ABSTRACT
Background
Urinary phosphorus excretion has been proposed as a recovery biomarker of dietary phosphorus intake. However, it is unclear whether phosphorus excretion is constant across a range of dietary and nondietary factors.
Objective
We assessed whether percentage urinary phosphorus excretion is constant across 3 dietary patterns in the Dietary Approaches to Stop Hypertension (DASH) trial.
Methods
DASH is a completed feeding study of 459 prehypertensive and stage 1 hypertensive adults (52% male, 56% black). After a 3-wk run-in on a typical American (control) diet, participants were randomly assigned to the control diet, a diet rich in fruits and vegetables (FV diet), or a diet rich in fruits, vegetables, and low-fat dairy with reduced saturated fat and cholesterol (DASH diet) for 8 wk. We estimated the percentage phosphorus excretion as urinary phosphorus excretion (from 24 h urine) divided by phosphorus intake (from analyzed food composites). Differences between group means for all 3 diets were compared by ANOVA followed by pairwise comparisons with Tukey's honest significant difference test.
Results
At the end of the intervention, the mean phosphorus intake was 1176 mg/d (95% CI: 1119, 1233 mg/d), 1408 mg/d (1352, 1464 mg/d), and 2051 mg/d (1994, 2107 mg/d) in the control, FV, and DASH diet, respectively (P < 0.001, all comparisons). The mean phosphorus excretion was 734 mg/d (682, 787 mg/d), 705 mg/d (654, 756 mg/d), and 872 mg/d (820, 923 mg/d) in the control, FV, and DASH diet, respectively (P = 0.74 control vs. FV, P < 0.001 all other comparisons). The mean percentage phosphorus excretion was 63% (60%, 67%), 51% (48%, 54%), and 43% (39%, 46%) in the control, FV, and DASH diet, respectively (P < 0.001, all comparisons).
Conclusions
These findings in prehypertensive and stage 1 hypertensive adults strongly suggest that urinary phosphorus excretion should not be used as a recovery biomarker for dietary phosphorus intake, given the wide range of urinary phosphorus excretion across dietary patterns. This trial is registered at clinicaltrials.gov as NCT0000054.
Keywords: phosphorus, feeding study, randomized trial, urinary excretion, diet, recovery biomarkers
Introduction
The average dietary phosphorus intake in US adults is twice the required amount to avoid deficiency (700 mg/d), and intake is increasing (1, 2). Conflicting studies, mainly small or cross-sectional in nature, have found associations of dietary phosphorus intake with both beneficial (3–5) and adverse (6–10) measures of health, ranging from blood pressure to all-cause mortality. The estimation of absolute phosphorus intake is critical to the study of dietary phosphorus intake. Phosphorus is nearly ubiquitous in the diet, with grain, meat, and milk products contributing >75% of the total intake (2). Additionally, phosphorus is added to processed foods in a variety of forms: sodium phosphates, calcium phosphates, and phosphoric acids (11). The total amount, chemical form (phosphates vs. phytates), and source (added vs. naturally occurring) of phosphorus in food vary greatly between broad food groups (grains vs. meat) and between very similar food products (crackers vs. cookies) (11). Nutrient database estimates of the phosphorus content of meats (12) and beverages (13) have been shown to be inaccurate.
Once consumed, phosphorus-containing molecules may be readily absorbed, broken down by human or bacterial enzymes in the gut, and then absorbed, or may pass into the feces unabsorbed (14, 15). Once absorbed, phosphorus may be incorporated into the skeleton and other bodily tissues or excreted in the urine (16). Several small or cross-sectional studies have suggested that the percentage of dietary phosphorus excreted in the urine varies in accordance with dietary and nondietary factors. Lower percentage phosphorus excretion in the urine has been seen in diets higher in vegetarian protein than meat protein (17), and from a higher consumption of oatmeal porridge, rye bread, and cheese than that of beef, pork, and sodium phosphate (18). Percentage phosphorus excretion has also been shown to vary by sex, smoking status, physical activity level, and BMI (19).
Despite this, urinary phosphorus excretion has been proposed as a recovery biomarker of total dietary phosphorus intake. An important assumption in the use of urinary phosphorus as a recovery biomarker is that dietary phosphorus intake is excreted at a constant rate in the urine for all individuals, regardless of dietary or nondietary factors (20–23). Several studies have used a percentage of dietary phosphorus excreted in the urine (percentage phosphorus excretion) of 65% to estimate intake for all individuals (20, 21, 24), whereas a recent meta-analysis of 14 studies found the mean ± SD percentage phosphorus excretion to be 62.7 ± 17.4% (19). However, the correlation between urinary excretion and self-reported dietary intake ranges from low to moderate in prior studies, with Spearman's correlation coefficients of 0.29–0.53 (19, 20, 25).
To inform the validity of urinary phosphorus excretion as a recovery biomarker for dietary phosphorus intake, we performed a secondary analysis of data from a randomized feeding study—the Dietary Approaches to Stop Hypertension (DASH) trial. We examined the association of dietary phosphorus intake with urinary phosphorus excretion across 3 dietary treatment arms of the trial. Additionally, we compared dietary phosphorus intake estimated from a nutrient database and chemically analyzed food composites collected during the DASH trial intervention.
