocatalysts in enantioselective halofunctionalization of alkenes and alkynes. Asian J. Org. Chem 2014, 3, 366. [Google Scholar]; (h) Cresswell AJ; Eey STC; Denmark SE Catalytic, stereoselective dihalogenation of alkenes: Challenges and opportunities. Angew. Chem. Int. Ed 2015, 54, 15642. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Chung WJ; Vanderwal CD Stereoselective halogenation in natural product synthesis. Angew. Chem. Int. Ed 2016, 55, 4396. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Liang XW; Zheng C; You SL Dearomatization through halofunctionalization reactions. Chem. - Eur. J 2016, 22, 11918. [DOI] [PubMed] [Google Scholar]; (k) Gieuw MH; Ke ZH; Yeung YY Lewis base catalyzed stereo- and regioselective bromocyclization. Chem. Rec 2017, 17, 287. [DOI] [PubMed] [Google Scholar]
- (4).Denmark SE; Burk MT; Hoover AJ On the absolute configurational stability of bromonium and chloronium ions. J. Am. Chem. Soc 2010, 132, 1232. [DOI] [PubMed] [Google Scholar]
- (5) (a).Hu DX; Shibuya GM; Burns NZ Catalytic enantioselective dibromination of allylic alcohols. J. Am. Chem. Soc 2013, 135, 12960. [DOI] [PubMed] [Google Scholar]; (b) Bucher C; Deans RM; Burns NZ Highly selective synthesis of halomon, plocamenone, and isoplocamenone. J. Am. Chem. Soc 2015, 137, 12784. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hu DX; Seidl FJ; Bucher C; Burns NZ Catalytic chemo-, regio-, and enantioselective bromochlorination of allylic alcohols. J. Am. Chem. Soc 2015, 137, 3795. [DOI] [PubMed] [Google Scholar]; (d) Burckle AJ; Vasilev VH; Burns NZ A unified approach for the enantioselective synthesis of the brominated chamigrene sesquiterpenes. Angew. Chem. Int. Ed 2016, 55, 11476. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Landry ML; Hu DX; McKenna GM; Burns NZ Catalytic enantioselective dihalogenation and the selective synthesis of (−)-deschloromytilipin A and (−)-danicalipin A. J. Am. Chem. Soc 2016, 138, 5150. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Seidl FJ; Burns NZ Selective bromochlorination of a homoallylic alcohol for the total synthesis of (−)-anverene. Beilstein J. Org. Chem 2016, 12, 1361. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Burckle AJ; Gal B; Seidl FJ; Vasilev VH; Burns NZ Enantiospecific solvolytic functionalization of bromochlorides. J. Am. Chem. Soc 2017, 139, 13562. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Landry ML; Burns NZ Catalytic enantioselective dihalogenation in total synthesis. Acc. Chem. Res 2018, 51, 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6) (a).Nugent TC, Ed.; Chiral Amine Synthesis, Wiley: Weinheim, 2010. [Google Scholar]; (b) Hantzsch A Ueber den Jodstickstoff N3J. Ber. Dtsch. Chem. Ges 1900, 33, 522. [Google Scholar]
- (7) (a).Hassner A; Levy LA Additions of iodine azide to olefins . Stereospecific introduction of azide functions. J. Am. Chem. Soc 1965, 87, 4203. [Google Scholar]; (b) Fowler FW; Hassner A; Levy LA Stereospecific introduction of azide functions into organic molecules. J. Am. Chem. Soc 1967, 89, 2077. [Google Scholar]; (c) Boerwinkle F; Hassner A Solvent participation in additions to olefins. Tetrahedron Lett 1968, 3921.; (d) Hassner A; Boerwinkle F Stereochemistry. Xxxix. Ionic and free-radical addition of bromine azide to olefins. J. Am. Chem. Soc 1968, 90, 216. [Google Scholar]; (e) Hassner A; Fowler FW A general synthesis of vinyl azides from olefins . Stereochemistry of elimination from beta-iodo azides. J. Org. Chem 1968, 33, 2686. [Google Scholar]; (f) Hassner A Regiospecific and stereospecific introduction of azide functions into organic molecules. Acc. Chem. Res 1971, 4, 9. [Google Scholar]; (g) Vanende D; Krief A New reagent for stereospecific synthesis of aziridines from olefins. Angew. Chem. Int. Ed 1974, 13, 279. [Google Scholar]; (h) Denis JN; Krief A New