Abstract
Fungi of the genus Trichoderma are economically important due to their plant growth- and performance-promoting effects, such as improved nutrient supply, mycoparasitism of plant-pathogens and priming of plant defense. Due to their mycotrophic lifestyle, however, they might also be antagonistic to other plant-beneficial fungi, such as mycorrhiza-forming species. Trichoderma spp. release a high diversity of volatile organic compounds (VOCs), which likely play a decisive role in the inter-species communication. It has been shown that Trichoderma VOCs can inhibit growth of some plant pathogens, but their inhibition potentials during early interactions with mutualistic fungi remain unknown. Laccaria bicolor is a common ectomycorrhizal fungus which in symbiotic relationship is well known to facilitate plant performance. Here, we investigated the VOC profiles of three strains of Trichoderma species, Trichoderma harzianum, Trichoderma Hamatum, and Trichoderma velutinum, as well as L. bicolor by stir bar sorptive extraction and gas chromatography – mass spectrometry (SBSE-GC-MS). We further examined the fungal performance and the VOC emission profiles during confrontation of the Trichoderma species with L. bicolor in different co-cultivation scenarios. The VOC profiles of the three Trichoderma species were highly species-dependent. T. harzianum was the strongest VOC emitter with the most diverse compound pattern, followed by T. hamatum and T. velutinum. Co-cultivation of Trichoderma spp. and L. bicolor altered the VOC emission patterns dramatically in some scenarios. The co-cultivations also revealed contact degree-dependent inhibition of one of the fungal partners. Trichoderma growth was at least partially inhibited when sharing the same headspace with L. bicolor. In direct contact between both mycelia, however, L. bicolor growth was impaired, indicating that Trichoderma and L. bicolor apply different effectors when defending their territory. Multivariate analysis demonstrated that all examined individual fungal species in axenic cultures, as well as their co-cultivations were characterized by a distinct VOC emission pattern. The results underline the importance of VOCs in fungal interactions and reveal unexpected adjustability of the VOC emissions according to the specific biotic environments.
Keywords: Trichoderma, volatile organic compounds, fungi–fungi interaction, antagonism, beneficial fungi, ectomycorrhiza, Laccaria bicolor, inhibition
Introduction
Biogenic volatile organic compounds (BVOCs) are a large group of chemically diverse small molecules emitted by plants, microbes, and fungi. Plant VOCs have well described functions in communication, interaction, and defense especially aboveground (Baldwin et al., 2006; Šimpraga et al., 2016). Belowground, the role of VOCs during plant-microbe interactions has only recently been recognized (Penuelas et al., 2014). Due to their volatility and their diffusiveness through air and liquid spaces, VOCs have ideal biophysical properties to function as signaling molecules aboveground but also belowground via pores in the soil matrix (Schulz-Bohm et al., 2017). So far, VOC emission profiles from around 600 microbial and fungal species have been obtained (Schulz-Bohm et al., 2017). Fungi emit a large spectrum of VOCs including acids, alcohols, aldehydes, aromatics, esters, heterocycles, ketones, thiols (Hung et al., 2015), and highly reactive terpenes (Weikl et al., 2016). Terpenes, and particularly SQTs, that fungi are a significant source of, play important roles also in atmospheric chemistry (Riipinen et al., 2012; Bourtsoukidis et al., 2018).
Recently, several research groups made intensive efforts to study the ecological and biological roles of fungal VOCs (Penuelas et al., 2014; Schenkel et al., 2015; Werner et al., 2016). Considering that over 5 million fungal species are predicted to live on earth (Blackwell, 2011), there is tremendous potential to find novel fungal bioactive compounds that are important in belowground interactions (Piechulla and Degenhardt, 2014). Especially the function of volatiles released from plant-beneficial fungi, such as symbiotic mycorrhizal and plant growth-promoting fungi (PGPF) has been of major interest (Morath et al., 2012; Ditengou et al., 2015; Schenkel et al., 2015). Fungal VOCs can aid plants by priming and activation of defense responses (Kishimoto et al., 2006, 2007; van Hulten et al., 2006), providing growth promotion of nearby plants (Morath et al., 2012), as well as by direct growth inhibition of phytopathogens (Strobel et al., 2001).
Trichoderma spp. are well-known PGPF having an ability to compete against pathogenic microbes and to promote plant fitness (Bitas et al., 2013). The genus Trichoderma comprises 254 identified species and 2 varieties (Bissett et al., 2015) which are ubiquitously present in forest and agricultural soils, where they are highly interactive with plant roots and rhizospheric microorganisms (Harman et al., 2004). Studies showed that Trichoderma spp. can act as elicitors promoting plant health by priming against pathogens (Bigirimana et al., 1997; Harman et al., 2004). In addition to the well-recognized induction of systemic and local plant immunity (Verma et al., 2007), Trichoderma species have been demonstrated to act as biological control agents for many soil-borne pathogens including several plant-pathogenic fungi, such as Armillaria, Chondrostereum, Phytophthora, Rhizoctonia, Sclerotinia, Verticillium, and others (Monte, 2001; Contreras-Cornejo et al., 2016).
The genomes of several Trichoderma species have been identified as being rich in genes encoding enzymes responsible for secondary metabolite production, which may contribute to a potential competitive advantage in their biocontrol activities, and of which those encoding for VOCs are an important subset (Kubicek et al., 2011; Mukherjee et al., 2012). Some studies have exploited the VOCs of Trichoderma spp., showing that the emission profiles depend on species/strains, substrate composition, and cultivation environment (Stoppacher et al., 2010; Crutcher et al., 2013). So far, approximately 480 different VOCs have been detected from Trichoderma species altogether. The detected Trichoderma VOCs comprise simple hydrocarbons, heterocycles, aldehydes, ketones, alcohols, phenols, thioalcohols, thioesters, and their derivatives (reviewed by Siddiquee, 2014). Some of these VOCs were shown to be detrimental to plant pathogens, indicating that VOCs may play a role in the biocontrol activity of Trichoderma spp. (Morath et al., 2012; Contreras-Cornejo et al., 2014; Li et al., 2018), and several studies have indicated an inhibitory effect of Trichoderma VOCs on wood decay fungi (Srinivasan et al., 1993; Bruce et al., 1996; Wheatley et al., 1997). In addition, some Trichoderma VOCs were reported to induce plant resistance, (Kottb et al., 2015) and to directly promote plant growth (Hung et al., 2013; Lee et al., 2016, 2019; Nieto-Jacobo et al., 2017).
In the present study, we explored and compared VOC emissions of three commercially relevant Trichoderma species: T. harzianum, T. hamatum, and T. velutinum. So far, no information exists on VOCs of T. hamatum or T. velutinum, whereas the previous studies on T. harzianum VOCs showed high discrepancies (Wheatley et al., 1997; Hung et al., 2013; Siddiquee, 2014; see also discussion). All three species are known mycoparasites on phytopathogenic fungi (Hung et al., 2013; Sharma et al., 2017), but so far, no studies have analyzed their performance in presence of other mutualistic fungi. Laccaria bicolor is an ectomycorrhizal fungus found throughout the temperate zones of the world and forms a symbiosis with several conifer roots (Courty et al., 2009) as well as, e.g., with Populus spp. (Plett et al., 2015). Also, Trichoderma spp. can be associated with Salix spp. and Populus spp. (Wuczkowski et al., 2003). Given their common occurrence and the overall benefits when these fungi are used for growth promotion purposes, their interactions are of interest and warrant a detailed investigation. While a few confrontation studies have been performed in the past (Summerbell, 1987; Rousseau et al., 1996; Werner et al., 2002), the results were mixed and the involvement of VOCs in the interactions between Trichoderma and mycorrhizal fungi is so far completely unknown. Within the present study, we thus examined different confrontation scenarios of the three Trichoderma species with L. bicolor and the involvement of VOCs in these interactions. Our results revealed distinct and species-dependent VOC emission profiles, which were found to be dynamically adjusted when Trichoderma was confronted with L. bicolor. Moreover, the antagonistic activities of the Trichoderma species were likewise unique, indicating a species-specific response to the mycorrhizal co-culture.
Materials and Methods
Fungal Strains and Cultivation
Trichoderma harzianum WM24a1, T. hamatum QL15d1, T. velutinum GL1561, and L. bicolor S238N strains were cultivated in a growth chamber with 23°C and permanent darkness on modified Melin-Norkrans synthetic medium (previously described by Müller et al., 2013). For VOC measurements and confrontation studies, fungal pieces of mycelium were punched out with a cork borer (1 cm diameter) and inoculated in glass Petri dishes (10 cm diameter) containing 40 ml modified Melin-Norkrans synthetic medium.