Methods
Study design
The DASH trial is a completed randomized feeding study of 459 prehypertensive and stage 1 hypertensive adult men and women assigned to 1 of 3 study diets for 8 wk with blood pressure as the main outcome. Details of the study design have been reported elsewhere (26–28). Briefly, DASH participants were adults ≥22 y old, not taking antihypertensive medication, with an average systolic blood pressure of <160 mm Hg and diastolic blood pressure of 80–95 mm Hg. Participants were enrolled in phases between September 1994 and January 1996 at 4 US centers (Baltimore, MD; Baton Rouge, LA; Boston, MA; Durham, NC), and received all meals and snacks from the study. All participants underwent a 3-wk run-in period on a typical American (control) diet, followed by an 8-wk intervention period where participants were randomly assigned to either stay on the control diet, or to consume a diet high in fruits and vegetables (FV diet), or one rich in fruits, vegetables, and low-fat dairy with reduced saturated fat and cholesterol (DASH diet) (Supplemental Figure 1). Each diet consisted of a 7-d menu cycle with 21 unique meals (Supplemental Table 1), and were designed with targets for fat (saturated, monounsaturated, and polyunsaturated), carbohydrates (total and fiber), protein, cholesterol, potassium, magnesium, calcium, and sodium, but not for phosphorus.
To keep participants at a constant weight, each diet was prepared at 4 calorie levels (1600, 2100, 2600, and 3100 kcal/d). Intermediate calorie levels were provided through the use of “unit foods” (muffins or cookies that provided 100 kcal and with the same levels of the targeted nutrients per 100 kcal as in the assigned diet). Urine was collected over a 24-h period once during screening, once within the last week of the run-in, and once within the last 13 d of the intervention. Serum samples were collected during the last week of the run-in and the last week of the intervention. Detailed protocols were developed for the recipes, ingredients, preparation, and delivery of foods to standardize the diets across the 4 centers (26, 27, 29).
Diet composition analysis
To validate the target nutrient contents in each diet, daily menus for an additional “participant” were collected at each center throughout the intervention, rotating the kilocalorie level collected each week for each diet, as described previously (30). In this study, phosphorus was analyzed in diet/kcalorie level composite samples, prepared through the use of archived aliquots of 7-d diet cycle composites from each center in blinded duplicate samples of each diet/kcalorie composite, at Medallion Laboratories (Minneapolis, MN) by inductively coupled plasma optical emission spectrometry through the use of AOAC method 2011.14 (31) (Supplemental Figure 2). The mean of each set of duplicate samples was used for analysis. The overall percentage difference of the nutrient database estimate of phosphorus for each diet was calculated as the mean of the percentage difference of the nutrient database estimate of phosphorus for the 4 calorie level composites. Mean percentage differences (nutrient database vs. composite analysis) for all 3 diets were compared by ANOVA, followed by pairwise comparisons with Tukey's honest significant difference test only if the ANOVA was statistically significant at the P < 0.05 level.
Moisture was analyzed to ensure that no water was lost during storage through the use of the same methodology as the original study (27). Control samples were also analyzed to ensure consistency of method performance between the analyses in 1994–1995 and 2018. Additionally, 4 reference materials with certified phosphorus concentrations were included, representing different food matrices and phosphorus levels: SRM 2383a Baby Food, SRM 1549a Whole Milk Powder, SRM 1546a Meat Homogenate, and SRM 1567b Wheat Flour (National Institute of Standards and Technology).
Analysis of dietary intake and urinary excretion of phosphorus
The phosphorus content of diet menus was evaluated by nutrient database estimate and composite analysis. The nutrient database estimate was from the original study menus built in Moore's Extended Nutrient database (MENu) version 1.0 (1994, Pennington Biomedical Research Foundation). There were no composites available for unit foods, so the phosphorus content of the unit foods was estimated through the use of the original MENu nutrient database values for the nutrient database estimate and the MENu nutrient database value adjusted by the mean percentage difference of the nutrient database estimate of phosphorus for the composite analysis.
We calculated each participant's mean phosphorus intake (separately by nutrient database estimate and composite analysis) as the mean of their daily intakes (menu + unit foods) over the run-in and intervention periods. We calculated each participant's percentage phosphorus excretion for each study period by dividing their 24-h urinary phosphorus excretion by their mean dietary phosphorus intake (separately by nutrient database estimate and composite analysis). If data on a participant's calorie intake were missing for a given day, we used the last known value carried forward. Completeness of 24-h urine samples was assessed through the use of the coefficient of variation for urinary creatinine excretion across all available 24-h samples (screening, run-in, and intervention) (32). Participants with missing urinary excretion data were excluded. The percentage of total phosphorus intake from specific dietary sources was estimated through the use of the MENu nutrient database estimates of the phosphorus content of each individual food in the 2100 kcal/d diet arm.
The correlation of dietary phosphorus intake with urinary phosphorus excretion was assessed through the use of Spearman's rank correlation coefficient. Group means and 95% CIs for dietary phosphorus intake (composite analysis and nutrient database values), urinary phosphorus excretion, and percentage phosphorus excretion were calculated with the use of marginal estimates from linear regression models both crude and adjusted for age, race (black vs. nonblack), sex, BMI, serum 1,25-dihydroxycholecalciferol (picograms per milliliter), and energy intake (kcalories per day). Group means for all 3 diets were compared by ANOVA, followed by pairwise comparisons with Tukey's honest significant difference test only if the ANOVA was statistically significant at the P < 0.05 level. We performed a subgroup analysis for dietary intake, urinary excretion, and percentage excretion by age, race (black vs. nonblack), sex, BMI, smoking status, and changes in serum 1,25-dihydroxycholecalciferol and energy intake. All statistical analyses were performed with Stata Version 14 (StataCorp). Figures were produced with R, Stata, and Inkscape Version 0.92 (The Inkscape Project).
