ABSTRACT
Background
The influence of long-term dietary patterns on weight gain and the underlying potential biological mechanisms are not fully understood.
Objective
We prospectively examined the association of changes in 2 empirical hypothesis-oriented dietary patterns (insulinemic and inflammatory) and weight gain over 24 y at 4-y intervals.
Methods
We followed 54,397 women in the Nurses’ Health Study and 33,043 men in the Health Professionals Follow-Up Study (1986–2010), and computed the empirical dietary index for hyperinsulinemia (EDIH) and empirical dietary inflammatory pattern (EDIP) scores from food frequency questionnaires administered every 4 y. Both scores are weighted sums of 18 food groups, which characterize dietary insulinemic or inflammatory potential based on plasma levels of insulin response or inflammatory biomarkers. We used multivariable-adjusted linear regression to examine 4-y changes in the dietary scores and weight change within the same period.
Results
The mean baseline body mass index (BMI, in kg/m2) was 25.4. Compared with participants who made minimal dietary changes (quintile 3) over 6 4-y periods; participants who changed their diets toward lower insulinemic or inflammatory potential (quintile 1) gained significantly less weight (in kilograms per 4 y) independent of total energy intake, BMI, physical activity, and smoking status: EDIH: −0.65 (95% CI: −0.73, −0.57), EDIP: −0.29 (−0.37, −0.21) among women; and EDIH: −0.60 (−0.71, −0.49), EDIP: −0.19 (−0.27, −0.07) among men. In contrast, those who changed their diets toward higher insulinemic or inflammatory potential (quintile 5) gained significantly more weight: EDIH: 0.43 (0.36, 0.51), EDIP: 0.15 (0.07, 0.23) among women; and EDIH: 0.49 (0.38, 0.59), EDIP: 0.22 (0.11, 0.33) among men (P-trend < 0.0001 for all comparisons). Associations were stronger among individuals who were overweight or obese, younger, less physically active, and had never smoked.
Conclusions
High dietary insulinemic and inflammatory potential is associated with substantial long-term weight gain in adult men and women independent of total energy intake. Dietary patterns with low insulinemic and inflammatory potential may aid in weight gain prevention.
Keywords: weight gain, dietary patterns, insulinemic diets, inflammatory diets, weight change
Introduction
Illnesses associated with overweight and obesity are major contributors to global morbidity and mortality (1), posing an immense and growing public health challenge. Few studies have examined the influence of long-term dietary patterns on weight gain (2–4), and the potential biological mechanisms underlying the influence of long-term dietary changes on weight gain are not fully understood. Some studies have provided support for the carbohydrate-insulin model of obesity (5, 6); however, the insulinemic potential of whole diets may be more important for weight gain than specific nutrients or foods (7–9). Given the links between inflammatory and metabolic processes in obesity (10), the inflammatory potential of whole diets may also be important in overweight and obesity. Examining overall dietary patterns rather than specific foods or nutrients enables the observation of the combined effect of multiple foods and nutrients on weight change.
Dietary insulinemic and inflammatory potential may be associated with body weight independent of total energy intake, which has been suggested as an important mediator of weight change (11). This would be consistent with previous findings that high insulinemia and high levels of systemic inflammation are associated with risk of weight gain (12–14). We previously developed 2 dietary indices: the empirical dietary index for hyperinsulinemia score (15), to assess the insulinemic potential of whole diets; and the empirical dietary inflammatory pattern score, to assess dietary inflammatory potential (16). In the current study, we investigated the associations of 4-y changes in these dietary scores with weight change over the same period, spanning a total of 24 y of follow-up in 2 cohorts of adult women and men. Given that several factors may modify the association of dietary patterns and weight change, we also conducted an in-depth analysis to identify population subgroups that may be most affected by changes in the insulinemic or inflammatory potential of the diet. We examined associations within strata of the following potential effect modifiers: body weight (2), physical activity (17), age (18), smoking status (19), menopausal status, and postmenopausal hormone use (20). Also, dietary changes during follow-up may be sensitive to the baseline dietary pattern.
Methods
Study population
We used data from 2 ongoing prospective cohort studies of women and men in the United States: the Nurses’ Health Study (NHS), initiated in 1976 with 121,701 female registered nurses, aged 30–55 y at enrollment; and the Health Professionals Follow-Up Study (HPFS), initiated in 1986 with 51,529 male health professionals, aged 40–75 y at enrollment. The baseline for the current study was 1986 for both cohorts—the follow-up cycle when detailed information on diet, weight, and key covariates were first available.
We excluded participants with cancer (except nonmelanoma skin cancer), respiratory diseases, self-reported diabetes, cardiovascular diseases, neurodegenerative disorders, gastric conditions, chronic kidney disease, or systemic lupus erythematosus at baseline. We made additional baseline exclusions for participants who reported implausible energy intake (<600 or >3500 kcal/d for women and <800 or >4200 kcal/d for men), or who had missing data on weight change or diet change. Weight loss at older ages may reflect loss of lean mass; therefore, we also excluded participants >65 y at baseline. During follow-up, we censored participants 6 y prior to diagnoses of any of the diseases listed. We also censored individuals when they reached 65 y, or had missing data on weight change or diet change over the follow-up period. After baseline (1986) exclusions, there were 54,397 women in the NHS and 33,043 men in the HPFS (Supplemental Figure 1). This study was approved by the institutional review boards of the Harvard T.H. Chan School of Public Health and Brigham and Women's Hospital.
Diet assessment and calculation of the dietary scores
Dietary data were collected every 4 y using a semi-quantitative food frequency questionnaire (FFQ), in which participants reported how often, on average, they had consumed defined portions of ∼130 food items over the previous year (21, 22). We calculated the dietary change scores, based on data from the FFQs, as the difference between the score at the beginning of the 4-y interval and the score at the end of the same 4-y interval.
The empirical dietary index for hyperinsulinemia (EDIH) score, described in detail elsewhere (15, 23, 24), was developed in a sample of 5812 women in the NHS to empirically create a score to measure the insulinemic potential of whole diets defined using food groups. Thirty-nine predefined food groups (servings per day) (25) were entered into stepwise linear regression analyses to identify the dietary pattern most predictive of plasma C-peptide levels as a surrogate marker for insulin secretion (26). The empirical dietary inflammatory pattern (EDIP) score was developed in a sample of 5230 women in the NHS (16). The goal was to empirically create a score for overall inflammatory potential of whole diets defined using food groups. The 39 food groups (25) were entered into reduced rank regression models to simultaneously predict levels of C-reactive protein, IL-6, and TNFα receptor 2. This was followed by stepwise linear regression analyses to identify the dietary pattern most predictive of the 3 plasma inflammation markers (16).
The EDIH is the weighted sum of food groups (n = 18 food groups) that remained statistically significant predictors from the initial 39 food groups; likewise, EDIP scores are weighted sums of the significant predictors for inflammation (n = 18 food groups). The EDIH and EDIP assess the insulinemic or inflammatory potential of a diet on a continuum from maximally low insulinemic or anti-inflammatory to maximally hyperinsulinemic or proinflammatory, respectively. That is, lower (more negative) scores indicate low insulinemic or anti-inflammatory diets and higher (more positive) scores indicate high insulinemic or proinflammatory diets. Both scores were evaluated for validity in independent samples of men and women using dietary and biomarker data from several cohorts (15, 16, 24, 27). The component foods, including serving sizes, for both scores are listed in Supplemental Table 1 and as a footnote in Table 1.
TABLE 1.
