Abstract
In plants, small RNA (sRNA)-mediated RNA interference (RNAi) is critical for regulating host immunity against bacteria, fungi, oomycetes, viruses, and pests. Similarly, sRNAs from pathogens and pests also play an important role in modulating their virulence. Strikingly, recent evidence supports that some sRNAs can travel between interacting organisms and induce gene silencing in the counter party, a mechanism termed cross-kingdom RNAi. Exploiting this new knowledge, host-induced gene silencing (HIGS) by transgenic expression of pathogen gene-targeting double-stranded (ds)RNA has the potential to become an important disease-control method. To circumvent transgenic approaches, direct application of dsRNAs or sRNAs (environmental RNAi) onto host plants or post-harvest products leads to silencing of the target microbe/pest gene (referred to spray-induced gene silencing, SIGS) and confers efficient disease control. This review summarizes the current understanding of cross-kingdom RNA trafficking and environmental RNAi and how these findings can be developed into novel effective strategies to fight diseases caused by microbial pathogens and pests.
Introduction
To meet the increasing food and energy demands of a fast-growing population, it will be necessary to roughly double crop yields worldwide over the next 40–50 years. Each year, pathogens and pests destroy 20–40% of attainable crop production globally. The demonstration that eukaryotic pathogens and pests are inhibited by small RNAs (sRNAs) targeting their essential and/or pathogenicity genes has raised the possibility that plants can be protected by a new generation of eco-friendly RNA-based fungicides or insecticides, which are highly specific and can be easily adapted to control multiple diseases simultaneously. The novel strategy employs the recent discoveries that sRNAs can move across the cellular boundaries between hosts and interacting pathogens and pests and induce gene silencing in trans, designated ‘cross-kingdom RNA interference (RNAi)’ [1●●,2,3,4●●] and that some pathogens and pests are capable of taking up RNAs from the environment, termed ‘environmental RNAi’ [5●●,6]. These mechanisms enable us to successfully control crop diseases by transgene-mediated cross-kingdom RNAi or spray-induced gene silencing (SIGS) that spraying pathogen gene-targeting dsRNAs and sRNAs on plant surfaces to suppress pathogen virulence [6]. We review here the current understanding and application of cross-kingdom RNA trafficking and environmental RNAi.
Pathogen-derived cross-kingdom sRNAs suppressing host immunity
Pathogen-derived sRNAs can move into host cells to suppress host immunity (Figure 1a). The grey mold fungal pathogen Botrytis cinerea (Bc) produces sRNA effectors, the majority of which derived from clusters within long-terminal repeat (LTR) retrotransposons in the fungal genome, which can migrate into and down-regulate Arabidopsis and tomato genes involved in immunity [1●●]. Some sRNA effectors can target multiple host immunity genes to enhance Bc pathogenicity. For example, Bc-siR37 suppresses host immunity by targeting at least 15 Arabidopsis genes, including WRKY transcription factors, receptor-like kinases, and cell wall-modifying enzymes [7●]. The Bc sRNAs utilize the host RNAi machinery by binding to Arabidopsis ARGONAUTE1 (AGO1) to silence host immunity genes [1●●,7●]. Consistent with this finding, Bc causes less disease symptoms on the Arabidopsis ago1–27 mutant compared to wild type plants. In addition, the Dicer (DCL) double mutant strain Bc-dcl1dcl2 can no longer produce these Bc-sRNAs also displays much reduced pathogenicity on various plant species [1●●,5●●], indicating that sRNA effectors are essential for Bcs pathogenicity. Similarly, the ago1–27 mutant is more resistant to the pathogenic ascomycete Verticillium dahlia (Vd), which causes Verticillium wilt disease on many plants [5●●]. An RNA immunoprecipitation (RIP) assay showed that Vd-sRNAs that have potential host targets are predominantly associated with Arabidopsis AGO1 during infection [5●●], suggesting that Vd also uses sRNAs to silence host target genes. One of the most destructive pathogens of wheat Puccinia striiformis (Ps) also delivers sRNAs, such as a novel microRNA-like RNA1 (milR1), into host cells and suppresses wheat Pathogenesis-related 2 gene in the defense pathway. Silencing of the Ps milR1 precursor led to enhanced wheat resistance to the virulent Ps isolate [8●●]. Cross-kingdom RNA silencing does not necessarily require canonical RNAi machinery in the pathogens or pests. For example, two non-coding RNAs, OxyS and DsrA, of Escherichia coli could enter and affect gene expression and physiology of its host Caenorhabditis elegans [9]. Moreover, the protozoan parasite Trypanosoma cruzi produces tRNA-derived sRNAs, which contribute to the ability to infect mammalian cells, though Trypanosoma cruzi lacks canonical sRNA pathways [10].