synthetic route to 9,10-imino-phenanthrene. Tetrahedron 1979, 35, 2901. [Google Scholar]; (i) Wasserman HH; Brunner RK; Buynak JD; Carter CG; Oku T; Robinson RP Total synthesis of (+/−)-ortho-methylorantine. J. Am. Chem. Soc 1985, 107, 519. [Google Scholar]; (j) Olah GA; Wang Q; Li XY; Prakash GKS Azidobromination of alkenes with azidotrimethylsilane n-bromosuccinimide. Synlett 1990, 487. [Google Scholar]; (k) Kirschning A; Hashem MA; Monenschein H; Rose L; Schoning KU Preparation of novel haloazide equivalents by iodine(iii)-promoted oxidation of halide anions. J. Org. Chem 1999, 64, 6522. [Google Scholar]; (l) Nair V; George TG; Sheeba V; Augustine A; Balagopal L; Nair LG A novel regioselective synthesis of azidoiodides from alkenes using cerium(iv) ammonium nitrate. Synlett 2000, 1597.; (m) Barluenga J; Alvarez-Perez M; Fananas FJ; Gonzalez JM A smooth and practicable azido-iodination reaction of alkenes based on ipy2bf4 and me3sin3. Adv. Synth. Catal 2001, 343, 335. [Google Scholar]; (n) Curini M; Epifano F; Marcotullio MC; Rosati O Simple and regioselective azidoiodination of alkenes using oxone (r). Tetrahedron Lett 2002, 43, 1201. [Google Scholar]; (o) Hajra S; Bhowmick M; Sinha D Highly regio- and stereoselective asymmetric bromoazidation of chiral α,β-unsaturated carboxylic acid derivatives: Scope and limitations. J. Org. Chem 2006, 71, 9237. [DOI] [PubMed] [Google Scholar]; (p) Hajra S; Sinha D; Bhowmick M Metal triflate catalyzed highly regio- and stereoselective 1,2-bromoazidation of alkenes using nbs and tmsn3 as the bromine and azide sources. Tetrahedron Lett 2006, 47, 7017. [Google Scholar]; (q) Saikia I; Phukan P Facile generation of vicinal bromoazides from olefins using tmsn3 and tsnbr2 without any catalyst. Tetrahedron Lett 2009, 50, 5083. [Google Scholar]; (r) Valiulin RA; Mamidyala S; Finn MG Taming chlorine azide: Access to 1,2-azidochlorides from alkenes. J. Org. Chem 2015, 80, 2740. [DOI] [PubMed] [Google Scholar]; (s) Chen L; Xing H; Zhang H; Jiang Z-X; Yang Z Copper-catalyzed intermolecular chloroazidation of α,β-unsaturated amides. Org. Biomol. Chem 2016, 14, 7463. [DOI] [PubMed] [Google Scholar]
- (8) (a).Kolb HC; Sharpless KB The growing impact of click chemistry on drug discovery. Drug Discovery Today 2003, 8, 1128. [DOI] [PubMed] [Google Scholar]; (b) Best MD Click chemistry and bioorthogonal reactions: Unprecedented selectivity in the labeling of biological molecules. Biochemistry 2009, 48, 6571. [DOI] [PubMed] [Google Scholar]; (c) Sletten EM; Bertozzi CR From mechanism to mouse: A tale of two bioorthogonal reactions. Acc. Chem. Res 2011, 44, 666. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Thirumurugan P; Matosiuk D; Jozwiak K Click chemistry for drug development and diverse chemical–biology applications. Chem. Rev 2013, 113, 4905. [DOI] [PubMed] [Google Scholar]
- (9).Gal B; Bucher C; Burns NZ Chiral alkyl halides: Underexplored motifs in medicine. Mar. Drugs 2016, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10) (a).Steinhagen H; Corey EJ A simple convergent approach to the synthesis of the antiviral agent virantmycin. Org. Lett 1999, 1, 823. [DOI] [PubMed] [Google Scholar]; (b) Hanessian S; Del Valle JR; Xue Y; Blomberg N Total synthesis and structural confirmation of chlorodysinosin a. J. Am. Chem. Soc 2006, 128, 10491. [DOI] [PubMed] [Google Scholar]; (c) Seiple IB; Su S; Young IS; Nakamura A; Yamaguchi J; Jørgensen L; Rodriguez RA; O’Malley DP; Gaich T; Köck M; Baran PS Enantioselective total syntheses of (−)-palau’amine, (−)-axinellamines, and (−)-massadines. J. Am. Chem. Soc 2011, 133, 14710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zhou PF; Lin LL; Chen L; Zhong X; Liu XH; Feng XM Iron-catalyzed asymmetric haloazidation of α,β-unsaturated ketones: Construction of organic azides with two vicinal stereocenters. J. Am. Chem. Soc 2017, 139, 13414. [DOI] [PubMed] [Google Scholar]
