Abstract
Context
No known previous study has focused on plant-based diet (PBD) to prevent relapse of ulcerative colitis (UC) except our previous educational hospitalization study.
Objective
To describe the relapse rate in a large case series of UC after incorporation of PBD into induction therapy.
Design
All patients with UC between 2003 and 2017 were admitted for induction therapy. Patients receiving educational hospitalization or treated with infliximab were excluded. A lacto-ovo-semivegetarian diet (PBD) together with medication prescribed according to UC guidelines was provided during hospitalization.
Main Outcome Measures
The primary endpoint was relapse during follow-up. The secondary endpoint was change over time in the plant-based diet score (PBDS), which evaluated adherence to the PBD.
Results
Ninety-two cases were studied, of which 51 were initial episodes and 41 were relapses. Cases varied in severity (31 mild, 48 moderate, 13 severe) and extent (15 proctitis, 22 left-sided colitis, 55 extensive colitis). More severe cases existed among the relapse cases than among the initial episode cases. Cumulative relapse rates at 1- and 5-year follow-up (Kaplan-Meier analysis) were 14% and 27%, respectively, for the initial episode cases, and 36% and 53%, respectively, for relapse cases. At long-term follow-up (6 years 4 months), PBDS was significantly higher than baseline PBDS (p < 0.0001).
Conclusion
Relapse rates in UC after induction therapy with PBD were far lower than those previously reported with conventional therapy. Adherence to PBD was significantly higher than baseline even at 6-year follow-up. We conclude PBD is effective for preventing UC relapse.
(Study identification no.: UMIN000019061: Registration: www.umin.ac.jp)
Keywords: Crohn disease, dietary habits, lifestyle medicine, plant-based diet, probiotics, relapse, ulcerative colitis, vegetarian diet
INTRODUCTION
Ulcerative colitis (UC) and Crohn disease have a common etiopathogenesis and features, and they fall under the collective term inflammatory bowel disease (IBD).1 Epidemiologic findings in Western countries show that since the start of the 21st century, the incidence of IBD has not accelerated at the same rate observed at the end of the 20th century but rather is more stable.2 It is natural, however, that the prevalence is increasing in Western countries. The incidence of IBD is rapidly increasing in newly industrialized countries in Asia, including Japan, South Korea, and China, and in Central and South America. There is a great concern for the increasing number of patients with IBD globally.2
Despite the recognition of westernization of lifestyle as a major driver of the growing incidence of IBD,3,4 no countermeasures such as lifestyle changes have been recommended, except that patients with Crohn disease should not smoke.5 Among various environmental risk factors, we concluded that the most ubiquitous environmental risk factor was westernized diet.6 Namely, we regard IBD as a lifestyle disease caused mainly by our omnivorous (Western) diet.7–10 We have designed a plant-based diet (PBD) to counter the westernized diet, and we have been providing PBD to all patients with IBD since 2003.8 We have achieved better outcomes than those reported in both the active stage and quiescent stage in Crohn disease.7,9 We suspect these outcomes are owing to the replacement of a westernized diet with a PBD. In a previous study, we reported relapse prevention in UC by PBD through educational hospitalization.10 Cumulative relapse rates for initial episode cases were 4% and 17% at 1 and 5 years, respectively, which were, again, better outcomes than those reported elsewhere. However, the clinical focus in this study was narrow. The subjects were those with mild disease or in remission who did not need immediate treatment. In the present study, our aim was to clarify whether treatment incorporating PBD during hospitalization was effective in prevention of relapse in ordinary patients with UC.
Biologics have revolutionized treatment of various conditions including IBD. In Japan, infliximab, the first biologic introduced for IBD, became available for treatment of UC in 2012. The guidelines for UC limit the use of infliximab or adalimumab to patients who are unresponsive to conventional therapy such as glucocorticoids.11 Considering the occurrence of corticosteroid dependence or surgical intervention at 1 year in nearly 50% of patients with UC treated with glucocorticoids,12 we changed the first choice of medication for cases with moderately severe (moderate) and severe UC from prednisolone (glucocorticoid) to infliximab in 2012. Comparison of our outcomes with those reported previously is possible under the same strategy. Therefore, cases with moderate and severe UC after 2012 were not included in this study.
IBD typically affects children, teens, young adults, and adults in their prime. It has an impact on physical and psychological well-being, education and/or work, quality of life, and socioeconomics.13 Prevention of relapse is a critical step to halting impairment caused by the disease. Our goal is the prevention of relapse during the follow-up period. We hypothesized that treatment incorporating PBD will decrease the relapse rate of UC.