Participant lifestyle and dietary characteristics1
| Nurses’ Health Study (n = 54,397) | Health Professionals Follow-up Study (n = 33,043) | |||
|---|---|---|---|---|
| Characteristic | Baseline (1986) | Change within each 4-y period (5th, 95th percentile) | Baseline (1986) | Change within each 4-y period (5th, 95th percentile) |
| Age, y | 57.1 ± 7.3 | — | 54.7 ± 8.1 | — |
| Alcohol, servings/d2 | 0.5 ± 0.9 | −0.001 (−0.58, 0.64) | 1.0 ± 1.2 | 0.04 (−0.98, 1.14) |
| Energy intake, kcal/d | 1760 ± 530 | −3.2 (−707, 699) | 1999 ± 606 | 19 (−762, 814) |
| Physical activity, MET/wk | 14.3 ± 21.9 | 0.25 (−30, 31) | 20.5 ± 28.3 | 2.7 (−46, 57) |
| Sitting and watching TV, h/wk | 14.2 ± 12.9 | — | 11.6 ± 8.6 | — |
| Sleep, h/d | 7.0 ± 1.1 | — | 7.1 ± 0.9 | — |
| Smoking, % | ||||
| Never | 44.2 | — | 45.2 | — |
| Past | 34.1 | — | 44.0 | — |
| Current | 21.6 | — | 10.8 | — |
| Dietary variables, servings/wk | ||||
| Vegetables | 18.5 ± 12.9 | 0.04 (−2.07, 2.16) | 19.3 ± 13.8 | 0.46 (−2.64, 4.00) |
| Fruits | 13.3 ± 9.4 | 0.004 (−1.51, 1.51) | 11.3 ± 9.0 | 0.11 (−1.46, 1.82) |
| Wholegrains | 8.9 ± 8.2 | −0.004 (−1.86, 1.78) | 10.1 ± 9.4 | 0.04 (−2.00, 2.28) |
| Refined grains | 8.3 ± 7.0 | −0.04 (−1.78, 1.58) | 8.5 ± 7.5 | −0.05 (−1.70, 1.48) |
| Red meat | 3.9 ± 2.6 | −0.06 (−0.57, 0.36) | 4.4 ± 3.2 | −0.02 (−0.64, 0.57) |
| Processed meat | 2.0 ± 2.1 | −0.02 (−0.43, 0.29) | 2.7 ± 3.1 | −0.03 (−0.49, 0.37) |
| Outcome-related variables | ||||
| Weight, kg | 68.0 ± 13.2 | 0.99 (−6.8, 9.1) | 81.2 ± 11.8 | 0.82 (−5.4, 6.8) |
| BMI, kg/m2 | 25.4 ± 4.7 | 0.37 (−2.57, 3.29) | 25.5 ± 3.2 | 0.25 (−1.7, 2.2) |
| BMI category (kg/m2), % | ||||
| 15–<25 | 55.7 | — | 45.9 | — |
| ≥25–<30 | 30.1 | — | 45.2 | — |
| ≥30–50 | 14.2 | — | 8.9 | — |
1Data are means (or mean changes) ± SDs for continuous variables, and percentages for categorical variables. The 4-y change data were averaged over the entire follow-up period. The food groups contributing to higher EDIH scores are: red meat, low-energy sugar-sweetened beverages (low-energy cola and other low-energy carbonated beverages), cream soups, processed meat, margarine, poultry, French fries, nondark fish, high-energy sugar-sweetened beverages (cola and other carbonated beverages with sugar, fruit drinks), tomatoes, low-fat dairy, and eggs; the food groups contributing to lower EDIH scores are: intakes of wine, coffee, whole fruit, high-fat dairy products, and green leafy vegetables; EDIP: processed meat, red meat, organ meat, nondark fish, other vegetables (i.e., vegetables other than green leafy vegetables and dark yellow vegetables), refined grains, high-energy beverages (cola and other carbonated beverages with sugar, fruit drinks), low-energy sugar-sweetened beverages (low-energy cola and other low-energy carbonated beverages), and tomatoes were positively related to concentrations of the inflammatory markers. Intakes of beer, wine, tea, coffee, dark-yellow vegetables, green leafy vegetables, snacks, fruit juice, and pizza were inversely related to concentrations of the inflammatory markers. EDIH, empirical dietary index for hyperinsulinemia score; EDIP, empirical dietary inflammatory pattern score; MET, metabolic equivalent task.
2Alcohol servings were defined as 1 bottle, 1 glass, or 1 can of beer, or a 4-oz (113 g) glass red or white wine, and are summed for each individual.
Weight change assessment
Participants reported their height in inches and weight in pounds at baseline, and provided updated information on weight in the biennial questionnaires. These self-reported weight data have been previously validated in these cohorts, with a Spearman correlation coefficient of 0.96 with measured weight (28). Weight change was calculated by subtracting the weight at the start of each 4-y interval from the weight at the end of the 4-y interval.
Assessment of covariates
The biennial questionnaires update information on disease diagnoses, medication use, and several lifestyle factors, including smoking, physical activity, sleep duration, and hours of sitting and TV watching in both cohorts, as previously described (25, 29). Among the women, updated information is also assessed on parity, menopausal status, and postmenopausal hormone use.
Statistical analysis
We adjusted the dietary scores for total energy intake using the residual method (30). The dietary scores are interval variables (i.e., without a true zero), and include both positive and negative score values. Therefore, to conduct diet change analyses, we first converted the dietary indices to nonnegative scores by ranking participants from 1 to n (n = 54,397 for NHS and 33,043 for HPFS), based on the actual intake scores. The nonnegative ranked scores then became the new scores for analyses. The exposure was 4-y changes in dietary scores (EDIH and EDIP) analyzed as a continuous variable (1 SD increment) and as quintiles. The quintiles of the score changes were qualitatively labelled as: quintile 1 = high improvement in diet quality; quintile 2 = moderate improvement; quintile 3 = relatively stable diet; quintile 4 = moderate worsening; and quintile 5 = high worsening of diet quality. We expected participants classified in quintile 3 of the change score to have the least change in their dietary scores compared with the other quintiles; we therefore used quintile 3 as the reference quintile. The outcome was absolute weight change over the same 4-y period.
We used multivariable-adjusted generalized linear regression models with robust variance and an unstructured correlation matrix to examine the associations of 4-y changes in EDIH and EDIP scores with concomitant 4-y weight changes. We had 6 4-y intervals for NHS and HPFS (1986–2010). In each 4-y interval, we constructed 3 statistical models as follows: 1) a minimally adjusted model; 2) a multivariable model with the covariates age, questionnaire cycle, baseline total energy intake, changes in total energy intake, baseline dietary insulinemic or inflammatory potential at the beginning of each 4-y period, basline BMI, baseline physical activity, changes in physical activity, baseline hours of sleep per day, hours of sitting and watching TV per week [baseline only in NHS; and also 4-y change in HPFS], 4-y change in alcohol consumption, 4-y change in smoking status, and among women only: baseline parity, menopausal status, and postmenopausal hormone use ; and 3) in the third model, we used the dietary score that was not adjusted for energy intake and did not include change in energy intake.
To identify which population subgroups may be most affected by changes in the insulinemic and inflammatory potential of the diet in relation to weight change, we examined potential effect modification by BMI (<25 kg/m2, ≥30 kg/m2), physical activity [metabolic equivalent hours per week (MET-h/wk): at or above the cohort-specific median, below the cohort-specific median], age (<55 y, ≥55 y), smoking status (ever smoker, never smoker), and baseline EDIH and EDIP scores (at or above the cohort-specific median, below the cohort-specific median). Among the women, we conducted additional stratified analyses by postmenopausal hormone use (ever, never) in categories of menopausal status (pre-, postmenopausal). All analyses were carried out separately for each cohort. We used Wald P values of the interaction terms to assess interaction of the dietary indices and potential effect modifier. Models were adjusted for all covariates in the primary analyses. When stratifying by BMI, physical activity, age, and baseline dietary insulinemic or inflammatory potential, we adjusted for the continuous variable within strata of the potential effect modifier, e.g., adjusting for continuous BMI among normal weight participants. Analyses were performed using SAS version 9.4 for Unix (SAS Institute Inc.), and statistical significance was set at a 2-tailed P value of <0.05.
Results
Table 1 shows the characteristics of the study population. The mean baseline age for the women was 57.1 y, with 54.7 y for the men. The mean BMI was 25.4 in the women and 25.5 in the men, and the mean physical activity level was 14.3 MET-h/wk among the women and 20.5 MET-h/wk among the men. The proportion of current smokers at baseline was 2 times higher in the women (21.6%) than in the men (10.8%). The mean weight change over each 4-y follow-up cycle was slightly lower among the men (0.82 kg) than among the women (0.99 kg) (Table 1). Supplemental Table 1 shows the constituent foods and serving sizes for the food groups in each dietary score. The EDIH and EDIP scores were moderately correlated, with a Spearman correlation coefficient ranging from 0.65 (men) to 0.71 (women).