Results
Phosphorus content of diets
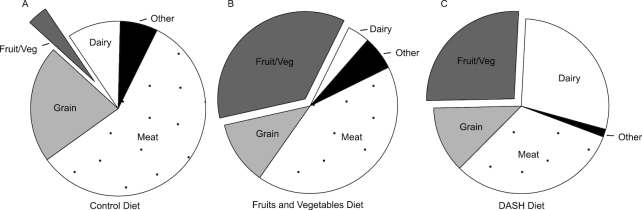
The phosphorus content from the composite sample analysis was lowest in the control diet (978 mg/2100 kcal), followed by the FV and DASH diets (1212 mg/2100 kcal and 1692 mg/2100 kcal, respectively) (Supplemental Table 2). Based on the nutrient database values for foods included in the diets, meats were the major source of phosphorus (58%) in the control diet. In the FV diet, meats and fruits and vegetables were the major sources of phosphorus (42% and 36%, respectively). In the DASH diet, the major sources were meats, dairy, and fruits and vegetables (32%, 28%, and 26%, respectively) (Figure 1 and Supplemental Table 2). Compared to the composite analysis, the nutrient database underestimated the phosphorus content of the FV and DASH diets by 15.9% (95% CI: 13.0%, 18.8%) and 15.5% (12.6%, 18.4%), respectively, but not the control diet, 1.2% (–1.7%, 4.1%). The percentage difference was significantly higher for the FV and DASH diets than for the control diet (P < 0.001, both comparisons), but not significantly different between the FV and DASH diets (P = 0.96).
FIGURE 1.
Food sources of phosphorus in the control (A), fruits and vegetables (B), and DASH (C) diets at the 2100 kcal/d energy level, estimated from nutrient database values. Values are percentage of total phosphorus contributed by a specific food source. DASH, Dietary Approaches to Stop Hypertension; Fruit/Veg, fruits and vegetables.
Quality control
Among participants with at least 2 urine collections, 99.5% had a coefficient of variation for urinary creatinine excretion below the 80% cutoff used in previous studies, suggesting high completeness of urine collections (32). The mean percentage difference between composite sample blind replicates was 0.62% (range 0.11–1.39%). There was high agreement between of the 2018 and 1994 composite analyses for sodium, with a median Horwitz ratio of 0.4 (range 0.0–0.9) (33). There was no significant moisture loss in the composite samples during storage. The phosphorus content of the reference materials was underestimated by 3.7%, 6.4%, 10.0%, and 5.6% for SRM 1546a Meat Homogenate, SRM 1549a Whole Milk Powder, SRM 1567b Wheat Flour, and SRM 2383a Baby Food, respectively. The estimate was below the approximate 95% CI for SRM 1549a Whole Milk Powder, SRM 1567b Wheat Flour, and SRM 2383a Baby Food.
Participant characteristics
Of the 397 participants with urinary phosphorus excretion data prior to run-in, the mean age was 44.9 y, 56.7% were black, 51.6% were male, and the mean BMI was 28.2 kg/m2 (Table 1). Of the 40% of participants with information on smoking status, 23.3% were current smokers. Complete dietary and urinary data were available for 341 of the 459 randomly assigned participants (Supplemental Table 3). Those missing dietary phosphorus intake and/or urinary phosphorus excretion data were younger, consumed fewer calories, had lower serum 1,25-dihydroxycholecalciferol, and were more likely to be black, female, current smokers, and from a particular study center.
TABLE 1.
Baseline characteristics in adults randomly assigned to consume the control, fruits and vegetables, or DASH diet (among participants with urinary phosphorus data)1
| Control | FV | DASH | Total | P 2 | |
|---|---|---|---|---|---|
| n | 136 (34.3) | 133 (33.5) | 128 (32.2) | 397 (100.0) | |
| Age, y | 44.9 ± 11.2 | 45.3 ± 10.7 | 44.5 ± 10.0 | 44.9 ± 10.7 | 0.83 |
| Black | 78 (57.4) | 74 (55.6) | 73 (57.0) | 225 (56.7) | 0.96 |
| Male | 73 (53.7) | 69 (51.9) | 63 (49.2) | 205 (51.6) | 0.77 |
| BMI, kg/m2 | 28.1 ± 3.7 | 28.2 ± 4.0 | 28.4 ± 3.9 | 28.2 ± 3.9 | 0.82 |
| Smoking status | 0.36 | ||||
| Current | 11 (21.6) | 17 (29.3) | 9 (18.0) | 37 (23.3) | |
| Noncurrent | 40 (78.4) | 41 (70.7) | 41 (82.0) | 122 (76.7) | |
| Study center | 0.99 | ||||
| A | 39 (28.7) | 39 (29.3) | 39 (30.5) | 117 (29.5) | |
| B | 29 (21.3) | 26 (19.5) | 22 (17.2) | 77 (19.4) | |
| C | 26 (19.1) | 27 (20.3) | 26 (20.3) | 79 (19.9) | |
| D | 42 (30.9) | 41 (30.8) | 41 (32.0) | 124 (31.2) | |
| Urinary phosphorus excretion, mg/d | 792 ± 326 | 799 ± 323 | 833 ± 339 | 807 ± 329 | 0.56 |
1Values are means ± SDs or frequency (%). DASH, Dietary Approaches to Stop Hypertension diet; FV, fruits and vegetables diet.
2Chi-square test for categorical variables, ANOVA for continuous variables.
Phosphorus intake and excretion
Dietary phosphorus intake (from the composite sample analysis) was positively correlated with urinary phosphorus excretion during both the run-in and intervention periods (Figure 2).
FIGURE 2.