- (12) (a).Blandy C; Choukroun R; Gervais D Synthesis of o-protected azidohydrins catalyzed by titanium and vanadium complexes. Tetrahedron Lett 1983, 24, 4189. [Google Scholar]; (b) Caron M; Sharpless KB Titanium isopropoxide-mediated nucleophilic openings of 2,3-epoxy alcohols. A mild procedure for regioselective ring-opening. J. Org. Chem 1985, 50, 1557. [Google Scholar]; (c) Sinou D; Emziane M Ouverture régiosélective d’époxydes par Me3SiN3 catalysée par Ti(OiPr)4. Tetrahedron Lett 1986, 27, 4423. [Google Scholar]; (d) Caron M; Carlier PR; Sharpless KB Regioselective azide opening of 2,3-epoxy alcohols by [Ti(o-i-Pr)2(N3)2]: Synthesis of α-amino acids. J. Org. Chem 1988, 53, 5185. [Google Scholar]; (e) Raifeld YE; Nikitenko AA; Arshava BM Compounds of (i-PrO)3 TiX as novel reagents for regioselective oxirane ring opening. Tetrahedron: Asymmetry 1991, 2, 1083. [Google Scholar]; (f) Raifeld YE; Nikitenko AA; Arshava BM; Mikerin IE; Zilberg LL; Vid GY; Lang SA Jr.; Lee VJ Synthesis of 3-substituted (azido, acylthio, chloro or fluoro)-2,3-dideoxy-d-erythro-pentoses and 3-methyl-3-substituted-2,3-dideoxy-d-erythro-pentoses. Tetrahedron 1994, 50, 8603. [Google Scholar]
- (13).Jat JL; Paudyal MP; Gao H; Xu Q-L; Yousufuddin M; Devarajan D; Ess DH; Kürti L; Falck JR Direct stereospecific synthesis of unprotected N-H and N-Me aziridines from olefins. Science 2014, 343, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Netherton MR; Fu GC Air-stable trialkylphosphonium salts: Simple, practical, and versatile replacements for air-sensitive trialkylphosphines. Applications in stoichiometric and catalytic processes. Org Lett 2001, 3, 4295. [DOI] [PubMed] [Google Scholar]
- (15).Vitaku E; Smith DT; Njardarson JT Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem 2014, 57, 10257. [DOI] [PubMed] [Google Scholar]
- (16).Kolb HC; Finn MG; Sharpless KB Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed 2001, 40, 2004. [DOI] [PubMed] [Google Scholar]
- (17) (a).Zhang W; Zheng SQ; Liu N; Werness JB; Guzei IA; Tang WP Enantioselective bromolactonization of conjugated (z)-enynes. J. Am. Chem. Soc 2010, 132, 3664. [DOI] [PubMed] [Google Scholar]; (b) Zhang W; Liu N; Schienebeck CM; Decloux K; Zheng SQ; Werness JB; Tang WP Catalytic enantioselective halolactonization of enynes and alkenes. Chem. - Eur. J 2012, 18, 7296. [DOI] [PubMed] [Google Scholar]; (c) Shunatona HP; Fruh N; Wang YM; Rauniyar V; Toste FD Enantioselective fluoroamination: 1,4-addition to conjugated dienes using anionic phase-transfer catalysis. Angew. Chem. Int. Ed 2013, 52, 7724. [DOI] [PubMed] [Google Scholar]; (d) Tripathi CB; Mukherjee S Catalytic enantioselective 1,4-lodofunctionalizations of conjugated dienes. Org. Lett 2015, 17, 4424. [DOI] [PubMed] [Google Scholar]
- (18) (a).Gagneux A; Winstein S; Young WG Rearrangement of allylic azides. J. Am. Chem. Soc 1960, 82, 5956. [Google Scholar]; (b) Feldman AK; Colasson B; Sharpless KB; Fokin VV The allylic azide rearrangement: Achieving selectivity. J. Am. Chem. Soc 2005, 127, 13444. [DOI] [PubMed] [Google Scholar]
- (19) (a).Gijsen HJM; Qiao L; Fitz W; Wong C-H Recent advances in the chemoenzymatic synthesis of carbohydrates and carbohydrate mimetics. Chem. Rev 1996, 96, 443. [DOI] [PubMed] [Google Scholar]; (b) Afarinkia K; Bahar A Recent advances in the chemistry of azapyranose sugars. Tetrahedron: Asymmetry 2005, 16, 1239. [Google Scholar]; (c) Horne G; Wilson FX; Tinsley J; Williams DH; Storer R Iminosugars past, present and future: Medicines for tomorrow. Drug Discovery Today 2011, 16, 107. [DOI] [PubMed] [Google Scholar]; (d) Gloster TM; Vocadlo DJ Developing inhibitors of glycan processing enzymes as tools for enabling glycobiology. Nat. Chem. Biol 2012, 8, 683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.