Table 2 shows the mean changes in weekly intakes per 4-y cycles over the 24 y of follow-up. Participants who changed to dietary patterns with the lowest insulinemic or inflammatory potential had, on average, lower intakes of red meat, processed meat, sugar-sweetened beverages, and refined grains, and higher intakes of green leafy vegetables, whole grains, whole fruit, wine, coffee, and high-fat dairy. This translated to an average change in macronutrient profile, with higher intake of carbohydrates and fiber and lower intakes of protein (including branched-chain amino acids) and total fat, especially saturated fat. Whereas most of these changes were several times larger for EDIH than for EDIP, some of the changes were more apparent in one index than in the other. For example, although wholegrain is not a component of any of the indices, EDIH seemed to capture the changes in wholegrain intake better than EDIP. In contrast, EDIP captured changes in refined grain intake (an EDIP component) better than EDIH. These differences in grain intake were also reflected in the changes in carbohydrate intake, with higher intake changes for EDIH and little or no change for EDIP.
TABLE 2.
Mean (5th, 95th percentile) changes in the weekly intakes of selected foods and nutrients per 4-y cycle over 24 y, across quintiles of dietary change scores1
| Quintiles of 4-y changes in EDIH scores | Quintiles of 4-y changes in EDIP scores | |||||
|---|---|---|---|---|---|---|
| Food group (servings/wk) or nutrient (g/wk) | Quintile 1 (high dietary improvement) | Quintile 3 (relatively stable diet) | Quintile 5 (high worsening in diet) | Quintile 1 (high dietary improvement) | Quintile 3 (relatively stable diet) | Quintile 5 (high worsening in diet) |
| Nurses’ Health Study (women) | ||||||
| Red meat | −1.2 (−5.0, 5.0) | −0.3 (−3.0, 2.0) | 0.4 (−2.5, 3.5) | −0.7 (−4.6, 2.0) | −0.3 (−3.0, 2.0) | −0.1 (−3.0, 3.0) |
| Processed meat | −0.7 (−4.0, 1.0) | −0.1 (−2.0, 1.5) | 0.4 (−1.5, 3.5) | −0.5 (−3.5, 1.5) | −0.1 (−2.0, 1.5) | 0.2 (−2.0, 3.0) |
| Low-energy sugary beverages | −1.8 (−13.5, 4.0) | 0.3 (−6.5, 4.5) | 0.7 (−5.5, 10.5) | −2.0 (−14.0, 3.0) | −0.4 (−6.0, 4.0) | 0.8 (−5.5, 10.5) |
| High-energy sugary beverages | −1.0 (−7.0, 2.5) | −0.1 (−3.0, 3.0) | 0.7 (−3.0, 6.5) | −1.1 (−7.0, 2.0) | −0.2 (−3.0, 2.5) | 0.9 (−2.9, 7.0) |
| Butter | −1.1 (−10.5, 3.0) | 0.2 (−3.0, 4.0) | 1.4 (-2.5, 12.0) | 0.1 (−5.5, 5.5) | 0.1 (−3.0, 4.0) | 0.3 (−5.0, 6.5) |
| Refined grains | −0.1 (−12.5, 12.5) | −1.1 (−13.9, 10.0) | −0.9 (−13.6, 11.0) | −2.5 (−17.4, 8.5) | −0.7 (−11.0, 8.5) | 1.1 (−10.0, 15.0) |
| Cream soup | −0.2 (−1.0, 0.5) | 0 (−0.5, 0.5) | 0.2 (−0.5, 1.0) | 0 (−0.5, 0.5) | 0 (−.5, 0.5) | 0 (−0.5, 0.5) |
| Green leafy vegetables | 0.7 (−5.5, 7.6) | −0.1 (−5.5, 5.0) | −1.0 (−8.1, 5.0) | 1.2 (−5.0, 8.5) | 0 (−5.0, 5.0) | −1.4 (−9.1, 4.6) |
| Whole fruit | 2.3 (−7.9, 15.1) | 0 (−8.5, 8.5) | −2.1 (−14.1, 8.0) | 0.3 (−11.0, 11.6) | 0.1 (8.5, 8.6) | −0.1 (−11.1, 11.1) |
| Whole grains | 1.2 (−11.5, 15.0) | −0.7 (−13.0, 10.5) | −0.6 (−13.5, 11.5) | 0.7 (−12.5, 14.1) | −0.3 (−11.5, 10.0) | −0.1 (−13.5, 12.5) |
| Wine | 1.1 (−1.0, 6.5) | 0.1 (−2.0, 2.5) | −0.8 (−5.5, 1.5) | 1.0 (−1.0, 6.0) | 0.2 (−1.5, 2.5) | −0.7 (−5.0, 2.0) |
| Coffee | 1.7 (−12.0, 17.5) | −0.8 (−15.0, 10.5) | −4.4 (−21.0, 8.0) | 2.9 (−10.5, 17.5) | −0.6 (−12.0, 10.0) | −5.6 (−24.5, 6.0) |
| High-fat dairy | 1.3 (−6.5, 14.5) | −0.1 (−6.5, 6.0) | −1.6 (−14.6, 6.0) | 0.1 (−8.5, 9.0) | −0.1 (−6.5, 6.0) | −0.1 (−9.0, 8.5) |
| Carbohydrates, g/wk | 80 (−283, 456) | 6.7 (−324, 333) | −64 (−431, 287) | 8.5 (−364, 376) | 2.4 (−333, 339) | 8.0 (−361, 378) |
| Protein, g/wk | −39 (−194, 106) | −10 (−142, 123) | 19 (−123, 166) | −21 (−172, 129) | −8 (−146, 129) | −0.7 (−146, 145) |
| Branched-chain amino acids, g/wk | −9.4 (−42.4, 21.4) | −1.7 (−30.7, 28.2) | 6.4 (−25.3, 39.1) | −4.7 (−37.4, 27.3) | −2.0 (−32.7, 27.9) | 1.3 (−30.7, 33.4) |
| Total fat, g/wk | −24 (−159, 110) | −0.9 (−123, 124) | 22 (−106, 155) | −3.1 (−137, 132) | −1.3 (−126, 125) | 2.1 (−133, 137) |
| Saturated fat, g/wk | −10.9 (−63.2, 40.7) | −3.3 (−49.0, 42.5) | 4.6 (−45.9, 55.3) | −3.9 (−55.3, 46.8) | −3.2 (−51.0, 43.0) | −2.4 (−53.6, 48.8) |
| Polyunsaturated fat, g/wk | 0.2 (−38.0, 41.5) | 2.9 (−33.1, 41.1) | 5.7 (−32.3, 44.9) | 3.6 (−33.6, 44.0) | 2.8 (−33.6, 41.2) | 2.5 (−36.4, 42.0) |
| Fiber, g/wk | 15.1 (−37.1, 73.7) | 5.1 (−43.9, 55.6) | −3.4 (−59.6, 49.3) | 7.9 (−46.3, 65.7) | 5.2 (v44.9, 57.4) | 4.1 (−51.7, 61.0) |
| Health Professionals Follow-up Study (men) | ||||||
| Red meat | −1.3 (−6.0, 2.5) | −0.1 (−4.1, 3.4) | 1.0 (−2.6, 5.9) | −0.7 (−5.5, 3.0) | −0.1 (−4.2, 4.0) | 0.5 (−3.6, 4.9) |
| Processed meat | −0.9 (−5.5, 1.5) | −0.1 (−3.0, 2.6) | 0.7 (−2.0, 4.6) | −0.6 (−4.6, 2.0) | −0.1 (−3.0, 2.6) | 0.3 (−2.6, 4.0) |
| Low-energy sugary beverages | −2.2 (−21, 8.1) | 1.4 (−9.0, 20.0) | 6.4 (−5.6, 41.4) | 0.4 (−15.1, 21.0) | 1.5 (−10.5, 23.5) | 3.3 (−7.0, 28.8) |
| High-energy sugary beverages | −1.1 (−7.8, 3.4) | −0.2 (−6.0, 5.0) | 0.7 (−4.1, 7.0) | −1.4 (−9.1, 3.0) | 0 (5.0, 5.3) | 0.9 (−3.6, 8.1) |
| Butter | −0.7 (−7.0, 3.0) | 0.1 (−3.0, 4.0) | 0.9 (−2.5, 7.0) | 0.1 (−4.0, 4.0) | 0.1 (−3.0, 4.6) | 0.3 (−3.0, 5.0) |
| Refined grains | 0.3 (−10.3, 12.1) | −0.4 (−11.3, 9.8) | −1.4 (−13.9, 8.7) | −2.7 (−16.9, 6.5) | −0.3 (−10.3, 9.7) | 1.5 (−7.6, 15.0) |
| Cream soup | −0.2 (−1.0, 0.6) | 0 (−0.6, 0.6) | 0.2 (−0.6, 1.0) | 0 (−0.6, 0.6) | 0 (−0.6, 0.6) | 0 (−0.6, 0.6) |
| Green leafy vegetables | 0.7 (−5.3, 7.1) | 0.1 (−5.6, 5.6) | −0.6 (−7.1, 5.0) | 1.2 (−4.5, 8.1) | 0 (−5.2, 5.6) | −1.3 (−8.1, 4.5) |
| Whole fruit | 3.4 (−7.5, 18.3) | 0.7 (−10.0, 12.1) | −1.7 (−15.1, 9.5) | 1.1 (−11.0, 14.4) | 0.5 (−10.4, 12.1) | 0.6 (−11.4, 13.2) |
| Whole grains | 1.8 (−11.1, 17.9) | −1.2 (−14.0, 7.9) | −1.0 (−15.5, 11.5) | 0.8 (−13.5, 16.8) | −0.4 (−12.5, 15.1) | −0.1 (−14.9, 14.8) |