Experimental Setup and Growth Analysis of the Fungi
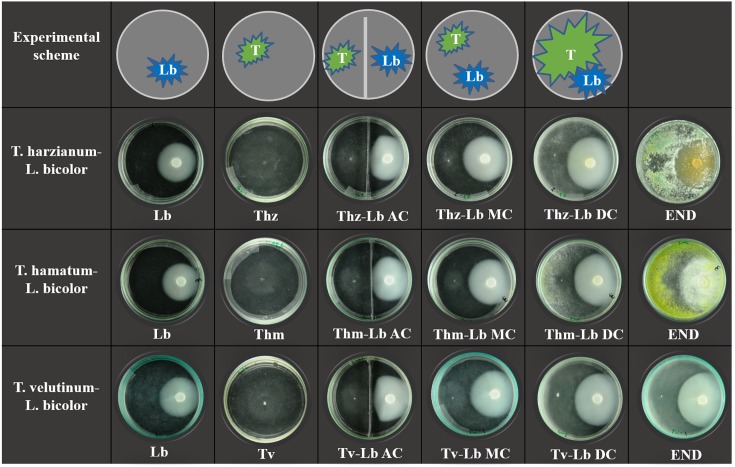
Initially, L. bicolor was inoculated on normal glass Petri dishes (Non-Split, 10 cm diameter) and bi-compartment Petri dishes (10 cm diameter, separated by a metal strip). After 14 days of cultivation (fungal mycelium area at that time point was approximately 15 cm-2), VOCs were collected from L. bicolor alone. Subsequently, the fast-growing T. harzianum, T. hamatum, or T. velutinum mycelia were inoculated onto the same Petri dishes. On normal Petri dishes, the two fungi had contact through solid media (MC) and headspace, whereas in bi-compartment dishes only AC was possible. By the end of the experiment, the MC contact turned to direct physical contact (DC) between the two fungal species. Eight days after Trichoderma inoculation, final pictures were taken with co-cultivations in normal Petri dishes (end). The visualization of the set-up is shown in Figure 1.
FIGURE 1.
Scheme of experimental setup and culture morphology. The top panel shows the experimental scheme where T indicates Trichoderma species, Lb indicates Laccaria bicolor. Three lower panels display the actual morphology of the individual fungus and co-cultures, where Thz, Trichoderma harzianum; Thm, Trichoderma hamatum; Tv, Trichoderma velutinum; Lb, Laccaria bicolor. The degree of contact in co-cultivation conditions in bi-partial Petri dishes is indicated as NC, no contact; AC, airborne contact and in direct confrontation set ups as MC, media contact; DC, direct contact. END indicates the final status of Trichoderma–L. bicolor co-cultures in direct physical contact (9 days after inoculation with Trichoderma species).
Two days post Trichoderma inoculation, VOC collection was performed on all the co-cultivations as well as the individual fungi. When Trichoderma spp. and L. bicolor got into physical contact (3 days post inoculation with Trichoderma), VOCs were collected to examine VOC profiles under DC of the two species. Six replicates of each treatment and control were performed. Control Petri dishes only contained media. Prior to each VOC collection, pictures of fungal mycelium were taken with a Nikon D300 camera (60 mm Nikkor AF-S Micro-Nikkor Lens, Nikon, Tokyo, Japan). The growth inhibition was calculated using the following formula (Raut et al., 2014):
Where D1 denotes the fungal area grown alone and D2 denotes the fungal area in co-cultivations.
Analysis of VOCs
Volatile organic compounds were collected from the headspace of fungal cultures for 16 h at 23°C in the dark by stir bar sorptive extraction (SBSE) technique (Twisters, Gerstel GmbH & Co.KG, Mülheim an der Ruhr, Germany) as previously described (Müller et al., 2013). The twisters were fixed inside the top of the Petri dishes with a magnet placed outside (Weikl et al., 2016). The samples were analyzed by thermo desorption-gas chromatography-mass spectrometry (TD-GC-MS), and VOC analyses followed established procedures (Ghirardo et al., 2012, 2016). The GC-MS parameters followed those given in Weikl et al. (2016) with the following exceptions: the VOCs were desorbed from twisters by changing the temperatures from 37 to 270°C at the rate of 280°C min-1 and holding for 2 min. Before chromatographic separation, samples were cryofocused in the trap of the injection system filled with Tenax TA (Gerstel) at -50°C, following by flash-heating the trap to 270°C at 12°C s-1 and holding for 2 min. The GC temperature program was: 40°C for 0 min followed by ramping at 10°C min-1 to 130°C and hold for 5 min, then 80°C min-1 to 175°C, 2°C min-1 to 200°C, 4°C min-1 to 220°C, 100°C min-1 to 300°C and hold for 6 min. Annotation was performed by comparison of the mass spectra against libraries of reference spectra (NIST 11, Wiley 275) and non-isothermal Kovats retention indices found in literature. Quantification was achieved using response factors calculated using the standards sabinene and α-pinene for MT, linalool for oMT, β-caryophyllene and α-humulene for SQT and geraniol and bornylacetate for oSQT. Other VOCs (oVOCs) were quantified following Kreuzwieser et al. (2014).
Statistics
For the visualization of the VOC patterns, heat map clusters were calculated using an R program (R Core Team, 2013) and the dendextend package (1.0.1) (Galili, 2015). Random forest analysis was performed using the package “randomForest” (Breiman, 2001) and network analysis using the package “qgraph” (Epskamp et al., 2012) (Fruchterman-Reingold algorithm was applied, p < 0.05). Principal component analysis (PCA) of VOCs was performed on SIMCA-P (SIMCA-P v13, Umetrics, Umeå, Sweden). Data was Hellinger transformed to meet the assumption PCA algorithm (Legendre and Legendre, 2012: Müller et al., 2013). First two important principle components were plotted. The bar plot of overall VOC emissions of all treatments and growth inhibition was created by OriginPro 9.0 (OriginLab, Northampton, MA, United States). Significance of growth inhibition was tested by a one-way ANOVA using SPSS (IBM SPSS Statistics 19.0, Duncan’s test, p < 0.05). The mycelium areas of the fungi were measured using ImageJ software1. Evolutionary analyses of fungi were conducted in MEGA7 (Kumar et al., 2016). The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei, 1987). The optimal tree with the branch length sum = 0.78308993 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al., 2004) and are in the units of the number of base substitutions per site. The analysis involved 4 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 455 positions in the final dataset. Data are shown as mean of 6 ± SEM.
Results
Unique Behavior of Selected Trichoderma Species in Co-cultivation With Laccaria bicolor
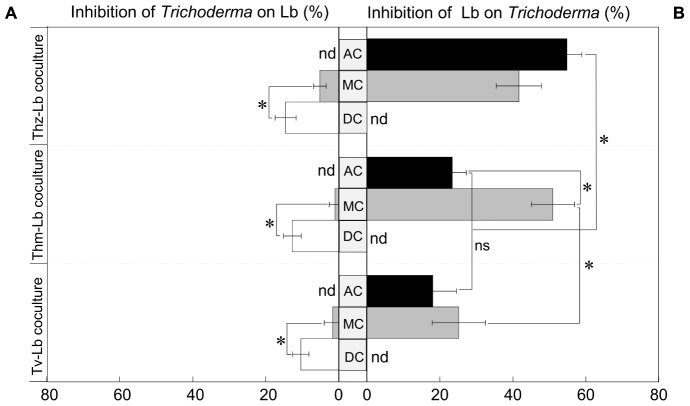
To test the performance of the three selected Trichoderma species in presence of L. bicolor as a non-pathogenic plant mutualistic fungus, we set up a series of direct and indirect confrontation assays (with common head-space) as visualized by representative pictures in Figure 1. Co-cultivations of Trichoderma and L. bicolor revealed that before direct physical contact on the plates, Trichoderma exerted only weak inhibition on L. bicolor (1.61–5.12%) (Figure 2A), whereas L. bicolor exhibited a much stronger inhibitory effect on all Trichoderma species (inhibition rate ranges from 25 to 51%) (Figure 2B). Likewise, also in the co-cultures with only aerial contact (AC), growth of T. harzianum was strongly inhibited by L. bicolor (54.79 ± 4.15%) with somewhat lower inhibition rates of 23.34 ± 3.89% and 18.02 ± 6.48% for T. hamatum and T. velutinum, respectively (Figure 2B). The L. bicolor-mediated inhibition on T. hamatum growth was higher in medium contact (MC) (50.95 ± 5.93%) compared to AC (25.15 ± 7.28%) co-cultures, whereas no differences were found between AC and MC for T. harzianum and T. velutinum (Figure 2B). When Trichoderma and L. bicolor got into direct physical contact (DC stage), the colony area of L. bicolor was significantly (p < 0.05) inhibited by ca. 10.35 ± 2.23% to 14.55 ± 2.88% compared to the MC stage (Figure 2A). By the end of the co-cultivation, T. harzianum and T. hamatum overgrew L. bicolor, whereas T. velutinum inhibited L. bicolor growth less drastically (Figure 1).
FIGURE 2.
Growth inhibition of (A) the three Trichoderma species on Lb and (B) vice versa. Black bar denotes the growth inhibition in air contact (AC) co-cultures, gray bar denotes the growth inhibition in media contact (MC), white bar denotes the growth inhibition in direct contact (DC); nd, not detected. Significances are denoted as asterisks (one-way ANOVA and Duncan’s test, p < 0.05), ns, no significance; mean ± SEM, n = 6.
VOC Emissions From Different Trichoderma Species Are Highly Species-Specific
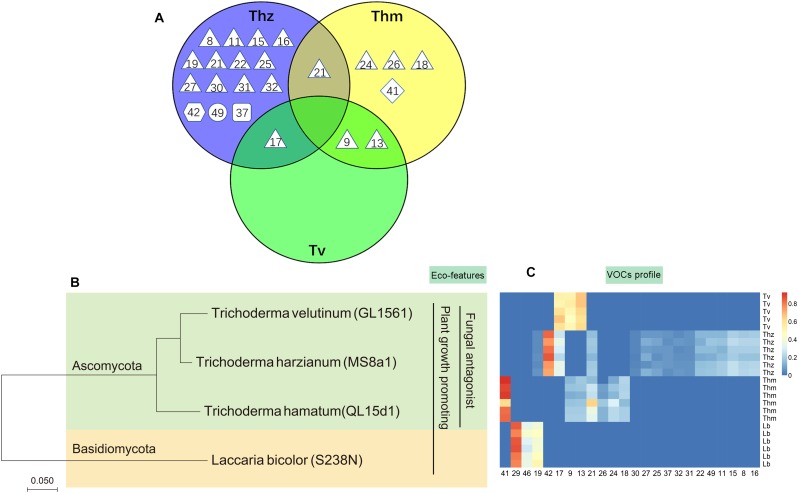
Overall, 16, 7 and 3 individual VOCs could be detected in the T. harzianum, T. hamatum, and T. velutinum emission profiles, respectively (Table 1 and Supplementary Table S1). Total emission rate from T. harzianum was 68.23 ± 7.68 pmol⋅cm-2 h-1, whereas T. hamatum showed a lower emission intensity of 18.18 ± 1.64 pmol⋅cm-2 h-1 and T. velutinum of 1.60 ± 0.20 pmol⋅cm-2 h-1 (Table 1). Surprisingly, the three Trichoderma species shared no common volatile compound and thus exhibited an extremely species-dependent VOC emission pattern (Figure 3).