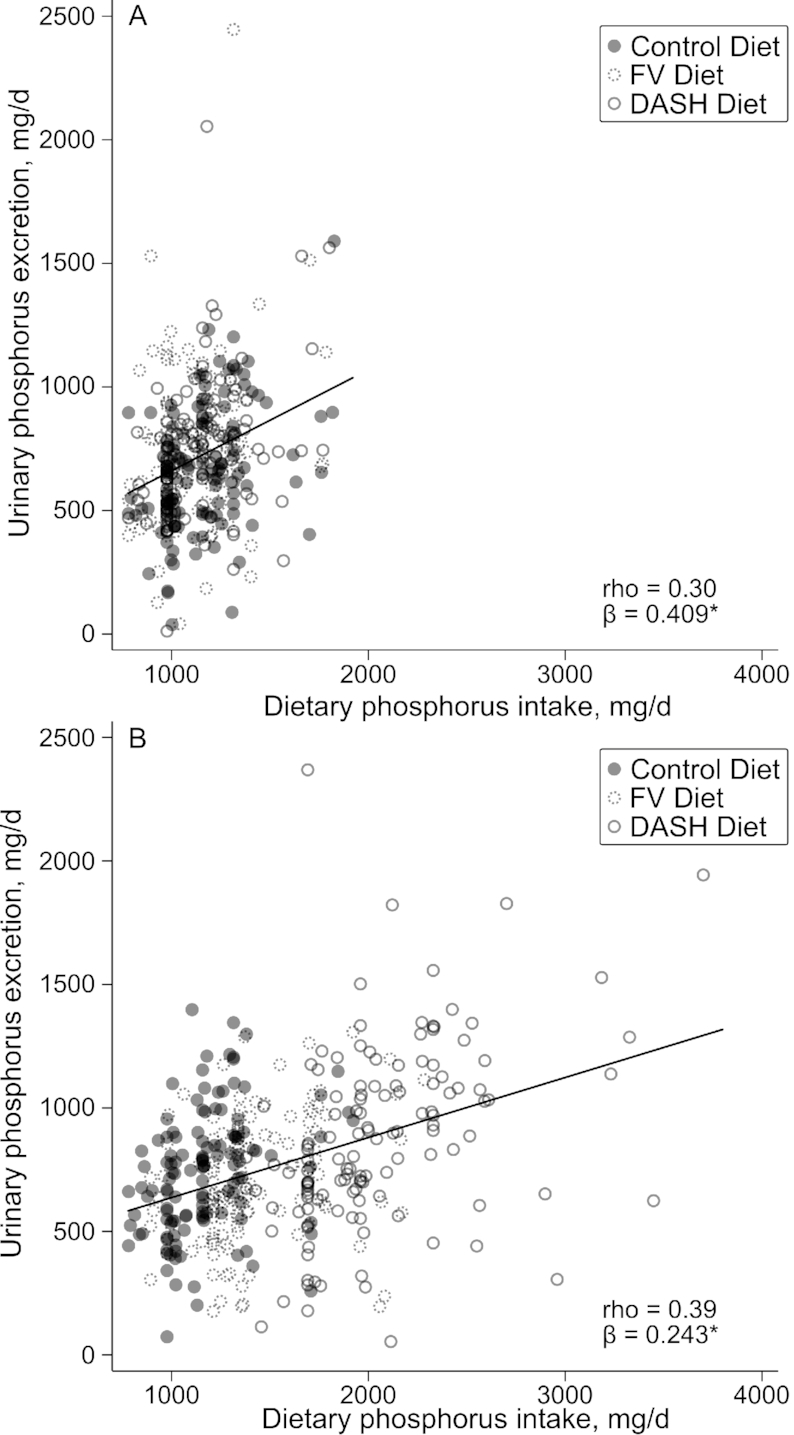
Correlation of urinary phosphorus excretion with dietary phosphorus intake, estimated from composite samples, at (A) the end of the run-in and (B) the end of the intervention in adults randomly assigned to consume the control, fruits and vegetables, or DASH diet. Values are mean individual dietary phosphorus intake for a given study period by composite sample analysis (milligrams per day) and urinary phosphorus excretion from a single 24-h urine collection (milligrams per day). β coefficient and trend line from linear regression of urinary phosphorus excretion on dietary phosphorus intake. *P < 0.001. DASH, Dietary Approaches to Stop Hypertension; FV, fruits and vegetables; rho, Spearman's correlation coefficient.
During the run-in, when all participants ate the control diet, the mean phosphorus intake was not significantly associated with the subsequent random diet assignment (P-interaction = 0.24 and 0.22 for nutrient database and composite sample analysis, respectively), as expected (Table 2). Likewise, mean urinary phosphorus excretion was not significantly different by randomly assigned group (P-interaction = 0.065). However, the mean percentage phosphorus excretion was significantly lower among participants who were subsequently randomly assigned to the control diet than those randomly assigned to the FV diet both by nutrient database and composite sample analysis (P = 0.003 and 0.004, respectively). After adjustment for study center, age, race, sex, BMI, calorie intake, and serum 1,25-dihydroxycholecalciferol, those randomly assigned to the control diet had significantly lower urinary phosphorus excretion than those randomly assigned to the FV or DASH diet (P = 0.015 and 0.014, respectively).
TABLE 2.