| Wine | 1.5 (−1.4, 9.2) | 0.3 (−2.6, 4.1) | −0.8 (−6.0, 2.5) | 1.4 (−1.1, 7.3) | 0.4 (−2.6, 5.0) | −0.8 (−6.0, 2.5) |
| Coffee | 1.0 (−21.5, 21.0) | −3.1 (−24.5, 14.0) | −7.5 (−31.5, 9.6) | −0.1 (v24.5, 18.6) | −3.0 (−24.5, 14.0) | −6.6 (−31.5, 10.5) |
| High-fat dairy | 1.1 (−6.0, 12.3) | −0.1 (−7.0, 6.6) | −1.1 (−12.8, 6.0) | 0 (−7.8, 7.8) | −0.1 (−7.6, 7.5) | −0.1 (8.1, 7.5) |
| Carbohydrates, g/wk | 89 (−345, 532) | −5.4 (−403, 376) | −96 (−543, 316) | −1.5 (−447, 426) | −1.3 (−400, 399) | −5.3 (−450, 430) |
| Protein, g/wk | −55 (−230, 111) | −10 (−164, 146) | 35 (−135, 213) | −26 (−202, 144) | −11 (−174, 148) | 7 (−162, 175) |
| Branched-chain amino acids, g/wk | −6.8 (−35.7, 20.8) | −1.7 (−26.5, 23.6) | 3.2 (−23.9, 31.1) | −3.4 (−32.0, 24.9) | −1.5 (−27.58, 24.0) | −0.3 (−28.1, 27.4) |
| Total fat, g/wk | −28.6 (−186, 129) | 2.6 (−141, 147) | 30.5 (−117, 195) | −4.6 (−165, 158) | −0.4 (−142, 142) | 9.6 (−145, 173) |
| Saturated fat, g/wk | −13.9 (−75.6, 45.2) | −2.3 (−55.9, 48.5) | 8.6 (−49.1, 68.3) | −5.0 (−67.3, 52.6) | −3.1 (−57.8, 50.5) | 0.8 (−56.8, 59.9) |
| Polyunsaturated fat, g/wk | 1.4 (−41.2, 48.0) | 4.4 (−37.7, 50.0) | 5.6 (−37.9, 49.8) | 4.4 (−38.1, 49.6) | 3.4 (−37.6, 47.0) | 3.3 (−40.5, 48.9) |
| Fiber, g/wk | 17.2 (−40.1, 85.1) | 5.4 (−51.3, 64.6) | −6.4 (−72.5, 51.6) | 8.8 (−54.0, 75.0) | 5.1 (−53.2, 61.7) | 3.4 (−60.9, 66.5) |
1Food groups were defined as follows: red meat (beef, pork, lamb, hamburger); processed meat (processed meats, bacon, hot dogs); low-energy beverages (low-energy cola, other low energy carbonated beverages); high-energy beverages (cola with sugar, other carbonated beverages with sugar, fruit punch drinks); cream soup (chowder, cream soups); butter (butter); refined grains (white bread, English muffins, bagels or rolls, muffins or biscuits, white rice, pasta, pancakes or waffles); green leafy vegetables (spinach, iceberg or head lettuce, romaine or leaf lettuce); whole fruit (raisins or grapes, avocado, bananas, cantaloupe, watermelon, fresh apples or pears, oranges, grapefruit, strawberries, blueberries, peaches, apricots, plums); whole grains (cooked oatmeal, other cooked breakfast cereal, dark bread, brown rice, other grains, bran added to food, wheat germ); wine (red wine, white wine); coffee (caffeinated, decaffeinated); high-fat dairy products (whole milk, cream, sour cream, ice cream, cream cheese, other cheese); branched-chain amino acids are the sum of the intakes of isoleucine, leucine, and valine. EDIH, empirical dietary index for hyperinsulinemia score; EDIP, empirical dietary inflammatory pattern score.
Table 3 presents results on the association between changes in the dietary scores and weight change over each 4-y period. Participants who changed their diets toward lower insulinemic or anti-inflammatory dietary patterns experienced less weight gain, whereas those who changed their diets toward hyperinsulinemic or proinflammatory dietary patterns gained more weight, compared with those who made minimal changes to their diets. For example, for the EDIH score, the multivariable-adjusted analyses that also adjusted for energy intake and changes in energy intake showed that women whose dietary changes had the lowest insulinemic potential (classified in quintile 1 of the EDIH change score) gained less weight (−0.65 kg; 95% CI: −0.73, −0.57 kg), whereas women whose dietary changes had the highest insulinemic potential (classified in quintile 5 of the EDIH change score) gained more weight (0.43 kg; 95% CI: 0.36, 0.51 kg; P-trend < 0.0001), than women who had minimal changes to their diets over the 4-y periods (quintile 3). Weight changes associated with dietary inflammatory potential were smaller in magnitude; however, the pattern of results was similar (Table 3). When we compared these results with the weight change estimates not adjusted for total energy intake, the weight change values were similar to, or slightly higher than, the estimates independent of energy intake (Table 3).
TABLE 3.
Multivariable-adjusted results for the associations between changes in dietary insulinemic and inflammatory potential and weight change over 4-y periods1
| Quintiles of 4-y changes in dietary index scores | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index | Group | Sample size in each 4-y period | Quintile 1 (high improvement) | Quintile 2 (moderate improvement) | Quintile 3 (relatively stable) | Quintile 4 (moderate worsening) | Quintile 5 (high worsening) | Per 1 SD increase | P-trend2 |
| Empirical dietary index for hyperinsulinemia score | NHS (women) | 1986–90 | 10,813 | 10,813 | 10,812 | 10,815 | 10,812 | — | |
| 1990–94 | 8410 | 8410 | 8411 | 8409 | 8410 | — | |||
| 1994–98 | 6317 | 6318 | 6317 | 6319 | 6318 | — | |||
| 1998–2002 | 4582 | 4584 | 4582 | 4583 | 4582 | — | |||
| 2002–06 | 2955 | 2957 | 2957 | 2956 | 2956 | — | |||
| 2006–10 | 1633 | 1635 | 1633 | 1634 | 1634 | — | |||
| Mean (95% CI) score change | −19.65 (−19.73, −19.56) | −5.48 (−5.50, −5.45) | −0.40 (−0.39, −0.42) | 4.98 (4.95, 5.00) | 18.62 (18.54, 18.71) | — | |||
| Mean (95% CI) weight change, kg/4 y | 0.75 (0.69, 0.80) | 1.04 (0.99, 1.09) | 1.29 (1.23, 1.34) | 1.34 (1.28, 1.40) | 1.50 (1.45, 1.56) | — | |||
| Minimally adjusted model | −0.52 (−0.59, −0.44) | −0.23 (−0.31, −0.16) | reference | 0.11 (0.03, 0.19) | 0.35 (0.28, 0.43) | 0.31 (0.29, 0.34) | <0.0001 | ||
| MV model | −0.65 (−0.73, −0.57) | −0.29 (−0.37, −0.22) | reference | 0.14 (0.06, 0.21) | 0.43 (0.36, 0.51) | 0.40 (0.38, 0.43) | <0.0001 | ||
| MV model without total energy intake | −0.74 (−0.82, −0.66) | −0.31 (−0.38, −0.23) | reference | 0.17 (0.09, 0.24) | 0.55 (0.47, 0.62) | 0.47 (0.45, 0.50) | <0.0001 | ||
| HPFS (men) | 1986–90 | 4091 | 4093 | 4091 | 4092 | 4091 | — | ||
| 1990–94 | 2554 | 2555 | 2555 | 2555 | 2555 | — | |||
| 1994–98 | 1657 | 1657 | 1658 | 1657 | 1657 | — | |||
| 1998–2002 | 1153 | 1154 | 1154 | 1154 | 1153 | — | |||
| 2002–06 | 754 | 755 | 755 | 754 | 755 | — | |||