Table 1.
Volatile organic compounds (VOCs) emitted by T. harzianum (Thz), T. hamatum (Thm), T. velutinum (Tv), mean ± SEM, n = 6; nd, not detected.
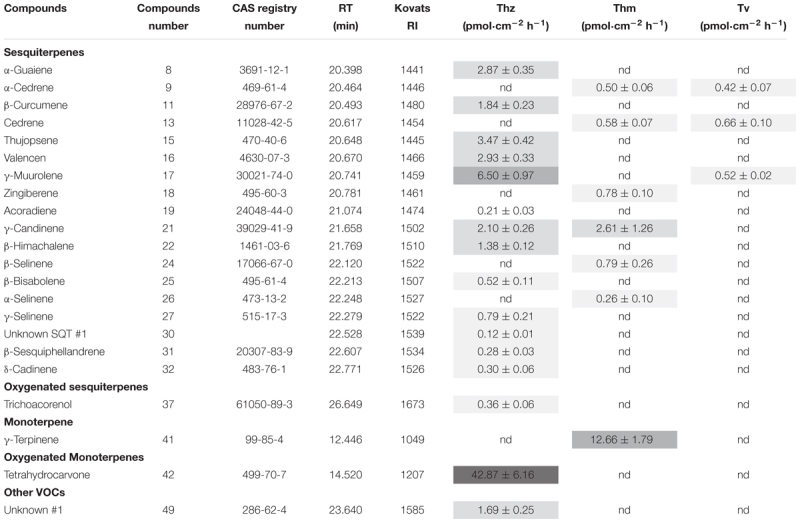 |
Colors indicate VOC emission strengths; dark gray: very high (>26 pmol⋅cm-2 h-1), medium gray: high (6–13 pmol⋅cm-2 h-1), gray: medium (1–5 pmol⋅cm-2 h-1), light gray: low (0–1 pmol⋅cm-2 h-1).
FIGURE 3.
Comparison of volatile organic compound profiles of T. harzianum (Thz), T. hamatum (Thm), and T. velutinum (Tv), and evolutionary relationship of the taxa. (A) Venn diagram depicting the VOC profiles of the Trichoderma species. Shape of the legend: diamond: monoterpene; hexagon: oxygenated monoterpene; triangle: sesquiterpene, square: oxygenated sesquiterpene; circle: other VOCs. The numbers refer to the compounds listed in Table 1. (B) Evolutionary relationships of taxa presented in a phylogenetic tree. The evolutionary history was inferred using the neighbor-joining method (Saitou and Nei, 1987). (C) Heat map analysis of the VOCs from the Trichoderma species. Data were Hellinger-transformed prior to heat map analysis. Compound numbers refer to Supplementary Table S2.
The VOCs of T. harzianum encompassed 13 SQTs and one major oxygenated sesquiterpene (oSQTs), which contributed to ca. 35% of the total emission. The emission profile of this species was further dominated by the oxygenated monoterpene (oMT) tetrahydrocarvone, which amounted up to 62.05 ± 0.04% of the total emission (Table 1). Of the 16 emitted VOCs from T. harzianum, 14 VOCs were unique compared to the two other Trichoderma species (Figure 3A). The most abundant compounds of T. hamatum were the MT γ-terpinene and the SQT γ-candinene with ca. 70 and 14% of the total emission, respectively. These compounds were not emitted by the two other Trichoderma species (Table 1 and Figure 3C).
Trichoderma velutinum emitted quantitatively and qualitatively the lowest number of VOCs compared to the two other Trichoderma species, and its emission pattern contained no unique compound compared to the other two species (Table 1 and Figure 3A). No significant differences were detected in the fungal area of the three Trichoderma species (the mean fungal area being 11.7 ± 0.78 cm2), and the VOC emissions were normalized to fungal area. Thus, the large differences in VOC emission we observed here among the Trichoderma species are unlikely to derive from species-specific growth performance or related fungal area density.
Different Contact Scenarios of Trichoderma spp. and Laccaria bicolor Trigger Changing Emission Profiles
Compared to axenic cultures, different co-cultivation scenarios were found to induce specific changes in the fungal VOC emissions. Already AC between the Trichoderma species and L. bicolor was sufficient to trigger some changes in the overall emission profile. Four new compounds [α-selinene, limonene, cyclohexane, 1,2,4-tris(methylene)-], were detected in AC co-culture of T. harzianum and L. bicolor, whereas T. harzianum-derived trichoacorenol could no longer be detected (Supplementary Figure S1B). Likewise, when T. velutinum and L. bicolor were grown together in AC, the two T. velutinum-originating SQTs α-cedrene and cedrene as well as (+)-cuparene from L. bicolor were now absent. In contrast, one new MT, limonene, was detected (Supplementary Figure S1C). Notably, the same SQT compounds (i.e., α-cedrene and cedrene) disappeared from the emission profile of T. hamatum when it was in AC with L. bicolor, as well as all compounds from the axenic emission profile of L. bicolor itself (Supplementary Figure S1).
Compared to AC, co-culturing in medium contact (MC) could additionally influence the VOC emissions by nutrient depletion and via communication through soluble secondary metabolites. The volatile profiles of the AC and MC co-cultured Trichoderma–L. bicolor mycelia were, however, nearly the same: As a new compound, the MT limonene was found in the emission profile of AC-cultured T. harzianum and AC-cultured T. velutinum, whereas this compound was not detected in MC cultivation. The two SQTs α-cedrene and cedrene originally emitted by T. velutinum and T. hamatum alone, where neither observed during AC nor during MC co-cultivation.
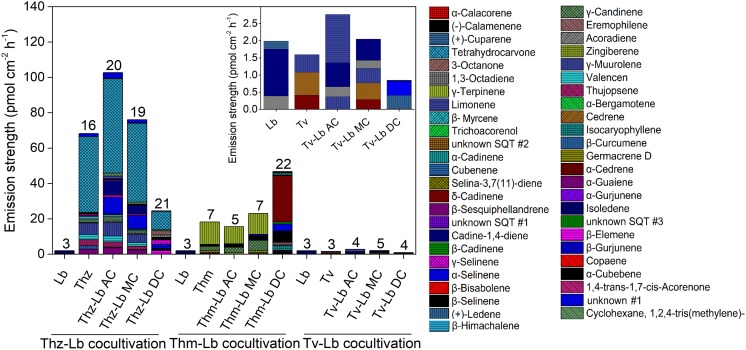
Direct contact between the fungi may induce specific communication- and defense-related signals within the fungi, which might also manifest themselves in the fungal emissions. Thus, we further compared the direct physical contact (DC) emission profiles to the AC and MC scenarios (Figure 4, 5). Considering the emission rates, the Trichoderma species behaved contrary to each other. For T. harzianum, a dramatic decrease for all VOC concentrations was measured in DC with L. bicolor, except for γ-selinene, which increased notably (Figure 5). Indeed, almost all the common compounds between AC and MC pattern had a higher emission rate in AC than in MC, and almost all the compounds common between DC and MC showed a higher emission rate in MC than in DC in the T. harzianum–L. bicolor co-culture. Though the emission intensity in T. harzianum–L. bicolor co-cultivation decreased and was only around one fourth of that measured in AC co-cultures, 21 compounds were detected in DC (Figure 5); 7 of them being new and unique for the DC confrontation scenario (Figure 4). Compared to axenic cultures of the individual fungi, a drastic change in T. harzianum–L. bicolor VOC profile was observed: emissions of 7 compounds originally detected from T. harzianum were absent (thujopsene, β-himachalene, β-bisabolene, β-sesquiphellandrene, δ-cadinene and unknown #1), while 10 new compounds were detected [β-elemene, α-bergamotene, α -selinene, selina-3,7(11)-diene, unknown SQT #2, 1,4-trans-1,7-cis-acorenone, limonene, 1,3-octadiene, 3-octanone, cyclohexane,1,2,4-tris(methylene)-] (Supplementary Figure S1A).
FIGURE 4.