Figure 1.
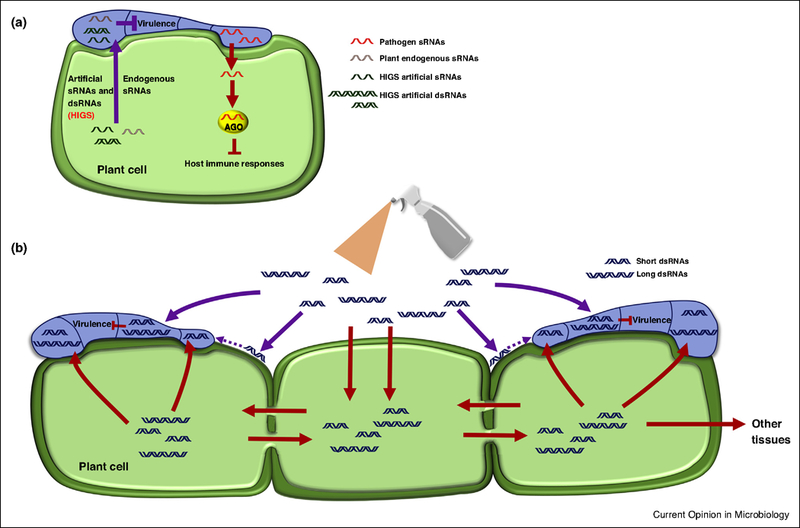
Cross-kingdom RNA trafficking and spray-induced gene silencing for plant protection against eukaryotic pathogens. (a) Cross-kingdom RNA transfer and gene silencing in a plant and an interacting pathogen. Plant pathogens deliver sRNAs into host plant cells, where they suppress host immune responses by hijacking host cell RNAi machinery (red block arrow). Host cells also deliver sRNAs into pathogen cells, either artificial HIGS sRNAs or endogenous sRNAs, to target virulence genes and other essential genes of pathogens (purple block arrow). (b) Mechanism of SIGS to counteract pathogen virulence. The sprayed short or long dsRNAs, which target pathogen virulence-related genes, can either translocate directly to the eukaryotic pathogen (purple arrows), via uptake from the plant surface (purple dotted arrows), or indirectly through the host cells (red arrows). These RNAs can also move systemically between cells or to other tissues in the plant, most likely through plasmodesmata and vascular bundles.
Host plant-derived cross-kingdom sRNAs regulate the outcome of microbial attacks
Recent discoveries that animals and plants deliver host sRNAs into interacting microbes to suppress their virulence has created new ideas for practical disease control [5●●,6,11,12●,13,14●●] (Figure 1a). Verticillium dahlia (Vd), recovered from infected cotton plants contained 28 miRNAs from cotton, implying that host-derived sRNAs were transmitted into the pathogen during infection [14●●]. Two of those cotton miRNAs, miR166 and miR159, target the fungal genes Ca2+-dependent cysteine protease calpain (VdClp-1) and Isotrichodermin C-15 hydroxylase (VdHiC-15), respectively. Consistent with host-mediated silencing, VdClp-1 and VdHiC-15 transcripts were reduced in the Vd hyphae recovered from V. dahliae-infected cotton. Moreover, fungal mutants vdclp-1 and vdhic-15 were reduced in virulence, confirming that these genes contribute to fungal pathogenicity [14●●]. Animal hosts also export sRNAs into interacting parasite cells to suppress their virulence [12●]. Sickle cell erythrocytes of anemia patients accumulate higher levels of miR-451 and lethal-7i (let-7i), which are transferred into the parasite Plasmodium falciparum. Although P. falciparum lacks RNAi machinery, the miRNAs fuse with targeted parasite messenger RNAs (mRNAs), cAMP-dependent protein kinase subunit (PKA-R) and Reduced expression 1 (REX1) at 50 UTR region and form chimeric structures that suppress mRNA translation [12●]. Furthermore, hosts can also deliver sRNAs into prokaryotic pathogens in the guts. Mouse and human miRNAs were found in gut bacteria E. coli and Fusobacterium nucleatum (Fn) and regulate transcript levels of bacterial target genes, thereby affecting bacteria growth [13]. In E. coli, host miRNAs hsa-miR-1224–5p and miR-623 downregulate the transcript levels of rutA and fucO respectively. In contrast to the negative regulation mediated by hsa-miR-1224–5p and miR-623, other transferred host miRNAs hsa-miR-1226–5p and hsa-miR-515–5p can elevate the transcript levels of other targets, yegH and Fn 16s rRNA, in E. coli and Fn respectively [13]. This regulation is miRNA-dependent because intestinal epithelial cell-miRNA deficient mice impaired regulation of bacterial mRNAs, and exhibited uncontrolled gut microbiota and exacerbated colitis. However, the underlying mechanism on how positive or negative regulation is determined and achieved is still unclear.