| 2006–10 | 491 | 491 | 491 | 491 | 491 | — | |||
| Mean (95% CI) score change | −11.52 (−11.61, −11.44) | −3.59 (−3.62, −3.57) | −0.07 (−0.09, −0.05) | 3.40 (3.38, 3.43) | 11.34 (11.25, 11.43) | — | |||
| Mean (95% CI) weight change, kg/4 y | 0.44 (0.36, 0.53) | 0.73 (0.65, 0.81) | 0.94 (0.86, 1.01) | 1.01 (0.93, 1.09) | 1.20 (1.11, 1.28) | — | |||
| Minimally-adjusted model | −0.47 (−0.58, −0.36) | −0.18 (−0.29, −0.08) | reference | 0.12 (0.05, 0.23) | 0.39 (0.28, 0.50) | 0.29 (0.26, 0.32) | <0.0001 | ||
| MV model | −0.60 (−0.71, −0.49) | −0.25 (−0.36, −0.14) | reference | 0.15 (0.05, 0.26) | 0.49 (0.38, 0.59) | 0.37 (0.34, 0.41) | <0.0001 | ||
| MV model without total energy intake | −0.54 (−0.65, −0.43) | −0.19 (−0.29, −0.08) | reference | 0.27 (0.17, 0.38) | 0.66 (0.55, 0.77) | 0.41 (0.37, 0.44) | <0.0001 | ||
| Empirical dietary inflammatory pattern score | NHS (women) | 1986–90 | 10,813 | 10,813 | 10,814 | 10,812 | 10,813 | — | |
| 1990–94 | 8409 | 8411 | 8410 | 8410 | 8410 | — | |||
| 1994–98 | 6317 | 6318 | 6318 | 6318 | 6318 | — | |||
| 1998–2002 | 4582 | 4583 | 4583 | 4583 | 4582 | — | |||
| 2002–06 | 2956 | 2956 | 2957 | 2956 | 2956 | — | |||
| 2006–10 | 1633 | 1634 | 1634 | 1634 | 1634 | — | |||
| Mean (95% CI) score change | −19.98 (−20.07, −19.89) | −5.52 (−5.54, −5.49) | −0.08 (−0.09, −0.07) | 5.15 (5.13, 5.18) | 19.88 (19.79, 19.97) | — | |||
| Mean (95% CI) weight change, kg/4y | 1.08 (1.02, 1.13) | 1.12 (1.06, 1.17) | 1.28 (1.22, 1.34) | 1.18 (1.13, 1.24) | 1.25 (1.20, 1.31) | — | |||
| Minimally-adjusted model | −0.18 (−0.26, −0.10) | −0.13 (−0.20, −0.05) | reference | −0.06 (−0.13, 0.02) | 0.06 (−0.02, 0.14) | 0.08 (0.05, 0.10) | <0.0001 | ||
| MV model | −0.29 (−0.37, −0.21) | −0.18 (−0.25, −0.10) | reference | −0.02 (−0.10, 0.06) | 0.15 (0.07, 0.23) | 0.15 (0.13, 0.18) | <0.0001 | ||
| MV model without total energy intake | −0.34 (−0.42, −0.27) | −0.22 (−0.30, −0.14) | reference | −0.002 (−0.08, 0.07) | 0.17 (0.09, 0.25) | 0.19 (0.16, 0.21) | <0.0001 | ||
| HPFS (men) | 1986–90 | 4092 | 4091 | 4092 | 4092 | 4091 | — | ||
| 1990–94 | 2554 | 2555 | 2555 | 2556 | 2554 | — | |||
| 1994–98 | 1657 | 1657 | 1658 | 1657 | 1657 | — | |||
| 1998–2002 | 1153 | 1154 | 1153 | 1155 | 1153 | — | |||
| 2002–06 | 754 | 755 | 756 | 754 | 754 | — | |||
| 2006–10 | 491 | 491 | 491 | 491 | 491 | — | |||
| Mean (95% CI) score change | −11.18 (−11.27, −11.09) | −4.82 (−4.87, −4.77) | −1.72 (−1.75, −1.68) | 1.36 (1.33, 1.39) | 7.68 (7.61, 7.76) | — | |||
| Mean (95% CI) weight change, kg/4y | 0.76 (0.68, 0.84) | 0.83 (0.75, 0.91) | 0.88 (0.81, 0.96) | 0.89 (0.81, 0.97) | 0.96 (0.87, 1.04) | — | |||
| Minimally-adjusted model | −0.12 (−0.23, −0.01) | −0.04 (−0.15, 0.06) | reference | 0.01 (−0.10, 0.12) | 0.13 (0.03, 0.24) | 0.08 (0.04, 0.12) | <0.0001 | ||
| MV model | −0.19 (−0.27, −0.07) | −0.08 (−0.19, 0.03) | reference | 0.07 (−0.04, 0.18) | 0.22 (0.11, 0.33) | 0.14 (0.10, 0.19) | <0.0001 | ||
| MV model without total energy intake | −0.22 (−0.33, −0.11) | −0.09 (−0.20, 0.02) | reference | 0.03 (−0.08, 0.14) | 0.21 (0.10, 0.32) | 0.15 (0.10, 0.19) | <0.0001 | ||
1Values are weight change (95% CIs) in kilograms. Mean score change values are in thousands. Minimally adjusted models were adjusted for age (years, continuous), questionnaire cycle (4-y intervals) and baseline energy intake (kcal/d), and 4-y changes in energy intake (kcal/d). Multivariable models were adjusted for age (years, continuous), questionnaire cycle (4-y intervals), baseline total energy intake (kcal/d), changes in total energy intake (kcal/d), baseline dietary insulinemic or inflammatory potential at the beginning of each 4-y period, baseline BMI (kg/m2, continuous), baseline physical activity (MET/wk, continuous), changes in physical activity (MET/wk, continuous), baseline hours of sleep per day (≤6, 7, 8, >8 h), hours of sitting and watching TV per week [baseline only in NHS (0–1, 2–5, 6–20, 21–40, >40 h); and also 4-y change in HPFS (continuous)], 4-y change in alcohol consumption, 4-y change in smoking status (stay never smoker, stay former smoker, stay current smoker, change from former to current smoker, change from never to current smoker, change from current to former smoker), and among women only: baseline parity (0, 1–2, 3, 4+ children), menopausal status, and postmenopausal hormone use (premenopausal, and postmenopausal never, current, past users). Number of participants missing weight change data in HPFS: 1986–90, 797; 1990–94, 7722; 1994–98, 6659; 1998–2002, 3964; 2002–06, 1835; 2006–10, 2704; and in NHS: 1986–90, 751; 1990–94, 3290; 1994–98, 1992; 1998–2002, 3438; 2002–06, 3292; 2006–10, 2280. HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent task; MV, multivariable; NHS, Nurses’ Health Study.
2The P value for trend was the P value of the 1 SD dietary score as a continuous variable in multivariable linear models.
The associations of EDIH and EDIP scores with 4-y weight change were stronger among the women (Figure 1 and Supplemental Table 2) and men (Figure 2 and Supplemental Table 3) who were overweight or obese, younger, less physically active, and never smokers, and among premenopausal women. Among the postmenopausal women, there did not appear to be much difference in weight change by hormone use status (ever versus never). Interestingly, when we stratified by baseline status of dietary insulinemic or inflammatory potential, we found that men and women who were consuming a low insulinemic or anti-inflammatory diet (below the median EDIH or EDIP) at baseline (ie, higher dietary quality) and then changed to diets with greater insulinemic or inflammatory potential gained the largest amount of weight. Similarly, participants who were above the median score at baseline (ie, lower dietary quality) and changed to low insulinemic or anti-inflammatory dietary patterns gained the least amount of weight (Supplemental Tables 2 and 3).
FIGURE 1.