Mean dietary phosphorus intake, urinary excretion, and percentage excretion at the end of the run-in in adults randomly assigned to consume the control, fruits and vegetables, or DASH diet (all participants received the control diet during the run-in)1
| Control | Fruits and vegetables | DASH | ||||
|---|---|---|---|---|---|---|
| Crude | Adjusted2 | Crude | Adjusted2 | Crude | Adjusted2 | |
| n | 120 | 117 | 128 | 127 | 119 | 119 |
| Dietary phosphorus intake, mg/d | ||||||
| Composite sample | 1164 (1128, 1200) | 1144 (1137, 1151) | 1120 (1084, 1155) | 1143 (1136, 1150) | 1144 (1108, 1181) | 1144 (1137, 1151) |
| Nutrient database | 1145 (1108, 1183) | 1125 (1119, 1131)a | 1100 (1064, 1137) | 1125 (1119, 1130) | 1123 (1086, 1161) | 1124 (1118, 1130) |
| Urinary phosphorus excretion, mg/d | 669 (619, 719)b | 655 (608, 701)b | 745 (697, 794)a | 747 (702, 791)a | 738 (688, 788)a | 748 (702, 794)a |
| Urinary phosphorus excretion, % of intake | ||||||
| Composite sample | 58 (54, 62)b | 58 (54, 62)b | 67 (63, 71)a | 66 (62, 70)a | 65 (61, 69)a,b | 66 (62, 70)a |
| Nutrient database | 59 (55, 63)b | 59 (55, 63)b | 69 (65, 73)a | 67 (64, 71)a | 66 (62, 70)a,b | 67 (63, 71)a |
1Values are means (95% CIs). DASH, Dietary Approaches to Stop Hypertension.
2Adjusted for age, black race, sex, serum 1,25-dihydroxycholecalciferol, BMI, study center, and calorie intake. Means within a row and adjustment category without a common letter differ, P < 0.05.
During the intervention period, the mean phosphorus intake by composite sample analysis was highest in those randomly assigned to the DASH diet, followed by those on the FV diet, and was lowest in those on the control diet (P < 0.001, all comparisons) (Table 3). The mean phosphorus intake by nutrient database was significantly higher in those randomly assigned to the DASH diet than in those on the control or FV diet (P = 0.001, both comparisons), but there was no significant difference between the control and FV diets (P = 0.61). The mean urinary phosphorus excretion was higher in those randomly assigned to the DASH diet than in those on the control or FV diets (both comparisons P < 0.001), but there was no significant difference between the control and FV diets (P = 0.70). The mean percentage phosphorus excretion (composite sample analysis) was lowest in the participants randomly assigned to the DASH diet, followed by the FV and control diets (P < 0.001, all comparisons). The mean percentage phosphorus excretion (nutrient database analysis) was lowest in participants randomly assigned to the DASH diet, followed by the FV and control diets (P < 0.001, both comparisons), respectively, but there was no significant difference between the control and FV diets (P = 0.26). Adjustment for study center, age, race, sex, BMI, calorie intake, and serum 1,25-dihydroxycholecalciferol did not substantively change the results.
TABLE 3.
Dietary phosphorus intake, urinary excretion, and percentage excretion at the end of the intervention in adults randomly assigned to consume the control, fruits and vegetables, or DASH diet1
| Control | Fruits and vegetables | DASH | ||||
|---|---|---|---|---|---|---|
| Crude | Adjusted2 | Crude | Adjusted2 | Crude | Adjusted2 | |
| n | 126 | 121 | 131 | 122 | 130 | 123 |
| Dietary phosphorus intake, mg/d | ||||||
| Composite sample | 1176 (1119, 1233)c | 1172 (1154, 1190)c | 1408 (1352, 1464)b | 1419 (1401, 1437)b | 2051 (1994, 2107)a | 2065 (2047, 2083)a |
| Nutrient database | 1158 (1108, 1207)b | 1155 (1142, 1168)c | 1191 (1143, 1239)b | 1200 (1188, 1213)b | 1755 (1707, 1804)a | 1768 (1755, 1780)a |
| Urinary phosphorus excretion, mg/d | 734 (682, 787)b | 728 (683, 773)b | 705 (654, 756)b | 709 (664, 754)b | 872 (820, 923)a | 862 (817, 907)a |
| Urinary phosphorus excretion, % of intake | ||||||
| Composite sample | 63 (60, 67)a | 63 (60, 66)a | 51 (48, 54)b | 51 (48, 54)b | 43 (39, 46)c | 42 (39, 45)c |
| Nutrient database | 64 (61, 68)a | 64 (61, 67)a | 60 (57, 64)a | 60 (57, 64)a | 50 (46, 53)b | 49 (45, 52)b |
1Values are means (95% CIs). DASH, Dietary Approaches to Stop Hypertension.
2Adjusted for age, black race, sex, serum 1,25-dihydroxycholecalciferol, BMI, study center, and calorie intake. Means within a row and adjustment category without a common letter differ, P < 0.05.
Between the end of the run-in and the end of the intervention, the mean dietary phosphorus intake did not change significantly for the control diet (P = 0.27 and 0.40 for the nutrient database and composite sample analysis, respectively), but it increased significantly for the FV and DASH diets (P < 0.001 for both the nutrient database and composite sample analysis) (Table 4). Phosphorus intake (by both nutrient database and composite sample analysis) increased the most for participants randomly assigned to the DASH diet, followed by the FV and control diets, respectively (P < 0.001, all comparisons). The mean urinary phosphorus excretion increased significantly for those on the control and DASH diets (P = 0.017 and P < 0.001, respectively), but did not change significantly for those on the FV diet (P = 0.095).
TABLE 4.