Host-derived sRNAs for plant protection against pathogens and pests
Evidence for cross kingdom RNAi has been supported by studies demonstrating delivery of artificially designed sRNA from plants into an interacting microbe/pest (Figure 1a). Such engineered RNA-based communication termed host-induced gene silencing (HIGS) [11,15●] has emerged as a promising strategy for crop protection. A wide range of transgenic crops expressing dsRNAs that are subsequently processed into sRNAs targeting essential and/or pathogenicity genes are more resistant to viruses [16], viroids [17], bacteria [18], fungi [5●●,15●,19,20●], oomycetes [21,22], nematodes [23–25], and insects [26–28,29●]. The broad applicability of the technique supports a basic evolutionary-conserved mechanism of sRNA trafficking.
The first successful report of HIGS on exploiting cross-kingdom RNAi using transgene-derived sRNAs against fungal microbes involved the biotrophic powdery mildew fungus [15●]. HIGS was shown to be also effective in controlling necrotrophic fungal pathogens [5●●], such as Botrytis and Verticillium. Thus, HIGS and RNA trafficking were observed in both biotrophic fungi that form effective interfaces for exchanging nutrients between the interacting partners, as well as necrotrophic fungi that induce host cell death and form lesions within dead tissue, suggesting that exchange of sRNA with the hosts happens in many fungal species regardless of their lifestyle.
HIGS has the potential to be extremely versatile for disease control because it can be easily designed to target multiple pathogens simultaneously. As a proof of concept, Wang et al. generated transgenic plants that expressed hairpin RNAs targeting DCL genes of both Bc and Vd. These transgenic plants displayed enhanced resistance to both Bc and Vd, which provided a successful example of controlling two fungal diseases using HIGS [5●●]. Furthermore, this study was the first to provide direct evidence that sRNAs are generated in planta and are at least one of the major mobile signals transported from the plant into Bc, because plant derived sRNAs were easily detected in the Bc-dcl1 dcl2 mutant where dsRNAs can no longer be processed inside Bc cells.
Host-derived sRNA for the control of cereal pathogens
Major diseases of cereal crops include powdery mildew, rusts, Fusarium head blight (FHB) and Fusarium seedling blight (FSB) [30]. DMI (demethylation inhibitors) fungicides, such as azoles, have dominated the agrochemical business in cereals, with 27% market share. DMI fungicides inhibit ergosterol biosynthesis of fungal membranes by binding to Cytochrome P450 lanosterol C-14 a-demethylase (CYP51) [31]. However, increasing insensitivity to fungicides is now widespread [32]. Exploitation of the new knowledge on cross-kingdom RNAi may provide a solution to fungicide resistance [33]. The use of dsRNA is expected to abrogate azole insensitivity of field strains as RNAi and CYP51 gene silencing would not be affected by known mechanisms for resistance development, including point mutations in the CYP51 gene, CYP51 overexpression, CYP51 gene duplication, or the overexpression of efflux transporters as explained in the next section.
Expression of a 791 nt long noncoding dsRNA (CYP3RNA), which targets the three Cytochrome P450 lanosterol C-14a-demethylase (CYP51) genes FgCYP51A, FgCYP51B and FgCYP51C of Fusarium graminearum (Fg) strongly inhibited fungal growth in barley and Arabidopsis [20●]. In wheat, expression of dsRNAs with sequence homology to Fg Chitin synthase Ch3b reduced fungal infection both on ears and seedlings [34●●]. Moreover, transgenic wheat carrying an RNAi hairpin construct against b-1, 3-Glucan synthase (FcGls1) of Fusarium culmorum or a triple combination against FcGls1, Mitogen-activated protein kinase1 (FcFmk1), and Myosin motor domain-containing chitin synthase V (FcChsV) showed enhanced FHB resistance under greenhouse and nearfield conditions [35]. Cross-kingdom RNAi strategies have also been successfully employed to control wheat rust diseases [19,36,37]. Expression of hairpin RNAi constructs with sequence homology to MAP kinase (PtMAPK1) or Cyclophilin1 (PtCYC1) resulted in silencing of the corresponding fungal genes and provided resistance to leaf rust Puccinia triticina [19]. Similarly, wheat-mediated silencing the pathogenicity factor PsCPK1 in Puccinia striiformis enhanced resistance to stripe rust [37].