METHODS
Design and Settings
We designed a single-group trial, which was conducted in Akita in northern Japan. Both Nakadori General Hospital and Akita City Hospital are tertiary care hospitals in Akita. The first author (MC) worked for the former between 2003 and 2012 and has been working for the latter since 2013. This study was approved by the ethical committees of Nakadori General Hospital and Akita City Hospital (Protocol no. 19–2003 and no. 15–2015). Written informed consent was obtained from all patients.
Patients
All patients with UC who needed immediate treatment between April 2003 and October 2017 were advised to be admitted for induction therapy. Patients were excluded if they had any of the following: 1) educational hospitalization,10 recommended because of mild disease or remission that did not need immediate treatment; 2) infliximab and PBD as first-line (IPF) therapy9 as of August 2012, when infliximab became available for treatment of UC in Japan (patients with moderate to severe UC were treated with prednisolone during the prebiologic era); and 3) proctocolectomy after the hospitalization caused by either severe disease refractory to prednisolone or mild disease intractable to medical treatments. The severity was judged according to the criteria of Truelove and Witts.14
Treatment During Hospitalization
Medication was initiated according to UC guidelines.11 The exception was some mild cases. The need for medication was judged on the basis of evaluation of patient history, endoscopic findings, laboratory findings, and circumstances. As a result, medication was not provided in a subset of mild cases.
In this study, the PBD provided during hospitalization was a lacto-ovo-semivegetarian diet that was described previously.7 The PBD (about 30 kcal/kg of standard body weight) included fish once a week and meat once every 2 weeks. During hospitalization, food other than the meal service was discouraged. The plant-based diet score (PBDS), which evaluated adherence to the PBD, was 35 during hospitalization.8
The same program provided during educational hospitalization for UC10 was provided during hospitalization. Namely, the program included lifestyle diseases, healthy lifestyle habits,15 pathogenesis of IBD, and information on the PBD. During hospitalization, patients were provided with answers to any questions they had. A registered dietitian also visited the patients and talked to them about the PBD and helped them get used to it.
Blood samples for measurement of C-reactive protein; erythrocyte sedimentation rate; complete blood cell counts; total protein, albumin, α2-globulin, transaminase, cholesterol, and cholinesterase levels; and so on were taken weekly to assess the clinical course. Remission was defined as a disappearance of bloody stool.14 Patients who achieved or almost achieved remission were discharged. Morphologic improvement was confirmed either by colonoscopy or double contrast-enhanced barium enema study.7
At the end of the hospitalization, a qualified dietitian gave dietary guidance for 30 to 40 minutes to the patient and the person who prepared the patient’s meals.7 Patients were advised to continue with the PBD after discharge.
Follow-Up Studies
Follow-up was continued as long as possible. The interval between visits to the Outpatient Department after discharge varied depending on the stability of the patient’s condition. For a patient who was in unstable remission, the interval was 4 to 6 weeks. For a patient who was in stable remission, the interval was 8 weeks. For a patient who was in remission for more than a few years without medication, the interval was 3 to 6 months. Patients were occasionally encouraged to adhere to the PBD without being asked about patient adherence to the PBD. When patients gained body weight above a standard body weight, they were advised to maintain the standard body weight. Patients were occasionally provided with information on lifestyle or IBD; for example, a summary sheet of an IBD lecture held biannually at Akita City Hospital.
Food-Frequency Questionnaire and Plant-Based Diet Score
A questionnaire of dietary habits and lifestyle behaviors before onset or relapse of the disease was obtained immediately after admission. On the basis of the questionnaire, a table was drawn that summarized the patient’s current and future recommended lifestyle and dietary habits.7,10 This table was given to the patient during hospitalization and was used by the dietitian when giving dietary guidance. The questionnaire was repeated during short-term (≤ 2 years) or long-term (> 2 years) follow-up.