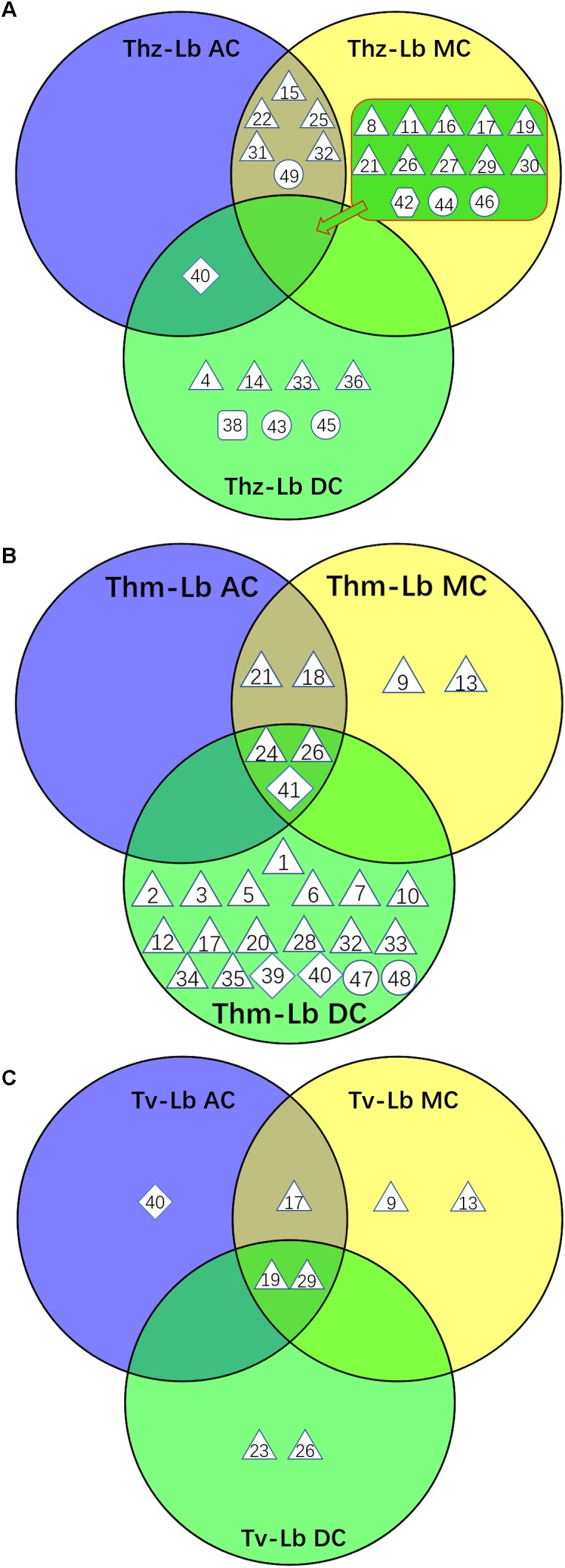
Venn diagram depicting the VOC profiles from the co-cultures of three Trichoderma species with L. bicolor. (A) VOCs from three T. harzianum – L. bicolor co-cultures, (B) VOCs from three T. hamatum – L. bicolor co-cultures, (C) VOCs from three T. velutinum – L. bicolor co-cultures. Shape legend: diamond: monoterpene; hexagon; oxygenated monoterpene; triangle: sesquiterpene, square: oxygenated sesquiterpene; circle: other VOCs. The numbers refer to the compounds listed in Supplementary Table S2.
FIGURE 5.
The volatile organic compound profiles of T. harzianum (Thz), T. hamatum (Thm), T. velutinum (Tv), and L. bicolor (Lb) alone and each Trichoderma strain in different co-cultivation set-ups with Lb. For further information on the abbreviations please see Figure 1. The numbers on the bars indicate the total individual VOCs detected. Insert: Magnification of the VOC profile of Tv and sole Lb culture. Emission rates of each compound calculated based on the fungal area and collection duration of VOCs; values are means of 6 replicates.
In contrast to T. harzianum, the overall T. hamatum emission rate notably increased in DC contact compared to other co-cultivation scenarios. By far the highest compound diversity was, moreover, detected in DC between both mycelia (22 compounds) compared to AC or MC (5 and 7 compounds, respectively) (Figure 4, 5). Of the 22 compounds detected in DC, 19 were new, while 4 of the 5 compounds originally detected in AC cultures were absent (Supplementary Figure S1A). Compared to MC cultures, three SQTs (β-selinene, α-selinene, and δ-cadinene) showed ca. 2.5-, 5-, and 26-fold concentration increase, respectively, in DC co-cultivation. In contrast, the MT γ-terpinene decreased from 11.79 pmol⋅cm-2 h-1 in MC to almost zero in DC co-cultivation.
In the context of T. velutinum and L. bicolor co-culture, 2 out of 5 detected SQTs [α-selinene and (+)-ledene] were specific for the DC culture. Moreover, three SQTs (α-cedrene, cedrene, and γ-muurolene) originally from T. velutinum were absent, and the emission of 2 compounds (acoradiene, and cadine-1,4-diene) (Figure 5 and Supplementary Figure S1C) was strongly decreased.
VOC-Based Characterization of Axenic and Co-cultured Trichoderma Species and Laccaria bicolor
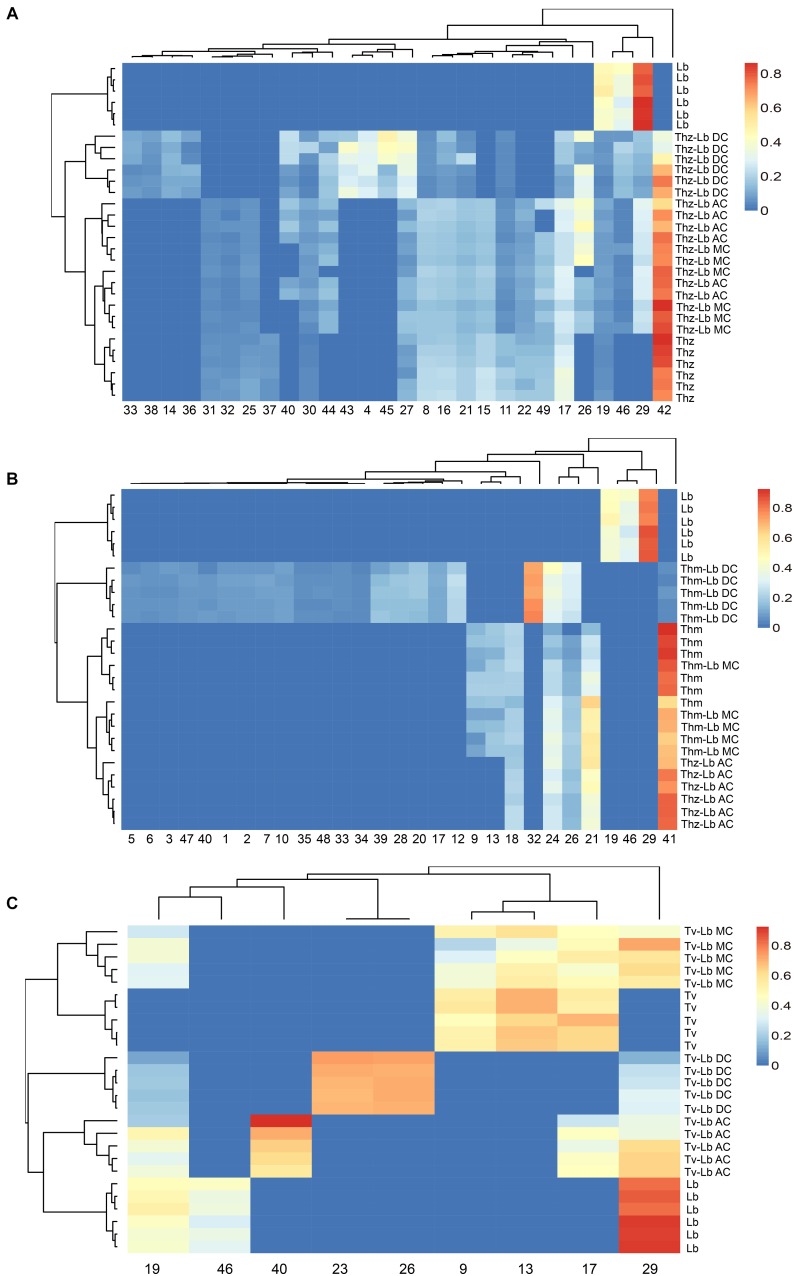
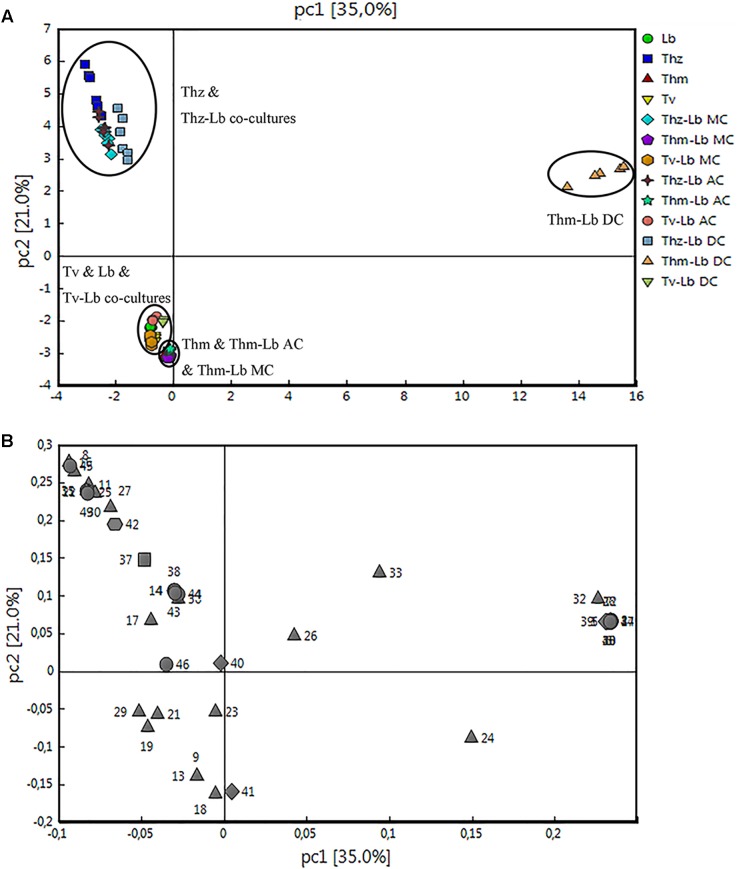
Hierarchical clustering (heat maps in Figure 6A–C) and a principal component analysis (PCA; Figure 7A,B) revealed a clear separation of the VOC profiles of individual Trichoderma species, L. bicolor and their interactions. PCA, for example, highlighted particularly the differences between the Trichoderma species (Figure 7A), while the heat maps clearly show that also the interactions of Trichoderma spp. with L. bicolor resulted in unique emission patterns, making it possible to separate the different confrontation scenarios according to the VOC profiles (Figure 6A–C). For T. velutinum and T. hamatum, the emission patterns were specific for each co-culture (AC, MC, and DC; Figure 6B,C) with the T. hamatum–L. bicolor DC condition clearly diverging the most from the others (see also separate cluster in PCA in Figure 7A). Also T. harzianum and L. bicolor DC co-culture was separated from the others, although a clear separation could not be detected between AC or MC (Figure 6A). Random forest analysis of the VOC emissions proposes a relative contribution of different compounds for separating groups (Supplementary Figure S2). In the co-cultivation of T. harzianum and L. bicolor, the MT limonene was the most important volatile accounting for the separation, whereas the same compound was rather inconsequential in the T. hamatum–L. bicolor interaction. The SQT cadine-1,4-diene, on the other hand, explained much of the differentiation in both T. harzianum–L. bicolor and T. velutinum–L. bicolor interactions (Supplementary Figure S2).