Environmental RNAi for the control of pathogens and pests
The delivery of inhibitory cross-kingdom RNAs by transgenic expression requires generation of transgenic plants, which has some limitations in agronomic application depending on the transformability and genetic stability of a crop species. Expression of transgenes is not always stable, they are often suppressed/silenced at the transcriptional level after a few generations. Even when transformation is applicable and genome integration is stable, considerable time is needed to develop and release an economically valuable cultivar, including a long regulatory process to obtain governmental approval, plus public concerns about genetically modified organisms (GMOs). Hence, environmental RNAi is an appealing alternative for disease control as it avoids any modification of crop genomes, bears a high flexibility for multi-target strategies and can be exploited with short time delays to virtually any microbial pathogen/pest that is sensitive to RNAi approaches. Most importantly, pathogens cannot easily generate sufficient target-resistance mutations to escape RNAi. RNAi would be still effective even with multiple mutations along the dsRNA because effective RNAi does not require 100% base pairing [38]. Although a critical agronomic evaluation, including activity in curative versus protective treatments, seed treatments versus whole plant spray, and cost efficiency of using environmental RNAi remains to be investigated, present knowledge already suggests that environmental RNAs have tremendous impact as a new generation of fungicides that are more sustainable than current chemical-based fungicides. RNAs are biomolecules that are found in almost all the food we consume. Topical application of RNAs does not leave toxic residues in the field or the environment, and does not cause any potential modifications of the crop genes. For these reasons, we would expect a much faster regulatory process of approval of RNA-based ‘fungicides’ than new chemical fungicides, and genetically modified organisms.
Uptake of environmental RNA has long been known for nematodes [39,40] and insects [27,41], while it was only recently discovered in fungi [5●●,42●●]. Some insects such as the Western Corn Rootworm (Diabrotica virgifera) seem to have a preference for >60 bp dsRNAs [43,44]. SID-2 (Systemic RNAi defective 2)-dependent dsRNA transport in nematodes requires an acidic extracellular environment and is selective for dsRNAs with at least 50 base pairs [45], although nematodes also can ingest small interfering (si)RNAs [46]. Similar preferences have not been seen for fungi as they can uptake 21 nt sRNA duplexes as well as long dsRNAs of at least up to 800 nt [5●●,42●●]. Fluorescein-labelled sRNAs or long dsRNAs can be easily observed inside Bc and Fg cells shortly after culturing on agar medium sprayed with Fluorescein-labelled RNAs, demonstrating that fungal cells are capable of taking up RNAs directly from the environment [5●●,34●●,42●●]. Possible application of this novel strategy to control fungal diseases is illustrated by inhibition of grey mold development upon spraying Bc Dcl-½-targeting long dsRNAs or sRNAs onto the surface of fruits, vegetables, and flowers [5●●]. Cumulative evidence suggest that these RNAs can either be taken up by fungal cells directly, or accumulate in plant cells from where they are transferred into fungal cells [47] (Figure 1b). The growth of Fusarium species was efficiently inhibited by treatment of dsRNAs and siRNAs [42●●]. CYP3RNA, the CYP51-targeting dsRNA, inhibited growth of Fg in vitro and in planta upon spraying on barley leaves at a concentration range of 1–20 ng/ml. Consistent with the knowledge that sRNA is mobile [48–50], compromised fungal growth was observed in directly sprayed (local) as well as non-sprayed (distal) parts of detached leaves. Efficient spray-induced gene silencing (SIGS) in the distal tissue required i. CYP3RNA passage via the vascular system, ii. CYP3RNA uptake into the interacting fungus, and iii. CYP3RNA processing into siRNAs by fungal FgDcl-1 [42●●]. The Fusarium Fgdcl-1 mutant are partially compromised in CYP3RNA-mediated SIGS, suggesting that the fungal RNAi machinery is required to generate inhibitory siRNAs. This finding raised the possibility that environmental dsRNAs and sRNAs can be taken up by fungal cells directly or indirectly via the plant cells, while transgene-derived dsRNAs are cleaved mostly by the plant silencing machinery and mainly the sRNAs are transferred to attack pathogens.