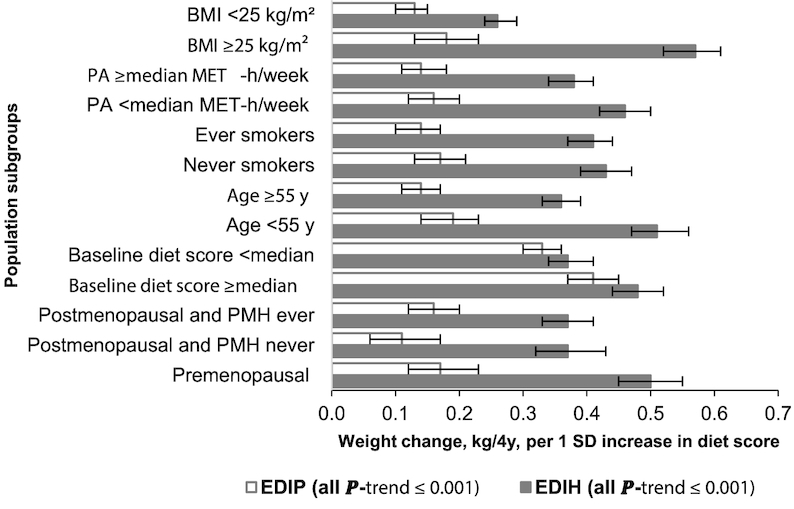
Multivariable-adjusted weight change (kilograms per 4 y) in women for each 1 SD increment in the change in dietary insulinemic (EDIH) and inflammatory (EDIP) potential, stratified by selected characteristics. Values are mean (95% CI) weight change in each 4-y interval. Analyses were adjusted for age, questionnaire cycle, baseline total energy intake, changes in total energy intake, baseline dietary insulinemic or inflammatory potential at the beginning of each 4-y period, baseline BMI, baseline physical activity, changes in physical activity, baseline hours of sleep per day, hours of sitting and watching TV per week (baseline only in NHS), 4-y change in alcohol consumption, 4-y change in smoking status, baseline parity, menopausal status, and postmenopausal hormone use. When stratifying by BMI, physical activity, age, and baseline dietary insulinemic or inflammatory potential, we adjusted for the continuous variable within strata of the potential effect modifier, e.g., adjusting for continuous BMI among normal weight women. The sample sizes in the strata of the potential effect modifiers (listed in order of the 4-y intervals: 1986–90, 1990–94, 1994–98, 1998–2002, 2002–06, 2006–10) were as follows: normal weight (BMI <25 kg/m2): 32,072, 22,343, 14,714, 9804, 5571, 3136; overweight or obese (BMI ≥25): 21,993, 18,195, 16,181, 12,615, 7853, 4460; high physical activity (at or above median, 20.8 MET-h/wk): 27,774, 20,289, 15,895, 11,979, 7791, 4599; low physical activity (below median) 26,291, 21,761, 15,694, 10,934, 6990, 3570; age ≥55 y: 20,927, 20,521, 19,147, 18,412, 14,781, 8169; age <55 y: 33,138, 21,529, 12,442, 4501, no woman was <55 y after 2002; ever smokers: 29,355, 22,631, 16,859, 12,065, 7621, 4143; never smokers: 24,610, 19,280, 14,601, 10,774, 7096, 4005; baseline EDIH score below median score: 27,028, 20,744, 15,400, 11,081, 7140, 4088; baseline EDIH score above or equal to median score: 27,037, 21,306, 16,189, 11,832, 7641, 4081; baseline EDIP score below median score: 27,060, 21,343, 16,155, 11,923, 7902, 4549; baseline EDIP score above or equal to median score: 27,005, 20,707, 15,434, 10,990, 6879, 3602; premenopausal women: 24,686, 15,388, 7706, 2502, 251, 202; postmenopausal and postmenopausal hormone use never: 14,192, 10,625, 7425, 4532, 2746, 1455; postmenopausal and postmenopausal hormone use ever: 13,887, 14,806, 15,764, 15,363, 11,349, 6112. EDIH, empirical dietary index for hyperinsulinemia score; EDIP, empirical dietary inflammatory pattern score; MET-h/wk, metabolic equivalent hours per week.
FIGURE 2.
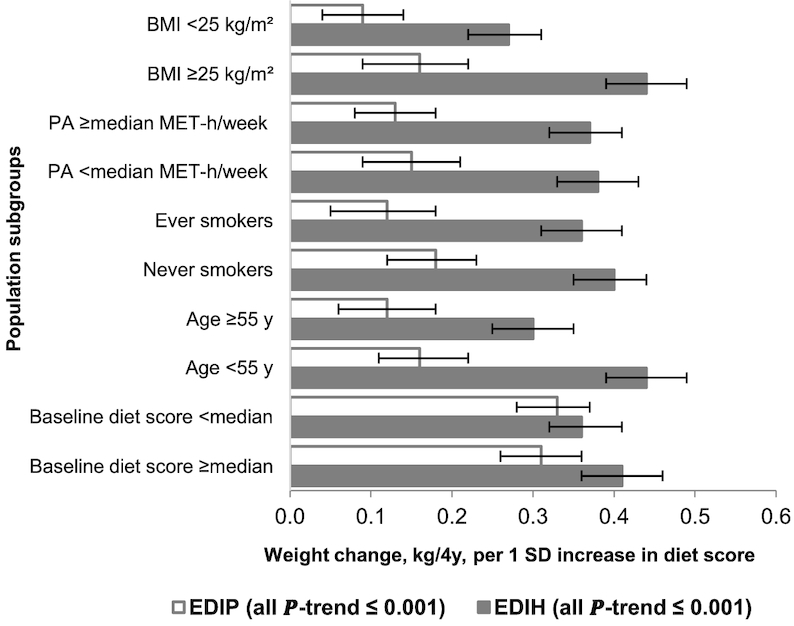
Multivariable-adjusted weight change (kilograms per 4 y) in men for each 1 SD increment in the change in dietary insulinemic (EDIH) and inflammatory (EDIP) potential, stratified by selected characteristics. Values are mean (95% CI) weight change in each 4-y interval. Analyses were adjusted for age, questionnaire cycle, baseline total energy intake, changes in total energy intake, baseline dietary insulinemic or inflammatory potential at the beginning of each 4-y period, baseline BMI, baseline physical activity, changes in physical activity, baseline hours of sleep per day, hours of sitting and watching TV per week (baseline only in NHS), 4-y change in alcohol consumption, and 4-y change in smoking status. When stratifying by BMI, physical activity, age, and baseline dietary insulinemic or inflammatory potential, we adjusted for the continuous variable within strata of the potential effect modifier, e.g., adjusting for continuous physical activity among men with physical activity below the median. The sample sizes in the strata of the potential effect modifiers (listed in order of the 4-y intervals: 1986–90, 1990–94, 1994–98, 1998–2002, 2002–06, 2006–10) were as follows: normal weight (BMI <25 kg/m2): 9855, 5955, 3396, 2251, 1404, 933; overweight or obese (BMI ≥25): 10,603, 6819, 4890, 3517, 2368, 1522; high physical activity (above or equal to median, 20.8 MET-h/wk): 10,240, 6468, 4209, 2926, 1969, 1262; low physical activity (below median): 10,218, 6306, 4077, 2842, 1808, 1163; age ≥55 y: 6734, 4949, 3766, 3571, 3773, 2455; age <55 y :13,724, 7825, 4520, 2156, no man was <55 y after 2002; ever smokers: 9735, 5788, 3599, 2406, 1471, 933; never smokers: 10,000, 6967, 4651, 3343, 2292, 1511; baseline EDIH score below median score: 10,312, 6386, 4146, 3025, 1997, 1238; baseline EDIH score above or equal to median score: 10,146, 6388, 4140, 2743, 1776, 1217; baseline EDIP score below median score: 10,400, 6584, 4328, 3071, 2077, 1367; baseline EDIP score above or equal to median score: 10,058, 6190, 3958, 2697, 1696, 1088. EDIH, empirical dietary index for hyperinsulinemia score; EDIP, empirical dietary inflammatory pattern score; MET-h/wk, metabolic equivalent hours per week.
Discussion
In this large study of men and women who were followed for up to 24 y and had updated weight and dietary data, we investigated the association of changes in the insulinemic and inflammatory potential of the diet and weight change during 4-y periods. Our major findings were the following: first, the men and women who changed and improved their diets the most toward lower insulinemic or inflammatory potential experienced the least long-term weight gain, whereas those who changed toward hyperinsulinemic or proinflammatory dietary patterns experienced the most long-term weight gain. Second, these weight changes remained significant even after accounting for total energy intake, changes in total energy intake, and other energy balance-related variables, including BMI, age, and physical activity. This suggests that diet may influence weight change through mechanisms involving inflammation and insulin response independent of energy intake. Third, the associations of changes in the insulinemic or inflammatory potential of the diet and weight change were stronger among men and women who were overweight or obese, younger, physically less active, and never smokers, and among premenopausal women.