Change in dietary phosphorus intake, urinary excretion, and percentage excretion between the end of the run-in and the end of the intervention in adults randomly assigned to consume the control, fruits and vegetables, or DASH diet1
| Control | Fruits and vegetables | DASH | ||||
|---|---|---|---|---|---|---|
| Crude | Adjusted2 | Crude | Adjusted2 | Crude | Adjusted2 | |
| n | 111 | 108 | 118 | 117 | 112 | 112 |
| Dietary phosphorus intake, mg/d | ||||||
| Composite sample | 15 (–19, 49)c | 2 (–28, 33)c | 280 (247, 313)b,* | 294 (265, 324)b,* | 933 (900, 967)a,* | 933 (903, 963)a,* |
| Nutrient database | 14 (–11, 40)c | 8 (–17, 34)c | 84 (59, 109)b,* | 91 (67, 116)b,* | 653 (627, 679)a,* | 653 (628, 677)a,* |
| Urinary phosphorus excretion, mg/d | 71 (15, 127)a,* | 83 (27, 139)a,* | –46 (–101, 8)b | –45 (–98, 9)b | 120 (64, 176)a,* | 112 (57, 166)a,* |
| Urinary phosphorus excretion, % of intake | ||||||
| Composite sample | 6 (1, 10)a,* | 7 (2, 12)a,* | –17 (–21, –12)b,* | –17 (–21, –12)b,* | –23 (–28, –19)b,* | –24 (–29, –20)c,* |
| Nutrient database | 6 (1, 11)a,* | 7 (2, 12)a,* | –9 (–13, –4)b,* | –9 (–13, –4)b,* | –18 (–23, –13)c,* | –19 (–23, –14)c,* |
1Values are means (95% CIs). DASH, Dietary Approaches to Stop Hypertension.
2Adjusted for age, black race, sex, serum 1,25-dihydroxycholecalciferol (at run-in), BMI (at run-in), study center, and calorie intake (at run-in). Means within a row and adjustment category without a common letter differ, P < 0.05.
The percentage phosphorus excretion increased significantly for those on the control diet (P = 0.017 and 0.014 for the nutrient database and composite sample analysis, respectively), and decreased significantly for those on the FV and DASH diets (P < 0.001 for both the nutrient database and composite sample analysis). The percentage phosphorus excretion decreased significantly more for the DASH diet than for the FV diet according to the nutrient database, but not the composite sample analysis (P = 0.019 and 0.095, respectively). After adjustment for study center, age, race, sex, BMI, calorie intake, and serum 1,25-dihydroxycholecalciferol, the percentage phosphorus excretion decreased significantly more for the DASH diet than for the FV diet according to the composite sample analysis (P = 0.047).
Subgroup analyses
We assessed the linear associations of age, BMI, black race, male sex, smoking status, and changes in serum 1,25-dihydroxycholecalciferol and energy intake with phosphorus intake, total excretion, and percentage excretion for the change from the end of the run-in to the end of the intervention, adjusted for only the randomization group (crude), and for age, BMI, black race, male sex, study center, randomization group, and changes in serum 1,25-dihydroxycholecalciferol and energy intake (fully adjusted) (Supplemental Table 4).
Increased phosphorus intake (nutrient database) was positively associated with baseline BMI (P < 0.001, fully adjusted), increased calorie consumption (P < 0.001, fully adjusted), and male sex (P < 0.001, fully adjusted), but was negatively associated with age (P = 0.0273, fully adjusted). Increase in phosphorus intake (composite analysis) was also positively associated with baseline BMI (P < 0.001, fully adjusted), increased calorie consumption (P < 0.001, fully adjusted), and male sex (P < 0.001, fully adjusted), and negatively associated with age (P = 0.004, fully adjusted). Increased urinary phosphorus excretion was positively associated with baseline BMI (P < 0.001, fully adjusted). Increased percentage phosphorus excretion (nutrient database) was positively associated with baseline BMI (P = 0.002, fully adjusted) and decreased calorie consumption (P = 0.002, fully adjusted). Increased percentage phosphorus excretion (composite analysis) was positively associated with baseline BMI (P = 0.003, fully adjusted) and decreased calorie consumption (P = 0.004, fully adjusted).
Discussion
In this secondary analysis of a randomized controlled feeding study, we found that the percentage of dietary phosphorus excreted in the urine was not constant across 3 dietary treatment arms. Our results strongly suggest that urinary phosphorus excretion should not be used as a recovery biomarker for dietary phosphorus intake. We also found that the nutrient database underestimated the phosphorus content in diets high in fruits and vegetables (the DASH and FV diets), but was relatively accurate for a typical American diet (the control diet).
We found that 63.3% of dietary phosphorus intake is excreted in the urine of participants on the control diet, which is very similar to the 62.7% found in a recent meta-analysis by Shinozaki and others (19), yet participants on the FV diet excreted 51.0% and those on the DASH diet excreted only 42.6%. Compared with the control diet, the FV diet had a greater proportion of phosphorus from nongrain fruits and vegetables, whereas the DASH diet had a greater proportion from nongrain fruits and vegetables as well as dairy. This is in line with research suggesting that some fruits and vegetables (specifically nuts) may be incompletely digested (34). Additionally, the higher calcium content of the DASH diet (from dairy) may have resulted in a further reduction in phosphorus absorption (3, 35).
We also found that the absolute and percentage urinary phosphorus excretion of those on the control diet increased significantly between the run-in and the intervention, suggesting that equilibrium was not reached after a full 3 wk on the control diet. This is in line with a study that found that urinary excretion of intravenously administered sodium phosphate progressively increased both during and after a 36-h administration (36) and another that found that fecal phosphorus excretion (specifically phytate phosphorus) did not stabilize in young adult women over a 10-d period (37).
The strengths of the DASH trial include the large sample size, diverse population, high follow-up rate, carefully controlled feeding conditions, large phosphorus contrasts between diets, and the randomized design. The strengths of the current study include our use of 2 estimates of phosphorus content, composite samples and nutrient database. There was relatively good agreement between blind replicates in the composite sample analysis, and the sodium values were similar between the 1997 and 2018 analyses. Although there were significant differences between the nutrient database estimates and our composite sample analysis, the direction of the results agree.