It has been shown that naked dsRNA and sRNAs sprayed on plants could protect vegetables and fruits against grey mold disease for 5–8 days. To further increase the efficacy and duration of plant protection by environmental RNAi, Mitter et al. developed non-toxic, degradable, layered double hydroxide (LDH) clay nanosheets that can carry dsRNA for virus protection for at least 20 days after a single spray [51●●]. Nanosheets and RNAs are more environmentally friendly than current chemical fungicides, and they are not toxic to humans and animals.
Conclusions
Pathogens and pests can be controlled by sRNAs targeting their essential genes or pathogenicity genes, which has raised the possibility that plants can be protected from diseases by novel eco-friendly, durable and highly specific RNA fungicides or pesticides. This new strategy employs the recent discovery that interaction of hosts with eukaryotic pathogens and pests rely on bidirectional trafficking of sRNAs and cross-kingdom RNAi. In agronomic practice, the inhibitory RNAs could be delivered either by transgenic expression or direct spray application, whereby both strategies lead to target gene knockdown by RNAi. Delivery of sRNA by transgenic expression of dsRNA (HIGS) requires generation of transgenic plants, which may cause delay in agronomic application depending on the transformability and genetic stability of a crop species as well as the complicated regulatory processes. Therefore, using environmental RNAi (SIGS) is an effective alternative for disease control as it bears a high flexibility for multi-target strategies and can be exploited with short time delays to virtually any microbial pathogen/pest that is sensitive to sRNA approaches. Overall, environmental RNAs have the capacity to change the current practice in crop protection with the potential of reducing pesticide usage and overcoming the obstacles faced by genetically modified crops.
Our knowledge on how pathogens and their plant hosts execute the sRNA transfer still is in its infancy. However, there is already enough evidence arguing for different mechanisms in different classes of organisms, for exam-ple, suggested by the fact that homologs for nematode SID genes have not been found in other groups of organisms, including insects, fungi and plants. Further studies will help elucidate the mechanisms of sRNA transfer between plant hosts and interacting pathogens and pests.
Acknowledgements
We apologize that we would not be able to include and cite many related interesting studies due to the limited space. Work in Jins laboratory was supported by grants from National Institute of Health (R01 GM093008), National Science Foundation (IOS-1257576, IOS-1557812). Kogels lab has received funding from the European Unions Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 674964.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
●of special interest
●●of outstanding interest
- 1. ●●.Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, Huang HD, Jin H: Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342:118–123.This study reveals for the first time that naturally occurring cross-kingdom RNAi that employed as an aggressive virulence mechanism by an aggressive fungal pathogen B. cinerea. B. cinerea small RNAs (Bc-sRNAs) can silence Arabidopsis and tomato genes involved in immunity by hijacking the host Arabidopsis Argonaute 1 (AGO1) and selectively silencing host immunity genes. These mobile fungal sRNAs serve as a novel class of effectors to suppress host immunity.
- 2.Knip M, Constantin ME, Thordal-Christensen H: Trans-kingdom cross-talk: small RNAs on the move. PLoS Genet 2014, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiberg A, Bellinger M, Jin H: Conversations between kingdoms: small RNAs. Curr Opin Biotechnol 2015, 32:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ●●.Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Le Bihan T, Kumar S, Abreu-Goodger C, Lear M, Harcus Y et al. : Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun 2014, 5:5488.This study shows that mice-infecting parasitic nematodes, secrete vesi-cles containing miRNAs. These miRNAs suppressed mice Type 2 innate immunity responses via cross-kingdom RNAi. The work revealed exo-somes as a weapon by which parasites manipulate their hosts and provides the first steps towards a mechanistic framework for RNA-mediated communication between animal species.
- 5. ●●.Wang M, Weiberg A, Lin FM, Thomma BP, Huang HD, Jin H: Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat Plants 2016, 2:16151.This study showed that bidirectional cross-kingdom RNAi and sRNA trafficking between plants and fungi. It also firstly showed that Botrytis can take up external sRNAs and double-stranded RNAs (dsRNAs) from the environment. Spraying sRNAs or dsRNAs that target Botrytis DCL1 and DCL2 genes on the surface of fruits, vegetables and flowers sig-nificantly inhibited grey mould disease. This RNAi-based new generation of pathogen gene-targeting RNAs could target multiple pathogens, which represent a new generation of environmentally friendly fungicides.