FIGURE 6.
Heat map clustering of VOC profiles from T. harzianum (Thz), T. hamatum (Thm), T. velutinum (Tv), L. bicolor (Lb), and Trichoderma- Lb co-cultures. (A) VOC profiles of Thz, Lb and their co-cultures; (B) VOC profiles of Thm, Lb and their co-cultures; (C) VOC profiles of Tv, Lb and their co-cultures. Data were Hellinger-transformed prior to clustering. NC, no contact; AC, airborne contact; MC, media contact; DC, direct contact. Compound numbers refer to Supplementary Table S2.
FIGURE 7.
Principal component analysis of the volatile organic compound emission patterns released from T. harzianum (Thz), T. hamatum (Thm), and T. velutinum (Tv) co-cultivated with L. bicolor (Lb). The scores (A) of the first two principal components (PC 1 and PC2) for the different species and co-cultivations are presented with the loadings (B). The detected compounds were divided into five different chemical groups (loading plot): diamond, monoterpene; hexagon, oxygenated monoterpene; triangle, sesquiterpene; square, oxygenated sesquiterpenes; circle, other VOCs.
The correlations of individual compounds are visualized in a correlation network (Supplementary Figure S3). In the interaction of T. harzianum and L. bicolor, all the compounds that were specific for direct physical contact displayed a positive correlation (p < 0.05). Most of the compounds shared by AC and MC co-cultures correlated positively as well, except for the SQTs γ-selinene and (+)-cuparene, that showed negative correlation. The compounds detected in the DC co-cultivation were negatively correlated with the compounds detected in AC and MC co-cultures. Only the SQTs γ-selinene and (+)-cuparene did not follow this pattern (Supplementary Figure S3A).
In the T. hamatum–L. bicolor co-cultivation, the compounds showed three groups of positive correlation (p < 0.05) (compounds from L. bicolor, compounds shared with AC and MC co-culture, and compounds specific for DC co-culture). However, these three clusters of compounds were negatively correlated to each other. The two SQTs β-selinene and α-selinene, which were detected in both, AC and MC, co-cultures, showed a positive correlation with DC-specific compounds but negative correlation with compounds in their own group (Supplementary Figure S3B). In the co-cultivations of T. velutinum and L. bicolor, the compounds also correlated in three groups (compounds from L. bicolor, compounds specific for MC co-culture and compounds specific for DC co-culture). In between each of these groups, the correlation was negative in all cases (Supplementary Figure S3C).
Discussion
Considering the enormous number of Trichoderma species and strains known to date, the VOC profiles of only a very limited number of fungi have been explored so far. We investigated the VOC emissions of three Trichoderma species, of which T. harzianum was the strongest emitter, followed by T. hamatum and T. velutinum. The VOC profiles of the three species were highly species-dependent and dominated by SQTs. With respect to T. harzianum, the abundance of SQTs was surprisingly high, as thus far only a few SQTs have been reported from this species (Lee et al., 2016; Li et al., 2018). Previous studies have shown, though, that the VOC profiles of different T. harzianum strains can vary tremendously (Nemcovic et al., 2008; Siddiquee et al., 2012; Lee et al., 2016; Nieto-Jacobo et al., 2017). In the study of Lee et al. (2016), T. harzianum CBS 227.95 emitted 27 VOCs, whereas T. harzianum CBS 226.95 only emitted 3 VOCs. Astonishingly, Siddiquee et al. (2012) detected even 278 VOCs in the T. harzianum strain FA1132. Apart from the genetic differences between strains, the variations in VOC patterns could also be due to discrepancies in nutrient availability (Nieto-Jacobo et al., 2017; Gonzáles-Pérez et al., 2018), developmental stage of the fungi (Weikl et al., 2016), and technique applied during VOC collection and analysis. In the present study, Melin-Norkrans synthetic growth medium was chosen as nutrient source based on previous experience. Müller et al. (2013), who screened several soil fungi, including Trichoderma spp. and L. bicolor, for their volatiles initially chose this medium based mainly on two criteria: (1) to avoid high background volatiles that could disturb the VOC analyses, and (2) to use a common medium for all tested strains for better comparability. The use of a common medium, despite being sub-optimal for some strains (Supplementary Figure S4), is essential when aiming to compare the VOC profiles across species (Polizzi et al., 2012). Nonetheless, if other non-chemotaxonomic aims are of interest, also screening of fungi under varying environmental conditions, including different media and in contact with other organisms, seems to be essential to reveal the whole VOC emission potential of fungal species.
So far, only scattered information exists on the VOCs of T. hamatum, and the VOC profiles of T. velutinum are completely uninvestigated. T. velutinum is a relatively new taxon of Trichoderma identified only in 2003 (Bissett et al., 2003). While this species has been reported to have good biocontrol potential (Matarese et al., 2012; Sharma et al., 2017), our results identified T. velutinum as a comparably low SQT emitter. Only few (3) SQTs at low emission rates were detected from axenic cultures of T. velutinum. For T. hamatum, 17 different VOCs have been previously reported (Siddiquee, 2014). In the present study, we detected seven VOCs composed of six SQTs and one MT, none of which having been reported previously from T. hamatum. Altogether therefore, 24 compounds are now reported from axenic cultures of T. hamatum. Notably, testing different strains of one species thus also appears to be a promising strategy to identify a more complete set of secondary metabolites this species is able to generate and thus to potentially activate otherwise silent genes or gene clusters.
Volatile organic compounds, and in particular SQTs, are able to quickly move through pores in the soil matrix and thus have the potential to mediate belowground, long-distance chemical signaling (Penuelas et al., 2014). Accordingly, several studies suggest that microbial VOCs are not a metabolic waste, but important signaling compounds in the soil (Schmidt et al., 2016, 2017; Weisskopf et al., 2016). Due to the biocontrol properties of Trichoderma spp., the functions of VOCs in Trichoderma interactions have been tested mostly in the context of controlling fungal pathogens of plants. Provided that Trichoderma spp. are antagonistic also against plant-beneficial fungi or other beneficial organisms in the soil, the benefit of commercial applications of Trichoderma spp. in forestry or agricultural practice might vary depending on the microbial communities already present at the site. Our study revealed that the outcome of the interaction between the three studied Trichoderma species and the ectomycorrhizal fungus L. bicolor depends strongly on the degree and type of contact. Surprisingly, we found that over longer distances, L. bicolor exerted more negative impact on the growth of Trichoderma than vice versa. As the growth inhibition of Laccaria on T. harzianum or T. velutinum did not differ between AC and MC cultivation, these results indicate that VOCs are important effectors in inhibiting Trichoderma growth over longer distances via airborne signaling. One or several of the detected three SQTs [acoradiene, cadine-1,4-diene and/or (+)-cuparene] might therefore be biologically active, even though also other undetected compounds might be involved. As the growth of Laccaria was only marginally affected at the same time, the VOCs emitted by Trichoderma at that stage probably have no or only a weak role in the antagonism against L. bicolor. The fact that L. bicolor deployed stronger inhibition on T. hamatum in MC compared to AC culture suggests the additional involvement of soluble compounds as effectors toward T. hamatum. However, the observed growth inhibition might also be due to competition of important nutrients. Interestingly, DC between Trichoderma species and L. bicolor resulted in an opposing outcome, as all the Trichoderma species showed antagonism to L. bicolor in this scenario. T. hamatum and T. harzianum visibly overgrew L. bicolor, whereas the antagonism of T. velutinum was milder. These observations indicate that Trichoderma antagonism is most effective at close distance, probably by involving enzyme-coupled attacks (such as by proteases and chitinases).