Although the absolute weight change over each 4-y interval may appear small, it is important to note that these are population means, which have been shown to be very linear over 6 4-y intervals (24 y). For example, the pooled estimates of weight gain among men and women who changed to consuming a low insulinemic dietary pattern were 0.60 and 1.35 kg, respectively, among those who changed to consuming the most hyperinsulinemic dietary patterns. This translates to 3.6 and 8.1 kg, respectively, when considered over the entire study period of 24 y. Therefore, changing from consuming the most hyperinsulinemic dietary patterns to the lowest insulinemic patterns could potentially reduce weight gain by more than half (from 8.1 to 3.6 kg). This is important considering that, within a population, some people will gain more and some less weight than the average for the same dietary change. For example, improvement in diet among participants with a poor dietary pattern at baseline, nonsmokers, or those who were overweight or obese was associated with significantly less weight gain compared with some other subgroups.
The 2 indices of dietary insulinemic (EDIH) and inflammatory (EDIP) potential emphasize the benefits of a lower intake of red meat, processed meat, sugar-sweetened beverages, and refined grains, and a higher intake of green leafy vegetables, whole grains, whole fruit, wine, coffee, and high-fat dairy. However, findings for the EDIH were more robust than for the EDIP, which could be related to the fact that, although all the changes in food intake were in the same direction for both indices, the changes were several times larger for the EDIH than for the EDIP. For example, among those who changed toward a low insulinemic or inflammatory dietary pattern, the changes in food intake led to an increase in total dietary fiber intake that was 3 times higher, lower total protein intake that was 2 times higher, or lower total fat intake that was 6–8 times higher for the EDIH than the EDIP. These differences may be partly explained by differences in some foods that are specific to each index. For example, cream soup, margarine, and butter, which increased insulin levels, and whole fruit and high-fat dairy, which reduced insulin secretion, are specific to EDIH, whereas refined grain is specific to EDIP. Some of these specificities may widen the differences between macronutrient profiles, making the EDIH more potent for weight loss than the EDIP.
The EDIH and EDIP scores assess dietary quality based on the insulinemic or inflammatory potential of the diet, respectively. Previous studies have found that higher dietary quality assessed using conventional diet quality indices such as the Dietary Approaches to Stop Hypertension (DASH) index, the Alternative Healthy Eating Index (AHEI-2010), or the Mediterranean Dietary Pattern Score (MED) is associated with less weight gain (2, 17, 31–33). The influence of dietary quality on weight change has, however, been explained mainly on the basis of satiety and imbalances in energy intake and expenditure, and some of these previous studies have therefore not adjusted for energy intake or changes in energy intake (2, 17). Although satiety and energy intake may play a role, our findings demonstrate that the potential of whole diets to contribute to long-term insulin exposure or to chronic systemic inflammation may also be important in weight change.
Divergent relationships between different foods and weight change also highlight the importance of examining overall eating patterns and the gaps in our understanding of the mechanisms of how specific dietary factors may influence weight change. For example, earlier studies of specific dietary factors that found inverse associations between dairy products (e.g., yogurt) and weight gain suggested potential confounding as a possible explanation of the apparently controversial findings (17). However, data have accumulated to support the inverse association of dairy products (including high-fat dairy products) with weight gain or overall health (34, 35). Our 2 hypothesis-oriented indices were created in a completely empirical manner and were based on biomarkers of specific biological pathways. They therefore suggest insulinemia and inflammation as potential underlying pathways that may partly account for why dietary patterns influence weight change. In addition, the empirical hypothesis-oriented dietary patterns have unique features not included in the conventional diets; for example, coffee, tomato sauce, and high-fat dairy are all beneficial factors. Also, in our previous studies in which we applied the empirical hypothesis-oriented scores and the commonly used scores in the same study populations using the same methods, we found the empirical hypothesis-oriented dietary scores to be more robustly associated with risk of developing colorectal cancer in both women and men (23, 36, 37).
There is debate about whether hyperinsulinemia precedes obesity (11). Our subgroup analyses among normal weight and overweight or obese men and women showed stronger associations among those who were overweight or obese. However, the significant associations among normal weight participants, although smaller in magnitude, support the hypothesis that high insulin secretion may be the main initiator of insulin-related weight gain (11). In response to a hyperinsulinemic or proinflammatory diet, glucose is preferentially metabolized as fuel and fat is stored (38), contributing to hyperlipidemia, insulin resistance, and weight gain (11). Dietary improvement among overweight or obese individuals may therefore be associated with greater weight loss than among normal weight individuals. Also, the change in dietary pattern may be dependent on, or sensitive to, the baseline dietary pattern, e.g., those who begin with poor dietary quality and change to a higher dietary quality may experience the least weight gain. Physiological changes associated with aging, such as changes in body composition, decreased physical fitness, and changes in hormones, may contribute to the impairment of insulin secretion and action (18). However, our findings showed that older adults (≥55 y) who improved their diets gained significantly less weight (although smaller in magnitude compared with younger adults). Smoking has also been implicated in weight change. Though smoking cessation has been associated with weight gain (39), cigarette smoking is also associated with insulin resistance, poor pancreatic β cell function and higher risk of type 2 diabetes (40). In the current study, ever smokers who improved their dietary patterns gained significantly less weight.
In the current study, we analyzed the long-term periodic data collected on weight, diet, and other lifestyle factors from 2 prospective cohorts to understand how changes in dietary insulinemic and inflammatory potential relate to weight change at 4-y intervals spanning 24 y. Another strength of our study is that we created empirical hypothesis-oriented dietary patterns based on unbiased associations of foods with biomarkers of insulin response and inflammation, and conducted analyses accounting for total energy intake, to provide insights on potential mechanisms of the associations of whole diets and weight change. In addition, the EDIH directly assesses long-term insulin exposure and may therefore be a more valid instrument than the glycemic index to assess the long-term insulinemic potential of a diet and its consequences on disease outcomes (24). Moreover, research has been inconclusive on the ability of the glycemic index to modify adiposity and body weight (41–43).
Some degree of error in assessment is inevitable in our self-reported data. In particular, even though we adjusted for baseline total energy intake and changes in total energy intake during follow-up, energy intake is not reliably measured through self-reported questionnaires and may therefore not accurately reflect energy balance (44, 45). However, weight change is the best population energy balance metric and partly captures energy intake. In addition, we adjusted for major determinants of energy balance, including age, BMI, and physical activity. Future well-controlled feeding studies may be needed to more accurately determine the role of energy balance. However, isocaloric diets of varying composition produce markedly different metabolic outcomes, indicating that changes in diet composition (or quality) based on the diet's inflammatory or insulinemic potential may influence weight change independent of total energy intake, i.e., the source of the calories matters as well as the amount. In addition, the dietary assessment method used (the FFQ) cannot measure which foods are consumed together, and this may be important in the insulinemic or inflammatory response. For example, if simple carbohydrates are consumed together with high fiber, the insulinemic response may be different than if the simple carbohydrates are consumed in isolation. Residual or unmeasured confounding cannot be completely ruled out in our study; however, we were able to control for several potential confounding factors, including changes in lifestyle factors. It is also possible that the associations reflect reverse causation, but our primary analytic approach excluded participants 6 y prior to the diagnoses of several major chronic diseases.
In summary, our findings support that diet quality may influence weight change in adult men and women through mechanisms involving insulin and inflammatory signaling pathways. Therefore, preventing high insulin secretion and chronic systemic inflammation via nutritional interventions may be a means to reduce overweight and obesity, and dietary patterns with low insulinemic and low inflammatory potential emphasize lower intake of red meat, processed meat, sugar-sweetened beverages, and refined grains, and higher intake of green leafy vegetables, whole grains, whole fruit, wine, coffee, and high-fat dairy.
Supplementary Material
Acknowledgments
We thank the participants and staff of the Nurses’ Health Study and Health Professionals Follow-up Study for their valuable contributions. The authors assume full responsibility for the analyses and interpretation of these data. FKT and ELG had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors’ responsibilities were as follows—FKT and ELG: designed the research; FKT: conducted the research and performed the statistical analysis; FKT, AS, TTF, SKC, and ELG: analyzed and interpreted the data and provided critical input; AS: reviewed the data for accuracy; FKT: wrote the paper; and all authors: read and approved the final content.