- 6.Wang M, Thomas N, Jin H: Cross-kingdom RNA trafficking and environmental RNAi for powerful innovative pre-and post-harvest plant protection. Curr Opin Plant Biol 2017, 38:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ●.Wang M, Weiberg A, Dellota E Jr, Yamane D, Jin H: Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol 2017, 14:421–428.This study demonstrated that one Bc-sRNA effector Bc-siR37 that, is predicted to target at least 15 Arabidopsis genes. Furthermore, the knockout mutants of the Bc-siR37 targets, At-WRKY7, At-PMR6, and At-FEI2, also exhibited enhanced disease susceptibility to B. cinerea.
- 8.Wang B, Sun YF, Song N, Zhao MX, Liu R, Feng H, Wang XJ, Kang ZS: Puccinia striiformis f. sp tritici microRNA-like RNA 1 (Pst-milR1), an important pathogenicity factor of Pst, impairs wheat resistance to Pst by suppressing the wheat pathogenesis-related 2 gene. New Phytol 2017, 215:338–350.This study shows a novel microRNA-like RNA (milRNA) from Puccinia striiformis f. sp. tritici (Pst), Pst-milR1, takes part in cross-kingdom RNAi events by binding the wheat pathogenesis-related 2 (PR2) gene, which suppresses wheat defenses during wheat–Pst interactions.
- 9.Liu HJ, Wang XR, Wang HD, Wu JJ, Ren J, Meng LF, Wu QF, Dong HS, Wu J, Kao TY et al. : Escherichia coli noncoding RNAs can affect gene expression and physiology of Caenorhabditis elegans. Nat Commun 2012, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Silva MR, das Neves RFC, Cabrera-Cabrera F, Sanguinetti J, Medeiros LC, Robello C, Naya H, Fernandez-Calero T, Souto-Padron T, de Souza W et al. : Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol Res 2014, 113:285–304. [DOI] [PubMed] [Google Scholar]
- 11.Nunes CC, Dean RA: Host-induced gene silencing: a tool for understanding fungal host interaction and for developing novel disease control strategies. Mol Plant Pathol 2012, 13:519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ●.LaMonte G, Philip N, Reardon J, Lacsina JR, Majoros W, Chapman L, Thornburg CD, Telen MJ, Ohler U, Nicchitta CV et al. : Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe 2012, 12:187–199.Two miRNAs, miR-451 and let-7i, were highly enriched in sickle cell erythrocytes of anemia patients and, which are transferred into parasite P. falciparum cells. miR-451 and let-7i binding to with essential parasite mRNAs and, resulted in translational inhibition by impaired ribosomal loading. This report showed that animal hosts can also export sRNAs into interacting parasite cells and thereby resulting in reduced in parasitism anemia.
- 13.Liu SR, da Cunha AP, Rezende RM, Cialic R, Wei ZY, Bry L, Comstock LE, Gandhi R, Weiner HL: The host shapes the gut microbiota via fecal MicroRNA. Cell Host Microbe 2016, 19:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ●●.Zhang T, Zhao YL, Zhao JH, Wang S, Jin Y, Chen ZQ, Fang YY, Hua CL, Ding SW, Guo HS: Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat Plants 2016, 2:16153.This study firstly revealed a novel defence strategy of host plants by exporting specific miRNAs to induce cross-kingdom gene silencing in pathogenic fungi and confer disease resistance.
- 15. ●.Nowara D, Gay A, Lacomme C, Shaw J, Ridout C, Douchkov D, Hensel G, Kumlehn J, Schweizer P: HIGS: host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 2010, 22:3130–3141.In this study it was shown for the first time that transgenic expression of target-specific double-stranded or antisense RNA in barley (Hordeum vulgare) and wheat (Triticum aestivum) inhibits development of the pow-dery mildew fungus Blumeria graminis.
- 16.Waterhouse PM, Graham HW, Wang MB: Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc Natl Acad Sci U S A 1998, 95:13959–13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwind N, Zwiebel M, Itaya A, Ding BA, Wang MB, Krczal G, Wassenegger M: RNAi-mediated resistance to Potato spindle tuber viroid in transgenic tomato expressing a viroid hairpin RNA construct. Mol Plant Pathol 2009, 10:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escobar MA, Civerolo EL, Summerfelt KR, Dandekar AM: RNAi-mediated oncogene silencing confers resistance to crown gall tumorigenesis. Proc Natl Acad Sci U S A 2001, 98:13437–13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panwar V, Jordan M, McCallum B, Bakkeren G: Host-induced silencing of essential genes in Puccinia triticina through transgenic expression of RNAi sequences reduces severity of leaf rust infection in wheat. Plant Biotechnol J 2017. [DOI] [PMC free article] [PubMed]
- 20. ●.Koch A, Kumar N, Weber L, Keller H, Imani J, Kogel KH: Host-induced gene silencing of cytochrome P450 lanosterol C14 alpha-demethylase-encoding genes confers strong resistance to Fusarium species. Proc Natl Acad Sci U S A 2013, 110:19324–19329.This article describes that host-induced gene silencing targeting the three fungal cytochrome P450 lanosterol C-14a-demethylase (CYP51) genes by one dsRNA restricts fungal infection. The work hints to the possibility to circumvent chemical fungicide resistance by RNA strategies as the CYP51 proteins are targets for azole fungicides.