In addition to the differences in antagonism toward each other, the co-cultivation scenarios also exhibited changing VOC profiles. In the case of T. hamatum, the VOC emission increased with increasing contact degree with L. bicolor. Not only the emission rate but also the diversity of VOCs in the emission blend was enhanced in DC compared to the situation when the fungi were without physical contact. Previously, it had been shown that fungal and microbial VOC emissions strongly depend on the abiotic and biotic environment (Schmidt et al., 2016, 2017). Microbial genomes possess large sets of functionally unknown genes that may be expressed only under specific environmental conditions (Zhou et al., 2004). For example, Schmidt et al. (2017) observed a strongly altered metabolism of the bacterium Serratia plymuthica when in contact with the VOCs from the fungal pathogen Fusarium culmorum. The rare terpene compound sodorifen, for instance, has been only detected form S. plymuthica when exposed to Fusarium volatiles (Schmidt et al., 2017). So far, only few studies addressed the VOCs in co-cultivation of two fungi. Weikl et al. (2016) investigated the VOCs of two in vitro co-cultured plant pathogenic fungi, Alternaria alternata and Fusarium oxysporum. Similar to T. hamatum in our study, the authors showed an increase of total VOC emissions when the two fungi were in DC. In contrast to Weikl et al. (2016), however, our results revealed that the VOC profiles completely changed in DC compared to other confrontation scenarios. For example, the MT γ-terpinene dominated the VOC profile of T. hamatum in all measurements except in DC, in which the compound accounted only for 0.3% of the total VOCs, while the SQT δ-cadinene was now the prevalent VOC. δ-cadinene synthase (CDNS) has been previously studied in Gossypium barbadense plants in which the CDNS activity and gene expression correlated with the resistance against cotton pathogens (Bianchini et al., 1999; Townsend et al., 2005). However, whether δ-cadinene may have a role in Trichoderma defense needs to be investigated in more detail. Similar to δ-cadinene, also isocaryophyllene was detected only when T. hamatum and L. bicolor grew in DC. Isocaryophyllene is an isomer of caryophyllene, whose microbial emission was previously shown to induce growth of lettuce (Minerdi et al., 2011).
In contrast to T. hamatum, more physical contact between T. harzianum or T. velutinum and L. bicolor decreased the overall VOC release. Nevertheless, interesting changes in the VOC profiles of both of these Trichoderma species in DC with L. bicolor appeared when compared to other confrontation scenarios. For example, the synthesis of tetrahydrocarvone, which dominated the emission profile of axenic T. harzianum cultures, was probably suppressed, as its emission decreased to only one fourth. Tetrahydrocarvone is an oxygenated MT and is to our knowledge reported for the first time from fungi. Also the emission of thujopsene, a SQT which was previously shown to induce lateral root growth in Arabidopsis and poplar (Ditengou et al., 2015), was suppressed in DC compared to all other tested growth conditions. In addition to the diminished compounds, DC between T. harzianum and L. bicolor also induced the emission of many new compounds, such as β-elemene, α-selinene, 1,3-octadiene and 3-octanone. Exposure to 3-octanone was previously shown to induce resistance in Arabidopsis against pathogenic bacteria (Naznin et al., 2014). In general, one possible explanation for the lower emission rates might be that the respective fungi rather invest in soluble than volatile compounds upon close-range (physical) contact. Also, nutrient competition between Trichoderma and L. bicolor or uptake and degradation of volatiles by one or both of the fungi might cause a decrease in the apparent VOC emission rates.
Regarding L. bicolor emission profiles, interesting discrepancies between different contact degrees were observed: the emission of (+)-cuparene, a SQT that has been previously shown to possess antimicrobial activity against fungi and bacteria (Ishikawa et al., 2001), was up-regulated when grown in DC with T. harzianum. This SQT, however, completely disappeared when L. bicolor was encountered by T. hamatum or T. velutinum, suggesting a species-specific response of L. bicolor. Alternatively, consumption of the (+)-cuparene by T. hamatum and T. velutinum is also possible. In L. bicolor, the emission of two other SQTs, acoradiene and cadine-1,4-diene were, moreover, significantly down-regulated in all direct confrontation scenarios compared to axenic cultures of Laccaria. However, whether these compounds are responsible for repressing Trichoderma growth in AC contact remains to be elucidated.
Overall, the present study demonstrates that Trichoderma species do not only differ in their emission profiles, but that the volatile emissions are also strongly adjusted according to the biotic environment. Previously Weikl et al. (2016) also showed altered VOC profiles when two fungi, A. alternata and F. oxysporum, grew in different contact degrees with each other. Interestingly, in the present study the strongest emission rates of single volatiles were measured when two beneficial species had highest distance to each other, suggesting ecological importance of VOCs in long-range interactions of plant-beneficial fungi. In particular, L. bicolor-emitted VOCs appear to be involved in the repression of Trichoderma before direct physical contact. Our results therefore verify the notion that fungi are able to regulate their VOC emissions according to the environmental constraints, supporting the hypothesis that fungal VOCs have important ecological functions in microbial interactions.
Multivariate analysis of our results furthermore revealed that different Trichoderma species possess individual VOC patterns, potentially allowing the utilization of VOCs as biomarkers for the identification of fungi (Neerincx et al., 2016). However, our study also demonstrated the complexity and adjustability of the fungal secondary metabolism in different environmental conditions. While this allowed us to differentiate between the three different co-cultivation scenarios, these dynamics also question the general suitability of VOCs as biomarkers in different labs and from different samples etc. Considering the enormous amount of different Trichoderma species (Bissett et al., 2015) as further challenge, a lot of efforts are still needed to understand the ecological function of VOCs from various Trichoderma strains and species.
The presented results reveal new aspects on possible functions of the Trichoderma genus used as biocontrol agent. The antagonistic nature of the here tested Trichoderma is apparently not limited to plant pathogens but may also affect plant-beneficial fungi. Previous studies on beneficial effects of co-inoculation of crop plants with arbuscular mycorrhizal (AM) fungi and Trichoderma spp. suggest varying compatibilities of different beneficial microbial species. For example, T. atroviride showed mycoparasitic behavior toward Gigaspora spp. when co-inoculated on Medicago truncatula plants (Lace et al., 2015). Similarly, Lagos et al. (2018) observed a strong mutual inhibition between Rhizophagus irregularis and Trichoderma viride. In contrast, in the study of Colla et al. (2015), R. irregularis co-inoculated with T. atroviride enhanced crop growth more than each of the microorganisms alone. Also Kabdwal et al. (2019) observed best plant performance when multiple plant-beneficial microorganisms, including T. harzianum and AM fungi, were applied. Since soil-born beneficial microbes play a pivotal role in the functioning of plants by influencing their physiology and development (Mendes et al., 2013), further studies are needed to investigate the possible outcome when a plant is involved in interactions between different beneficial agents, including ectomycorrhizal fungi. Clearly, the beneficial microbiome of the rhizosphere might be affected due to application of commercial biocontrol species (Schulz-Bohm et al., 2018). In our study, T. harzianum and T. hamatum showed antagonistic behavior against L. bicolor in DC, whereas the low VOC emitter T. velutinum was least antagonistic against the mycorrhizal fungus. Still, T. velutinum was already shown to be very effective against several plant-pathogenic microbes (Matarese et al., 2012; Sharma et al., 2017). Should it be able to grow concomitantly with other plant beneficial fungi, the use of this species might be highly advantageous in biocontrol. These results suggest that by choosing the right Trichoderma species for biocontrol purposes, agriculture and forest management could be further optimized.
Author Contributions
YG, JB, J-PS, and MR designed the study. YG performed the experiments and analyzed the data. YG coordinated cultivation and VOC sampling. YG performed GC-MS analysis together with AG and BW. YG and MR wrote the first draft of the manuscript and prepared the figures. All authors contributed to data analysis, interpretation of the findings, and edited and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors sincerely thank Monika Schmoll (AIT Austrian Institute of Technology GmbH, Austria) for the gift of the used Trichoderma strains and for critical reading of the manuscript.