This study also has some important limitations. First, we were not able to determine which aspects of the FV diet and DASH diet led to lower percentage phosphorus excretion than the control diet. However, plant sources of phosphorus (FV diet and DASH diet) and calcium (DASH diet) have both been found to lower urinary excretion in several studies (17, 18, 38). Our results are in line with these findings. Second, we only had 1 urine collection at each time point, giving us a less reliable estimate of each individual's urinary phosphorus excretion. However, this would be expected to bias results towards the null, and the study was sufficiently powered to find differences between the intervention groups. Third, the analyzed values for the reference standards were lower than expected, possibly suggesting a loss of composite sample during analysis. However, this would be expected to result in an underestimate of the phosphorus content of the composite samples, biasing our results towards the null.
All of these findings strongly suggest that urinary phosphorus excretion neither stabilizes quickly to changes in intake nor stabilizes uniformly across dietary patterns. Although it could be possible to take individual characteristics (such as age, race, or sex) into account when estimating dietary phosphorus intake from urinary phosphorus excretion, it would be impractical to require characterization of diet in an equation meant to estimate dietary intake. The use of urinary phosphorus excretion as a recovery biomarker would lead to the underestimation of phosphorus intake in those consuming diets similar to the FV or DASH diet. Additionally, nutrient databases appear to underestimate the phosphorus content of diets high in fruits and vegetables, but not a typical American diet. Such underestimation may lead to spurious inverse associations between phosphorus intake and the health benefits associated with diets rich in fruits and vegetables, or ones rich in fruits, vegetables, and low-fat dairy with reduced saturated fat and cholesterol.
In summary, given the errors in nutrient databases, urinary biomarkers are a tempting alternative for nutrition researchers. For urinary excretion of phosphorus to serve as a recovery biomarker for dietary phosphorus intake, it must be excreted at a predictable rate across individuals. The current analysis strongly suggests that urinary phosphorus excretion does not meet this requirement. Wherever possible, researchers should use food samples to directly measure phosphorus intake. Furthermore, updating nutrient database estimates (and including measurement of error in those estimates) is critical to the accurate study of dietary phosphorus.
In this secondary analysis of a randomized controlled feeding study, we found that the percentage of dietary phosphorus excreted in the urine varies significantly by dietary pattern. These data suggest that urinary phosphorus excretion is not a valid recovery biomarker for dietary phosphorus intake.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—STM, CMR, ES, and LJA: provided input on the design of the analysis; KMP: ran the composite analysis; CMC: gathered food-level nutrition data; STM: analyzed the data and drafted the manuscript; and all authors: edited the manuscript and read and approved the final version.
Notes
STM was supported by the NIH/National Heart, Lung & Blood Institute grant T32 HL007024 and a grant from the Johns Hopkins School of Public Health Department of Epidemiology Doctoral Research Fund. CMR was supported by a Mentored Research Scientist Development Award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782). ES was supported by NIH/NIDDK grant K24DK106414.
Author disclosures: STM, CMR, KMP, CMC, ES, and LJA, no conflicts of interest.
Supplemental Figures 1 and 2 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: DASH, Dietary Approaches to Stop Hypertension; FV, fruits and vegetables; MENu, Moore's Extended Nutrient database.
References
- 1. Intakes DR. Dietary Reference Intakes. Vol 55: 2009. doi:10.1111/j.1753-4887.1997.tb01621.x. [Google Scholar]
- 2. McClure ST, Chang AR, Selvin E, Rebholz CM, Appel LJ. Dietary sources of phosphorus among adults in the United States: results from NHANES 2001–2014. Nutrients. 2017;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takeda E, Yamamoto H, Yamanaka-Okumura H, Taketani Y. Dietary phosphorus in bone health and quality of life. Nutr Rev. 2012;70:311–21. [DOI] [PubMed] [Google Scholar]
- 4. Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, Brown IJ, Zhao L, Stamler J. Dietary phosphorus and blood pressure: international study of macro- and micro-nutrients and blood pressure. Hypertension. 2008;51:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alonso A, Nettleton JA, Ix JH, De Boer IH, Folsom AR, Bidulescu A, Kestenbaum BR, Chambless LE, Jacobs DR. Dietary phosphorus, blood pressure, and incidence of hypertension in the Atherosclerosis Risk in Communities study and the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55:776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang AR, Lazo M, Appel LJ, Gutiérrez OM, Grams ME. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr. 2014;99:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamamoto KT, Robinson-Cohen C, de Oliveira MC, Kostina A, Nettleton JA, Ix JH, Nguyen H, Eng J, Lima JAC, Siscovick DS et al.. Dietary phosphorus is associated with greater left ventricular mass. Kidney Int. 2013;83:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Itkonen ST, Karp HJ, Kemi VE, Kokkonen EM, Saarnio EM, Pekkinen MH, Kärkkäinen MUM, Laitinen EKA, Turanlahti MI, Lamberg-Allardt CJE. Associations among total and food additive phosphorus intake and carotid intima-media thickness—a cross-sectional study in a middle-aged population in southern Finland. Nutr J. 2013;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y et al.. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishi T, Shuto E, Ogawa M, Ohya M, Nakanishi M, Masuda M, Katsumoto M, Yamanaka-Okumura H, Sakai T, Takeda E et al.. Excessive dietary phosphorus intake impairs endothelial function in young healthy men: a time- and dose-dependent study. J Med Invest. 2015;62:167–72. [DOI] [PubMed] [Google Scholar]