- 21.Eschen-Lippold L, Landgraf R, Smolka U, Schulze S, Heilmann M, Heilmann I, Hause G, Rosahl S: Activation of defense against Phytophthora infestans in potato by down-regulation of syntaxin gene expression. New Phytol 2012, 193:985–996. [DOI] [PubMed] [Google Scholar]
- 22.Govindarajulu M, Epstein L, Wroblewski T, Michelmore RW: Host-induced gene silencing inhibits the biotrophic pathogen causing downy mildew of lettuce. Plant Biotechnol J 2015, 13:875–883. [DOI] [PubMed] [Google Scholar]
- 23.Huang G, Allen R, Davis EL, Baum TJ, Hussey RS: Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc Natl Acad Sci U S A 2006, 103:14302–14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairbairn DJ, Cavallaro AS, Bernard M, Mahalinga-Iyer J, Graham MW, Botella JR: Host-delivered RNAi: an effective strategy to silence genes in plant parasitic nematodes. Planta 2007, 226:1525–1533. [DOI] [PubMed] [Google Scholar]
- 25.Shivakumara TN, Chaudhary S, Kamaraju D, Dutta TK, Papolu PK, Banakar P, Sreevathsa R, Singh B, Manjaiah KM, Rao U: Host-induced silencing of two pharyngeal gland genes conferred transcriptional alteration of cell wall-modifying enzymes of meloidogyne incognita vis-a-vis perturbed nematode infectivity in eggplant. Front Plant Sci 2017, 8:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY: Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 2007, 25:1307–1313. [DOI] [PubMed] [Google Scholar]
- 27.Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, Ilagan O, Johnson S, Plaetinck G, Munyikwa T, Pleau M et al. : Control of coleopteran insect pests through RNA interference. Nat Biotechnol 2007, 25:1322–1326. [DOI] [PubMed] [Google Scholar]
- 28.Coleman AD, Wouters RHM, Mugford ST, Hogenhout SA: Persistence and transgenerational effect of plant-mediated RNAi in aphids. J Exp Bot 2015, 66:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. ●.Zhang J, Khan SA, Hasse C, Ruf S, Heckel DG, Bock R: Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015, 347:991–994.The plant chloroplast is a cellular compartment that appears to lack an RNAi machinery. The study shows that long dsRNAs, which targets a b-actin gene of the Colorado potato beetle, can be stably produced in chloroplasts of potato plants. As a result, this dsRNA can trigger a lethal RNAi response in the insect.
- 30.Broekaert N, Devreese M, De Baere S, De Backer P, Croubels S: Modified Fusarium mycotoxins unmasked: from occurrence in cereals to animal and human excretion. Food Chem Toxicol 2015, 80:17–31. [DOI] [PubMed] [Google Scholar]
- 31.Kuck KH, Stenzel K, Vors JP: Sterol biosynthesis inhibitors. edn 2. Modern Crop Protection Compounds 2012:761–805.
- 32.Price CL, Parker JE, Warrilow AG, Kelly DE, Kelly SL: Azole fungicides – understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag Sci 2015, 71:1054–1058. [DOI] [PubMed] [Google Scholar]
- 33.Machado AK, Brown NA, Urban M, Kanyuka K, Hammond-Kosack KE: RNAi as an emerging approach to control Fusarium head blight disease and mycotoxin contamination in cereals. Pest Manag Sci 2017. [DOI] [PMC free article] [PubMed]
- 34. ●●.Cheng W, Song XS, Li HP, Cao LH, Sun K, Qiu XL, Xu YB, Yang P, Huang T, Zhang JB et al. : Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol J 2015, 13:1335–1345.This work demonstrates that the expression of dsRNA derived from an essential Fusarium graminearum virulence gene, chitin synthase (Chs) 3b is an effective strategy for enhancing resistance in crop plants under near field test conditions.