Abbreviations
- AC
airborne contact
- DC
direct contact
- MC
media contact
- MT
monoterpene
- SQT
sesquiterpene
- VOCs
volatile organic compounds
Funding. This work was supported by a China Scholarship Council (CSC) studentship to YG.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00891/full#supplementary-material
References
- Baldwin I. T., Halitschke R., Paschold A., Von Dahl C. C., Preston C. A. (2006). Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 311 812–815. 10.1126/science.1118446 [DOI] [PubMed] [Google Scholar]
- Bianchini G. M., Stipanovic R. D., Bell A. A. (1999). Induction of δ-cadinene synthase and sesquiterpenoid phytoalexins in cotton by Verticillium dahliae. J. Agric. Food Chem. 47 4403–4406. 10.1021/jf990195y [DOI] [PubMed] [Google Scholar]
- Bigirimana J., De Meyer G., Poppe J., Elad Y., Höfte M. (1997). Induction of systemic resistance on bean (Phaseolus vulgaris) by Trichoderma harziamum. Mededelingen van de Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen, Universiteit Gent. 62 1001–1007. [Google Scholar]
- Bissett J., Gams W., Jaklitsch W., Samuels G. J. (2015). Accepted Trichoderma names in the year 2015. IMA Fungus 6 263–295. 10.5598/imafungus.2015.06.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett J., Szakacs G., Nolan C. A., Druzhinina I., Gradinger C., Kubicek C. P. (2003). New species of Trichoderma from Asia. Can. J. Bot. 81 570–586. 10.1139/B03-051 [DOI] [Google Scholar]
- Bitas V., Kim H. S., Bennett J. W., Kang S. (2013). Sniffing on microbes: diverse roles of microbial volatile organic compounds in plant health. Mol. Plant Microbe Interact. 26 835–843. 10.1094/MPMI-10-12-0249-CR [DOI] [PubMed] [Google Scholar]
- Blackwell M. (2011). The Fungi: 1, 2, 3… 5.1 million species?. Am. J. Bot. 98 426–438. 10.3732/ajb.1000298 [DOI] [PubMed] [Google Scholar]
- Bourtsoukidis E., Behrendt T., Yañez-Serrano A. M., Hellén H., Diamantopoulos E., Catão E., et al. (2018). Strong sesquiterpene emissions from Amazonian soils. Nat. Commun. 9:2226. 10.1038/s41467-018-04658-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. (2001). Random forests. Mach. Learn. 45 5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- Bruce A., Kundzewicz A., Wheatley R. (1996). Influence of culture age on the volatile organic compounds produced by Trichoderma aureoviride and associated inhibitory effects on selected wood decay fungi. Mater. Org. 30 79–94. [Google Scholar]
- Colla G., Rouphael Y., Di Mattia E., El-Nakhel C., Cardarelli M. (2015). Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a Biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 95 1706–1715. 10.1002/jsfa.6875 [DOI] [PubMed] [Google Scholar]
- Contreras-Cornejo H. A., Macias-Rodriguez L., Del-Val E., Larsen J. (2016). Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: interactions with plants. FEMS Microbiol. Ecol. 92 fiw036. 10.1093/femsec/fiw036 [DOI] [PubMed] [Google Scholar]
- Contreras-Cornejo H. A., Macias-Rodriguez L., Herrera-Estrella A., Lopez-Bucio J. (2014). The 4-phosphopantetheinyl transferase of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission. Plant Soil 379 261–274. 10.1007/s11104-014-2069-x [DOI] [Google Scholar]
- Courty P.-E., Hoegger P., Kilaru S., Kohler A., Buée M., Garbaye J., et al. (2009). Phylogenetic analysis, genomic organization, and expression analysis of multi-copper oxidases in the ectomycorrhizal basidiomycete Laccaria bicolor. New Phytol. 182 736–750. 10.1111/j.1469-8137.2009.02774.x [DOI] [PubMed] [Google Scholar]
- Crutcher F. K., Parich A., Schuhmacher R., Mukherjee P. K., Zeilinger S., Kenerley C. M. (2013). A putative terpene cyclase, vir4, is responsible for the biosynthesis of volatile terpene compounds in the biocontrol fungus Trichoderma virens. Fungal Genet. Biol. 56 67–77. 10.1016/j.fgb.2013.05.003 [DOI] [PubMed] [Google Scholar]
- Ditengou F. A., Muller A., Rosenkranz M., Felten J., Lasok H., Van Doorn M. M., et al. (2015). Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nat. Commun. 6:6279. 10.1038/ncomms7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S., Cramer A. O. J., Waldorp L. J., Schmittmann V. D., Borsboom D. (2012). qgraph: network visualizations of relationships in psychometric data. J. Stat. Softw. 48 1–18. [Google Scholar]
- Galili T. (2015). dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 31 3718–3720. 10.1093/bioinformatics/btv428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardo A., Heller W., Fladung M., Schnitzler J. P., Schroeder H. (2012). Function of defensive volatiles in pedunculate oak (Quercus robur) is tricked by the moth Tortrix viridana. Plant Cell Environ. 35 2192–2207. 10.1111/j.1365-3040.2012.02545.x [DOI] [PubMed] [Google Scholar]
- Ghirardo A., Xie J. F., Zheng X. H., Wang Y. S., Grote R., Block K., et al. (2016). Urban stress-induced biogenic VOC emissions and SOA-forming potentials in Beijing. Atmospher. Chem. Phys. 16 2901–2920. 10.5194/acp-16-2901-2016 [DOI] [Google Scholar]
- Gonzáles-Pérez E., Ortega-Amaro M. A., Salazar-Badillo F. A., Bautista E., Douterlungne D., Jiménez-Bremont J. F. (2018). The Arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Sci. Rep. 8:16427. 10.1038/s41598-018-34500-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman G. E., Howell C. R., Viterbo A., Chet I., Lorito M. (2004). Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2:43. 10.1038/nrmicro797 [DOI] [PubMed] [Google Scholar]
- Hung R., Lee S., Bennett J. W. (2013). Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecol. 6 19–26. 10.1016/j.funeco.2012.09.005 [DOI] [Google Scholar]
- Hung R., Lee S., Bennett J. W. (2015). Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 99 3395–3405. 10.1007/s00253-015-6494-4 [DOI] [PubMed] [Google Scholar]
- Ishikawa N. K., Fukushi Y., Yamaji K., Tahara S., Takahashi K. (2001). Antimicrobial cuparene-type sesquiterpenes, enokipodins C and D, from a mycelial culture of flammulina v elutipes. J. Nat. Prod. 64 932–934. 10.1021/np000593r [DOI] [PubMed] [Google Scholar]
- Kabdwal B. C., Sharma R., Tewari R., Tewari A. K., Singh R. P., Dandona J. K. (2019). Field efficacy of different combinations of Trichoderma harzianum, Pseudomonas fluorescens, and arbuscular mycorrhiza fungus against the major diseases of tomato in Uttarakhand (India). Egypt J. Biol. Pest Control. 29:1 10.1186/s41938-018-0103-7 [DOI] [Google Scholar]
- Kishimoto K., Matsui K., Ozawa R., Takabayashi J. (2007). Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. J. Gen. Plant Pathol. 73 35–37. 10.1007/s10327-006-0314-8 [DOI] [Google Scholar]
- Kishimoto K., Matsui K., Wawa R., Takabayashi J. (2006). Components of C6-aldehyde-induced resistance in Arabidopsis thaliana against a necrotrophic fungal pathogen. Botrytis Cinerea. Plant Sci. 170 715–723. 10.1016/j.plantsci.2005.11.002 [DOI] [Google Scholar]
- Kottb M., Gigolashvili T., Großkinsky D. K., Piechulla B. (2015). Trichoderma volatiles effecting Arabidopsis: from inhibition to protection against phytopathogenic fungi. Front. Microbiol. 6:995. 10.3389/fmicb.2015.00995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzwieser J., Scheerer U., Kruse J., Burzlaff T., Honsel A., Alfarraj S., et al. (2014). The venus flytrap attracts insects by the release of volatile organic compounds. J. Exp. Bot. 65 755–766. 10.1093/jxb/ert455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek C. P., Herrera-Estrella A., Seidl-Seiboth V., Martinez D. A., Druzhinina I. S., Thon M., et al. (2011). Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 12:1. 10.1186/gb-2011-12-4-r40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lace B., Genre A., Woo S., Faccio A., Lorito M., Bonfante P. (2015). Gate crashing arbuscular mycorrhizas: in vivo imaging shows the extensive colonization of both symbionts by Trichoderma atroviride. Environ. Microbiol. Reports 7 64–77. 10.1111/1758-2229.12221 [DOI] [PubMed] [Google Scholar]
- Lagos C., Larsen J., Correa E. S., Almonacid L., Herrera H., Fuentes A., et al. (2018). Dual inoculation with mycorrhizal and saprotrophic fungi suppress the maize growth and development under phenanthrene exposure. J. Soil Sci. Plant Nutr. 18 721–734. 10.4067/S0718-95162018005002102 [DOI] [Google Scholar]
- Lee S., Behringer G., Hung R., Bennett J. (2019). Effects of fungal volatile organic compounds on Arabidopsis thaliana growth and gene expression. Fungal Ecol. 37 1–9. 10.1016/j.funeco.2018.08.004 [DOI] [Google Scholar]
- Lee S., Yap M., Behringer G., Hung R., Bennett J. W. (2016). Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 3:7. 10.1186/s40694-016-0025-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Legendre L. (2012). Numerical Ecology, 3rd Edn. Amsterdam: Elsevier Science BV press. [Google Scholar]
- Li N., Alfiky A., Wang W., Islam M., Nourollahi K., Liu X., et al. (2018). Volatile compound-mediated recognition and inhibition between Trichoderma biocontrol agents and Fusarium oxysporum. Front. Microbiol. 9:2614. 10.3389/fmicb.2018.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandels M., Andreotti R. E. (1978). Problems and challenges in the cellulose to cellulose fermentation. Process Biochem. 13 6–13. [Google Scholar]
- Matarese F., Sarrocco S., Gruber S., Seidl-Seiboth V., Vannacci G. (2012). Biocontrol of Fusarium head blight: interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 158 98–106. 10.1099/mic.0.052639-0 [DOI] [PubMed] [Google Scholar]
- Mendes R., Garbeva P., Raaijmakers J. M. (2013). The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37 634–663. 10.1111/1574-6976.12028 [DOI] [PubMed] [Google Scholar]