A PBDS was calculated from the questionnaire. The method for how the PBDS was calculated has been described previously.8 In brief summary, 8 items considered to be preventive factors for IBD (vegetables, fruits, pulses [beans, soybeans, peas, etc], potatoes, rice, miso soup, green tea, and plain yogurt) contributed to a positive score (PBDS+), whereas 8 items considered to be IBD risk factors (meat, minced or processed meat, cheese/butter/margarine, sweets, soft drinks, alcohol, bread, and fish) contributed to a negative score (PBDS−). Scores of 5, 3, and 1 were given according to the frequency of consumption: Every day, 3 to 5 times per week, and 1 to 2 times per week, respectively. The PBDS was calculated as the sum of the positive and negative scores, and it ranged between −40 and +40. A higher PBDS indicated greater adherence to the PBD.8
Assessment of Efficacy
The primary endpoint was relapse during the follow-up period. Relapse was defined as a flare-up that required more aggressive medical treatment.16–19 Reappearance of streak blood, a small volume of blood, or bloody stool was not counted as relapse if the blood disappeared or was controlled with previous medication and/or modification of the diet or a lifestyle behavior.
The secondary endpoint was change over time in PBDS. Short− (≤ 2 years after discharge) and long-term (> 2 years) chronological change in the PBDS were studied.
Safety Evaluations
Safety assessments included vital signs, patient symptoms, findings during daily practitioner rounds, weekly laboratory data, and physical examination findings.
Statistical Analysis
Demographic parameters are expressed as mean and standard deviation (SD) and/or median (interquartile range), as appropriate. The frequency of categorical variables between initial episode cases and relapse cases was assessed using the χ2 test. Chronologic changes in PBDS+, PBDS−, and PBDS in identical patients were compared using the paired t-test or Wilcoxon test. Kaplan-Meier survival analysis was used to calculate the cumulative proportion of patients who had a relapse. Comparison of cumulative relapse rates between initial episode cases and relapse cases was tested using the log rank test. All directional tests were 2-tailed. A p value ≤ 0.05 was considered statistically significant. Statistical analyses were performed using JMP 8 software (SAS Institute Inc, Cary, NC, USA).
RESULTS
Patients
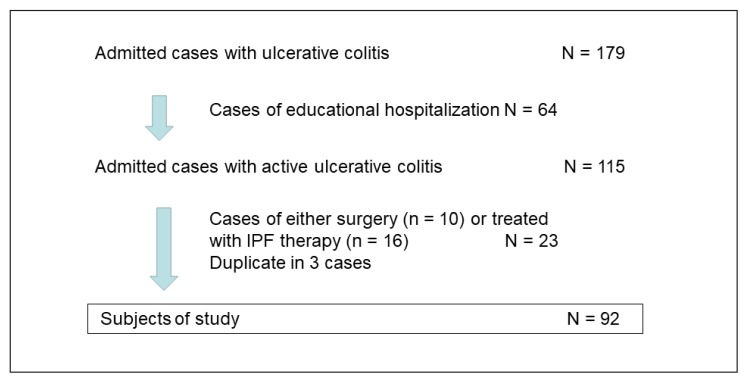
A total of 179 patients with UC were admitted (Figure 1). Sixty-four cases of educational hospitalization were excluded. Ten surgical colectomy cases were excluded, of which 9 cases were relapses and 1 was an initial episode. Sixteen cases of IPF therapy were also excluded, but 3 of these cases overlapped with excluded colectomy cases (duplicates). Therefore, 92 cases were ultimately included in this study (Figure 1).
Figure 1.
Enrollment of inpatients with ulcerative colitis. Cases of educational hospitalization were either mild in severity or in the quiescent phase (remission), and they did not need immediate treatment.10 Admitted cases with active ulcerative colitis needed immediate treatment. Infliximab and a plant-based diet as first-line (IPF) therapy replaced glucocorticoid therapy for induction in severe or moderate cases since August 2012.
Demographic characteristics of the 92 patients are presented in Table 1. Of the 92 cases, 51 cases were initial episodes and 41 cases were relapses. The male-to-female ratio was 1:1 in both groups. The median age of the initial episode cases was 35 years, and the median age of the relapse cases was 36 years. Extensive colitis20 was the most frequent extent with more than half of cases, followed by left-sided colitis and proctitis in both groups. Although moderate cases were most frequent and severe cases the least frequent in both groups, there were more severe cases among the relapse cases than among initial episode cases (p = 0.0346, Table 1). The difference between the mean disease duration for the initial episode cases (3 months) compared with that for the relapse cases (75 months) was statistically significant (p < 0.0001, Table 1).
Table 1.