- 35.Chen WX, Kastner C, Nowara D, Oliveira-Garcia E, Rutten T, Zhao YS, Deising HB, Kumlehn J, Schweizer P: Host-induced silencing of Fusarium culmorum genes protects wheat from infection. J Exp Bot 2016, 67:4979–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Guo J, Voegele RT, Zhang JS, Duan YH, Luo HY, Kang ZS: Functional characterization of calcineurin homologs PsCNA1/PsCNB1 in Puccinia striiformis f. sp tritici using a host-induced RNAi system. PLoS ONE 2012, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi T, Zhu X, Tan C, Liu P, Guo J, Kang Z, Guo J: Host-induced gene silencing of an important pathogenicity factor PsCPK1 in Puccinia striiformis f. sp. tritici enhances resistance of wheat to stripe rust. Plant Biotechnol J 2017. [DOI] [PMC free article] [PubMed]
- 38.Waterhouse PM, Wang MB, Lough T: Gene silencing as an adaptive defence against viruses. Nature 2001, 411:834–842. [DOI] [PubMed] [Google Scholar]
- 39.Timmons L, Court DL, Fire A: Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001, 263:103–112. [DOI] [PubMed] [Google Scholar]
- 40.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC: Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391:806–811. [DOI] [PubMed] [Google Scholar]
- 41.Mulot M, Boissinot S, Monsion B, Rastegar M, Clavijo G, Halter D, Bochet N, Erdinger M, Brault V: Comparative analysis of RNAi-based methods to down-regulate expression of two genes expressed at different levels in Myzus persicae. Viruses 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. ●●.Koch A, Biedenkopf D, Furch A, Weber L, Rossbach O, Abdellatef E, Linicus L, Johannsmeier J, Jelonek L, Goesmann A et al. : An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLOS Pathogens 2016, 12.This study showed for the first time the control of a fungal disease by spray-induced gene silencing in a cereal host. It was shown that envir-onmental RNAi in pathogens can by induced by direct fungal uptake of dsRNA or sRNA duplexes from the plant surface; or upon accumulation of sprayed dsRNA and sRNAs into the plant by fungal uptake from the plant tissue. Moreover, the study shows that sprayed dsRNAs can be translo-cated in the plant tissue and taken up by the fungus from distal, non-sprayed tissue.
- 43.Bolognesi R, Ramaseshadri P, Anderson J, Bachman P, Clinton W, Flannagan R, Ilagan O, Lawrence C, Levine S, Moar W et al. : Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 2012, 7:e47534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winston WM, Sutherlin M, Wright AJ, Feinberg EH, Hunter CP: Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc Natl Acad Sci U S A 2007, 104:10565–10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McEwan DL, Weisman AS, Hunter CP: Uptake of extracellular double-stranded RNA by SID-2. Mol Cell 2012, 47:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arguel MJ, Jaouannet M, Magliano M, Abad P, Rosso MN: siRNAs trigger efficient silencing of a parasitism gene in plant parasitic root-knot nematodes. Genes (Basel) 2012, 3:391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M, Jin H: Spray-induced gene silencing: a powerful innovative strategy for crop protection. Trends Microbiol 2017, 25:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molnar A, Melnyk CW, Bassett A, Hardcastle TJ, Dunn R, Baulcombe DC: Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 2010, 328:872–875. [DOI] [PubMed] [Google Scholar]
- 49.Melnyk CW, Molnar A, Baulcombe DC: Intercellular and systemic movement of RNA silencing signals. EMBO J 2011, 30:3553–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mermigka G, Verret F, Kalantidis K: RNA silencing movement in plants. J Integr Plant Biol 2016, 58:328–342. [DOI] [PubMed] [Google Scholar]
- 51. ●●.Mitter N, Worrall EA, Robinson KE, Li P, Jain RG, Taochy C, Fletcher SJ, Carroll BJ, Lu GQ, Xu ZP: Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat Plants 2017, 3:16207.The instability of naked dsRNA sprayed on plants has been a major challenge towards its practical application for plant protection from viral infection. In this study, it was demonstrated that dsRNA can be loaded on layered double hydroxide (LDH) clay nanosheets. After loading, the dsRNA does not wash off, shows sustained release and can protect plant more 20 days after application. This study provides an excellent tool to gain fundamental insights into the mechanism of systemic transport of dsRNA to protect plants with the potential of reducing pesticide usage and overcoming the public concern by genetically modified crops.