- Minerdi D., Bossi S., Maffei M. E., Gullino M. L., Garibaldi A. (2011). Fusarium oxysporum and its bacterial consortium promote lettuce growth and expansin A5 gene expression through microbial volatile organic compound (MVOC) emission. FEMS Microbiol. Ecol. 76 342–351. 10.1111/j.1574-6941.2011.01051.x [DOI] [PubMed] [Google Scholar]
- Monte E. (2001). Understanding Trichoderma: between biotechnology and microbial ecology. Int. Microbiol. 4 1–4. 10.1007/s101230100001 [DOI] [PubMed] [Google Scholar]
- Morath S. U., Hung R., Bennett J. W. (2012). Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol. Rev. 26 73–83. 10.1016/j.fbr.2012.07.001 [DOI] [Google Scholar]
- Mukherjee P. K., Horwitz B. A., Kenerley C. M. (2012). Secondary metabolism in Trichoderma–a genomic perspective. Microbiology 158 35–45. 10.1099/mic.0.053629-0 [DOI] [PubMed] [Google Scholar]
- Müller A., Faubert P., Hagen M., Zu Castell W., Polle A., Schnitzler J. P., et al. (2013). Volatile profiles of fungi–chemotyping of species and ecological functions. Fungal Genet. Biol. 54 25–33. 10.1016/j.fgb.2013.02.005 [DOI] [PubMed] [Google Scholar]
- Naznin H. A., Kiyohara D., Kimura M., Miyazawa M., Shimizu M., Hyakumachi M. (2014). Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS One 9:e86882. 10.1371/journal.pone.0086882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerincx A. H., Geurts B. P., Habets M. F., Booij J. A., Van Loon J., Jansen J. J., et al. (2016). Identification of Pseudomonas aeruginosa and Aspergillus fumigatus mono- and co-cultures based on volatile biomarker combinations. J. Breath. Res. 10:016002. 10.1088/1752-7155/10/1/016002 [DOI] [PubMed] [Google Scholar]
- Nemcovic M., Jakubikova L., Viden I., Farkas V. (2008). Induction of conidiation by endogenous volatile compounds in Trichoderma spp. FEMS Microbiol. Lett. 284 231–236. 10.1111/j.1574-6968.2008.01202.x [DOI] [PubMed] [Google Scholar]
- Nieto-Jacobo M. F., Steyaert J. M., Salazar-Badillo F. B., Nguyen D. V., Rostás M., Braithwaite M., et al. (2017). Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 8:102. 10.3389/fpls.2017.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuelas J., Asensio D., Tholl D., Wenke K., Rosenkranz M., Piechulla B., et al. (2014). Biogenic volatile emissions from the soil. Plant Cell Environ. 37 1866–1891. 10.1111/pce.12340 [DOI] [PubMed] [Google Scholar]
- Piechulla B., Degenhardt J. (2014). The emerging importance of microbial volatile organic compounds. Plant Cell Environ. 37 811–812. 10.1111/pce.12254 [DOI] [PubMed] [Google Scholar]
- Plett J. M., Tisserant E., Brun A., Morin E., Grigoriev I. V., Kuo A., et al. (2015). The mutualist Laccaria bicolor expresses a core gene regulon during the colonization of diverse host plants and a variable regulon to counteract host-specific defenses. Mol. Plant Microbe Interact. 28 261–273. 10.1094/MPMI-05-14-0129-FI [DOI] [PubMed] [Google Scholar]
- Polizzi V., Adams A., Malysheva S. V., De Sager S., Van Peteghem C., Moretti A., et al. (2012). Identification of volatile markers for indoor fungal growth and chemotaxonomic classification of Aspergillus species. Fungal Biol. 116 941–953. 10.1016/j.funbio.2012.06.001 [DOI] [PubMed] [Google Scholar]
- R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Raut I., Badea-Doni M., Calin M., Oancea F., Vasilescu G., Sesan T. E., et al. (2014). Effect of volatile and non-volatile metabolites from Trichoderma spp. against important phytopathogens. Revista. De. Chimie. 65 1285–1288. 28602402 [Google Scholar]
- Riipinen I., Yli-Juuti T., Pierce J. R., Petaja T., Worsnop D. R., Kulmala M., et al. (2012). The contribution of organics to atmospheric nanoparticle growth. Nat. Geosci. 5 453–458. 10.1038/ngeo1499 [DOI] [Google Scholar]
- Rousseau A., Benhamou N., Chet I., Piche Y. (1996). Mycoparasitism of the extramatrical phase of Glomus intraradices by Trichoderma harzianum. Phytopathology 86 434–443. 10.1094/Phyto-86-434 [DOI] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4 406–425. 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- Schenkel D., Lemfack M. C., Piechulla B., Splivallo R. (2015). A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Front. Plant Sci. 6:707. 10.3389/fpls.2015.00707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Etalo D. W., De Jager V., Gerards S., Zweers H., De Boer W., et al. (2016). Microbial small talk: volatiles in fungal–bacterial interactions. Front. Microbiol. 6:1495 10.3389/fmicb.2015.01495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Jager V., Zuhlke D., Wolff C., Bernhardt J., Cankar K., et al. (2017). Fungal volatile compounds induce production of the secondary metabolite Sodorifen in Serratia plymuthica PRI-2C. Sci. Rep. 7:862. 10.1038/s41598-017-00893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Bohm K., Geisen S., Wubs E. J., Song C., De Boer W., Garbeva P. (2017). The prey’s scent–volatile organic compound mediated interactions between soil bacteria and their protist predators. ISME J. 11:817. 10.1038/ismej.2016.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Bohm K., Gerards S., Hundscheid M., Melenhorst J., De Boer W., Garbeva P. (2018). Calling from distance: attraction of soil bacteria by plant root volatiles. ISME J. 12 1252–1262. 10.1038/s41396-017-0035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Magotra A., Manhas R. S., Chaubey A. (2017). Antagonistic potential of a psychrotrophic fungus: Trichoderma velutinum ACR-P1. Biol. Control. 115 12–17. 10.1016/j.biocontrol.2017.08.024 [DOI] [Google Scholar]
- Siddiquee S. (2014). “Recent advancements on the role and analysis of volatile compounds (VOCs) from Trichoderma,” in Biotechnology and Biology of Trichoderma, eds Gupta V. K., Schmoll M., Herrera-Estrella A., Upadhyay R. S., Druzhinina I., Tuohy M. G. (Cambridge, MA: Elsevier press; ), 139–175. 10.1016/b978-0-444-59576-8.00011-4 [DOI] [Google Scholar]
- Siddiquee S., Cheong B. E., Taslima K., Kausar H., Hasan M. M. (2012). Separation and identification of volatile compounds from liquid cultures of Trichoderma harzianum by GC-MS using three different capillary columns. J. Chromatogr. Sci. 50 358–367. 10.1093/chromsci/bms012 [DOI] [PubMed] [Google Scholar]
- Šimpraga M., Takabayashi J., Holopainen J. K. (2016). Language of plants: where is the word? J. Integr. Plant Biol. 58 343–349. 10.1111/jipb.12447 [DOI] [PubMed] [Google Scholar]
- Srinivasan U., Staines H., Bruce A. (1993). Influence of media type on antagonistic modes of Trichoderma spp. against wood decay basidiomycetes. Mater. Org. 27 301–321. [Google Scholar]
- Stoppacher N., Kluger B., Zeilinger S., Krska R., Schuhmacher R. (2010). Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods. 81 187–193. 10.1016/j.mimet.2010.03.011 [DOI] [PubMed] [Google Scholar]
- Strobel G. A., Dirkse E., Sears J., Markworth C. (2001). Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147 2943–2950. 10.1099/00221287-147-11-2943 [DOI] [PubMed] [Google Scholar]
- Summerbell R. C. (1987). The inhibitory effect of Trichoderma species and other soil microfungi on formation of mycorrhiza by Laccaria bicolor in vitro. New Phytol. 105 437–448. 10.1111/j.1469-8137.1987.tb00881.x [DOI] [PubMed] [Google Scholar]
- Tamura K., Nei M., Kumar S. (2004). Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U.S.A. 101 11030–11035. 10.1073/pnas.0404206101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend B. J., Poole A., Blake C. J., Llewellyn D. J. (2005). Antisense suppression of a (+)-δ-cadinene synthase gene in cotton prevents the induction of this defense response gene during bacterial blight infection but not its constitutive expression. Plant Physiol. 138 516–528. 10.1104/pp.104.056010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hulten M., Pelser M., Van Loon L. C., Pieterse C. M., Ton J. (2006). Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 103 5602–5607. 10.1073/pnas.0510213103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M., Brar S. K., Tyagi R. D., Surampalli R. Y., Valero J. R. (2007). Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochem. Eng. J. 37 1–20. 10.1016/j.bej.2007.05.012 [DOI] [Google Scholar]
- Weikl F., Ghirardo A., Schnitzler J. P., Pritsch K. (2016). Sesquiterpene emissions from Alternaria alternata and Fusarium oxysporum: effects of age, nutrient availability, and co-cultivation. Sci. Rep. 6:22152. 10.1038/srep22152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf L., Ryu C. M., Raaijmakers J. M., Garbeva P. (2016). Smelly fumes: volatile-mediated communication between bacteria and other organisms. Front. Microbiol. 7:2031. 10.3389/fmicb.2016.02031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Zadworny M., Idzikowska K. (2002). Interaction between Laccaria laccata and Trichoderma virens in co-culture and in the rhizosphere of Pinus sylvestris grown in vitro. Mycorrhiza 12 139–145. 10.1007/s00572-002-0159-8 [DOI] [PubMed] [Google Scholar]
- Werner S., Polle A., Brinkmann N. (2016). Belowground communication: impacts of volatile organic compounds (VOCs) from soil fungi on other soil-inhabiting organisms. Appl. Microbiol. Biotechnol. 100 8651–8665. 10.1007/s00253-016-7792-1 [DOI] [PubMed] [Google Scholar]
- Wheatley R., Hackett C., Bruce A., Kundzewicz A. (1997). Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. inhibitory to wood decay fungi. Int. Biodeterior. Biodegrad. 39 199–205. 10.1016/S0964-8305(97)00015-2 [DOI] [Google Scholar]
- Wuczkowski M., Druzhinina I., Gherbawy Y., Klug B., Prillinger H., Kubicek C. P. (2003). Species pattern and genetic diversity of Trichoderma in a mid-European, primeval floodplain-forest. Microbiol. Res. 158 125–133. 10.1078/0944-5013-00193 [DOI] [PubMed] [Google Scholar]
- Zhou J., Thompson D. K., Xu Y., Tiedje J. M. (2004). Microbial Functional Genomics. Michigan: John Wiley & Sons press, 10.1002/0471647527 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.