Notes
FKT was supported by National Cancer Institute grant #K99CA207736 and R00CA207736. The HPFS and NHS cohorts are supported by NIH grants UM1CA167552 (HPFS) and UM1CA186107 (NHS).
Author disclosures: FKT, AS, TTF, SKC, and ELG, no conflicts of interest.
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: EDIH, empirical dietary index for hyperinsulinemia score; EDIP, empirical dietary inflammatory pattern score; HPFS, Health Professionals Follow-up Study; MET-h/wk, metabolic equivalent hours per week; NHS, Nurses' Health Study.
References
- 1. WHO. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva (Switzerland): World Health Organization; 2009. [Google Scholar]
- 2. Fung TT, Pan A, Hou T, Chiuve SE, Tobias DK, Mozaffarian D, Willett WC, Hu FB. Long-term change in diet quality is associated with body weight change in men and women. J Nutr. 2015;145:1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cespedes Feliciano EM, Tinker L, Manson JE, Allison M, Rohan T, Zaslavsky O, Waring ME, Asao K, Garcia L, Rosal M et al.. Change in dietary patterns and change in waist circumference and DXA trunk fat among postmenopausal women. Obesity. 2016;24:2176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang T, Heianza Y, Sun D, Huang T, Ma W, Rimm EB, Manson JE, Hu FB, Willett WC, Qi L. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ. 2018;360:j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Astley CM, Todd JN, Salem RM, Vedantam S, Ebbeling CB, Huang PL, Ludwig DS, Hirschhorn JN, Florez JC. Genetic evidence that carbohydrate-stimulated insulin secretion leads to obesity. Clin Chem. 2018;64:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out”. JAMA Intern Med. 2018;178:1098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forsythe LK, Wallace JMW, Livingstone MBE. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21:117–33. [DOI] [PubMed] [Google Scholar]
- 8. Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BWJH, Loeser RF, Palla S, Bleecker E, Pahor M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–51. [DOI] [PubMed] [Google Scholar]
- 9. Mason C, Foster-Schubert KE, Imayama I, Kong A, Xiao L, Bain C, Campbell KL, Wang C-Y, Duggan CR, Ulrich CM et al.. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes. 2003;27:S53. [DOI] [PubMed] [Google Scholar]
- 11. Erion KA, Corkey BE.. Hyperinsulinemia: a cause of obesity?. Curr Obes Rep. 2017;6:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tong J, Fujimoto WY, Kahn SE, Weigle DS, McNeely MJ, Leonetti DL, Shofer JB, Boyko EJ. Insulin, C-peptide, and leptin concentrations predict increased visceral adiposity at 5- and 10-year follow-ups in nondiabetic Japanese Americans. Diabetes. 2005;54:985. [DOI] [PubMed] [Google Scholar]
- 13. Morrison JA, Glueck CJ, Wang P. Preteen insulin levels interact with caloric intake to predict increases in obesity at ages 18 to 19 years: a 10-year prospective study of black and white girls. Metabolism. 2010;59:718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lourenço BH, Cardoso MA. C-reactive protein concentration predicts change in body mass index during childhood. PLoS One. 2014;9:e90357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tabung FK, Wang W, Fung TT, Hu FB, Smith-Warner SA, Chavarro JE, Fuchs CS, Willett WC, Giovannucci EL. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br J Nutr. 2016;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, Chan AT, Willett WC, Giovannucci EL. Development and validation of an empirical index of dietary inflammatory potential. J Nutr. 2016;146:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Møller N, Gormsen L, Fuglsang J, Gjedsted J. Effects of ageing on insulin secretion and action. Horm Res. 2003;60(Suppl 1):102–4. [DOI] [PubMed] [Google Scholar]
- 19. Tian J, Venn A, Otahal P, Gall S. The association between quitting smoking and weight gain: a systemic review and meta-analysis of prospective cohort studies. Obes Rev. 2015;16:883–901. [DOI] [PubMed] [Google Scholar]
- 20. Simkin-Silverman LR, Wing RR. Weight gain during menopause. Postgrad Med. 2000;108:47–56. [DOI] [PubMed] [Google Scholar]
- 21. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 22. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185:570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tabung FK, Wang W, Fung TT, Smith-Warner SA, Keum N, Wu K, Fuchs CS, Hu FB, Giovannucci EL. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am J Clin Nutr. 2018;108:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabung FK, Nimptsch K, Giovannucci EL. Postprandial duration influences the association of insulin-related dietary indices and plasma C-peptide concentrations in adult men and women. J Nutr. 2018;Epub ahead of print (doi: 10.1093/jn/nxy239). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food frequency questionnaire. Am J Clin Nutr. 1999;69:243–9. [DOI] [PubMed] [Google Scholar]
- 26. Bonser A, Garcia-Webb P.. C-peptide measurement: methods and clinical utility. Crit Rev Clin Lab Sci. 1984;19:297–352. [DOI] [PubMed] [Google Scholar]
- 27. Tabung FK, Giovannucci EL, Giulianini F, Liang L, Chandler PD, Balasubramanian R, Manson JE, Cespedes Feliciano EM, Hayden KM, Van Horn L et al.. An empirical dietary inflammatory pattern score is associated with circulating inflammatory biomarkers in a multi-ethnic population of postmenopausal women in the United States. J Nutr. 2018;148:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH. Cigarette smoking, relative weight and menopause. Am J Epidemiol. 1983;117:651–8. [DOI] [PubMed] [Google Scholar]
- 29. Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–6. [DOI] [PubMed] [Google Scholar]
- 30. Willett W, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S. [DOI] [PubMed] [Google Scholar]
- 31. Romaguera D, Norat T, Vergnaud A-C, Mouw T, May AM, Agudo A, Buckland G, Slimani N, Rinaldi S, Couto E et al.. Mediterranean dietary patterns and prospective weight change in participants of the EPIC-PANACEA project. Am J Clin Nutr. 2010;92:912–21. [DOI] [PubMed] [Google Scholar]
- 32. Beunza J-J, Toledo E, Hu FB, Bes-Rastrollo M, Serrano-Martínez M, Sánchez-Villegas A, Martínez JA, Martínez-González MA. Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2010;92:1484–93. [DOI] [PubMed] [Google Scholar]
- 33. Buckland G, Bach A, Serra‐Majem L. Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes Rev. 2008;9:582–93. [DOI] [PubMed] [Google Scholar]
- 34. Fogelholm M, Anderssen S, Gunnarsdottir I, Lahti-Koski M. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: a systematic literature review. Food Nutr Res. 2012;56: 10.3402/fnr.v56i0.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, Iqbal R, Gupta R, Lear S, Wentzel-Viljoen E, Avezum A et al.. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet North Am Ed. 2018; 392:(10161):2288–97. [DOI] [PubMed] [Google Scholar]
- 36. Tabung FK, Liu L, Wang W, Fung TT, Wu K, Smith-Warner SA, Cao Y, Hu FB, Ogino S, Fuchs CS et al.. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. 2018;4:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petimar J, Smith-Warner SA, Fung TT, Rosner B, Chan AT, Hu FB, Giovannucci EL, Tabung FK. Recommendation-based dietary indices and risk of colorectal cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Am J Clin Nutr. 2018;108:1092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bessesen DH, Rupp CL, Eckel RH. Dietary fat is shunted away from oxidation, toward storage in obese Zucker Rats. Obes Res. 1995;3:179–89. [DOI] [PubMed] [Google Scholar]
- 39. Bush T, Lovejoy JC, Deprey M, Carpenter KM. The effect of tobacco cessation on weight gain, obesity, and diabetes risk. Obesity (Silver Spring, MD). 2016;24:1834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res. 2017;184:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2013;23:699–706. [DOI] [PubMed] [Google Scholar]
- 42. de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Zazpe I, Vazquez-Ruiz Z, Benito-Corchon S, Bes-Rastrollo M. Glycemic load, glycemic index, bread and incidence of overweight/obesity in a Mediterranean cohort: the SUN project. BMC Public Health. 2014;14:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hall KD, Guyenet SJ, Leibel RL. The carbohydrate-insulin model of obesity is difficult to reconcile with current evidence. JAMA Intern Med. 2018;178(8):1103–5. [DOI] [PubMed] [Google Scholar]
- 44. Willett W. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998:137–8. [Google Scholar]
- 45. Hébert JR, Hurley TG, Steck SE, Miller DR, Tabung FK, Peterson KE, Kushi LH, Frongillo EA. Considering the value of dietary assessment data in informing nutrition-related health policy. Adv Nutr. 2014;5:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