- 11. Lampila LE. Applications and functions of food-grade phosphates. Ann N Y Acad Sci. 2013;1301:37–44. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr. 2007;17:350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krekel C, McClure ST, Chang AR. Improving estimates of phosphorus additive content: manufacturers needed. J Ren Nutr. 2016;26:e27–30. [Google Scholar]
- 14. St-Jules DE, Jagannathan R, Gutekunst L, Kalantar-Zadeh K, Sevick MA. Examining the proportion of dietary phosphorus from plants, animals, and food additives excreted in urine. J Ren Nutr. 2016; 27:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlemmer U, Frølich W, Prieto RM, Grases F. Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res. 2009;53(Suppl 2):S330–75. [DOI] [PubMed] [Google Scholar]
- 16. Elia M, Ljungqvist O, Stratton R, Lanham-New S. Clinical nutrition. 2nd ed[Internet] West Sussex (UK): John Wiley & Sons; 2013. Available from: http://ebookcentral.proquest.com/lib/jhu/reader.action?docID=1152684&ppg=1. [Google Scholar]
- 17. Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karp HJ, Vaihia KP, Kärkkäinen MUM, Niemistö MJ, Lamberg-Allardt CJE. Acute effects of different phosphorus sources on calcium and bone metabolism in young women: a whole-foods approach. Calcif Tissue Int. 2007;80:251–8. [DOI] [PubMed] [Google Scholar]
- 19. Shinozaki N, Murakami K, Asakura K, Uechi K, Kobayashi S, Masayasu S, Sasaki S. Dietary phosphorus intake estimated by 4-day dietary records and two 24-hour urine collections and their associated factors in Japanese adults. Eur J Clin Nutr. 2018;72:517–25. [DOI] [PubMed] [Google Scholar]
- 20. Morimoto Y, Sakuma M, Ohta H, Suzuki A, Matsushita A, Umeda M, Ishikawa M, Taketani Y, Takeda E, Arai H. Estimate of dietary phosphorus intake using 24-h urine collection. J Clin Biochem Nutr. 2014;55:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakuma M, Morimoto Y, Suzuki Y, Suzuki A, Noda S, Nishino K, Ando S, Ishikawa M, Arai H. Availability of 24-h urine collection method on dietary phosphorus intake estimation. J Clin Biochem Nutr. 2017;60:125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Selamet U, Tighiouart H, Sarnak MJ, Beck G, Levey AS, Block G, Ix JH. Relationship of dietary phosphate intake with risk of end-stage renal disease and mortality in chronic kidney disease stages 3–5: the Modification of Diet in Renal Disease Study. Kidney Int. 2016;89:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaaks R, Ferrari P, Ciampi A, Plummer M, Riboli E. Uses and limitations of statistical accounting for random error correlations, in the validation of dietary questionnaire assessments. Public Health Nutr. 2002;5:969–76. [DOI] [PubMed] [Google Scholar]
- 24. Noori N, Kalantar-Zadeh K, Kovesdy CP, Murali SB, Bross R, Nissenson AR, Kopple JD. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, Karanja N, Lin PH, Steele P, Proschan MA. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5:108–18. [DOI] [PubMed] [Google Scholar]
- 27. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM et al.. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 28. Moore TJ, Vollmer WM, Appel LJ, Sacks FM, Svetkey LP, Vogt TM, Conlin PR, Simons-Morton DG, Carter-Edwards L, Harsha DW. Effect of dietary patterns on ambulatory blood pressure : results from the Dietary Approaches to Stop Hypertension (DASH) trial. DASH Collaborative Research Group. Hypertens (Dallas, Tex 1979). 1999;34:472–7. [DOI] [PubMed] [Google Scholar]
- 29. Swain JF, Windhauser MM, Hoben KP, Evans MA, McGee BB, Steele PD. Menu design and selection for multicenter controlled feeding studies: process used in the Dietary Approaches to Stop Hypertension trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99(8 Suppl):S54–9. [DOI] [PubMed] [Google Scholar]
- 30. Phillips KM, Stewart KK, Karanja NM, Windhauser MM, Champagne CM, Swain JF, Lin PH, Evans MA. Validation of diet composition for the Dietary Approaches to Stop Hypertension trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99(8 Suppl):S60–8. [DOI] [PubMed] [Google Scholar]
- 31. AOAC International. Official methods of analysis of AOAC International. 20th ed Gaithersburg (MD): AOAC International; 2016. [Google Scholar]
- 32. Turban S, Thompson CB, Parekh RS, Appel LJ. Effects of sodium intake and diet on racial differences in urinary potassium excretion: results from the Dietary Approaches to Stop Hypertension (DASH)-Sodium trial. Am J Kidney Dis. 2013;61:88–95. [DOI] [PubMed] [Google Scholar]
- 33. Horwitz W, Albert R. The Horwitz ratio (HorRat): a useful index of method performance with respect to precision. J AOAC Int. 89:1095–109. [PubMed] [Google Scholar]
- 34. Novotny JA, Gebauer SK, Baer DJ. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am J Clin Nutr. 2012;96:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takeda E, Taketani Y, Sawada N, Sato T, Yamamoto H. The regulation and function of phosphate in the human body. Biofactors. 2004;21:345–55. [DOI] [PubMed] [Google Scholar]
- 36. Scanni R, VonRotz M, Jehle S, Hulter HN, Krapf R. The human response to acute enteral and parenteral phosphate loads. J Am Soc Nephrol. 2014;25:2730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joung H, Jeun BY, Li SJ, Kim J, Woodhouse LR, King JC, Welch RM, Paik HY. Fecal phytate excretion varies with dietary phytate and age in women. J Am Coll Nutr. 2007;26:295–302. [DOI] [PubMed] [Google Scholar]
- 38. Zemel MB, Linkswiler HM. Calcium metabolism in the young adult male as affected by level and form of phosphorus intake and level of calcium intake. J Nutr. 1981;111(2):315–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.