Demographics of 92 patients
| Characteristic | Total | Initial episode cases | Relapse cases | p valuea |
|---|---|---|---|---|
| Male, no. (%) | 45 (49) | 25 | 20 | 0.9818 |
| Female, no. (%) | 47 (51) | 26 | 21 | |
| Age, y | ||||
| Range | 11–81 | 11–80 | 14–81 | |
| Mean (SD) | 39 (19) | 39 (19) | 40 (18) | 0.5876 |
| Median (IQR) | 36 (23–54) | 35 (22–56) | 36 (26–52) | |
| Extent of ulcerative colitis,20 no. (%) | 0.9267 | |||
| Proctitis | 15 (16) | 9 | 6 | |
| Left-sided colitis | 22 (24) | 12 | 10 | |
| Extensive colitis | 55 (60) | 30 | 25 | |
| Severity14,20 | 0.0346 | |||
| Mild | 31 (34) | 18 | 13 | |
| Moderate | 48 (52) | 30 | 18 | |
| Severe | 13 (14) | 3 | 10 | |
| Disease duration, mo | ||||
| Range | 1–296 | 1–24 | 1–296 | |
| Mean (SD) | 35 (63) | 3 (4) | 75 (78) | < 0.0001 |
| Median (IQR) | 4 (1–35) | 1 (1–4) | 46 (8–127) | |
| Case referral status, no. (%) | 0.5575 | |||
| Referred | 53 (58) | 28 | 25 | |
| Nonreferred | 39 (42) | 23 | 16 | |
| Smoking status, no. (%) | 0.8190 | |||
| Current smoker | 17 (18) | 9 | 8 | |
| Nonsmoker | 75 (82) | 42 | 33 | |
| Medication during hospitalization, no. (%) | 0.0033 | |||
| None | 11 (12) | 6 | 5 | |
| A: Local (suppository, enema) | 9 (10) | 7 | 2 | |
| B: 5-aminosalicylic acids, oral | 27 (29) | 21 | 6 | |
| C: A and B | 15 (16) | 8 | 7 | |
| Immunomodulator | 30 (33) | 9 | 21 | |
| Prednisolone alone or with any combination of A, B, C, and tacrolimus | 22 | 8 | 14 | |
| Prednisolone and azathioprine and any combination of A, B, C, and CAP | 6 | 1 | 5 | |
| Azathioprine and B or C | 2 | 0 | 2 | |
| Corticosteroid dependent | 5 (5) | 1 | 4 | 0.0950 |
| Previous proctocolectomy | 0 (0) | 0 | 0 | |
| Hospitalization, d | ||||
| Range | 7–125 | 13–125 | 7–121 | |
| Mean (SD) | 37 (27) | 34 (25) | 42 (29) | 0.1142 |
| Median (IQR) | 29 (22–45) | 25 (20–39) | 32 (23–50) | |
| Follow-up period after discharge | ||||
| Mean (SD) | 5 y 11 mo (4 y 3 mo) | 5 y 2 mo (3 y 9 mo) | 6 y 10 mo (4 y 7mo) | 0.0935 |
| Median (IQR) | 5 y 1 mo (2 y 2 mo–9 y 3 mo) | 4 y 1 mo (2 y 1 mo–8 y 1 mo) | 6 y 4 mo (2 y 8 mo–11 y 3 mo) | |
Comparison between initial attack cases and relapse cases (χ2 test).
CAP = cytapheresis; IQR = interquartile range; SD = standard deviation.
Medication was not provided during the hospitalization in 11 cases (12%). They were mild cases, except for 2 moderate cases. Adverse events caused by 5-aminosalicylic acid prescribed by referring physicians exacerbated 2 patients’ diarrhea, and withdrawal of the drug therapy resulted in remission. Immunomodulators (systemic prednisolone and/or azathioprine) were used in 9 (18%) of 51 initial episode cases, whereas they were used in 51% of relapse cases (21 of 41 cases, Table 1).
Periods of hospitalization were shorter for initial episode cases (median = 25 days) than those for relapse cases (32 days). Median hospital days in nonmedication cases (n = 11) was 15.0 days (interquartile range = 13–19 days). All 92 patients achieved remission or near-remission by the time of discharge. Of 10 patients excluded from this study because of colectomy, 1 patient was experiencing an initial episode of UC. The colectomy rate for initial episode cases during the first hospitalization was 1.9% (1/52). The median follow-up period for initial episode cases and relapse cases was 4 years 1 month and 6 years 4 months, respectively (Table 1).
Efficacy
Primary Endpoint: Relapse Rate
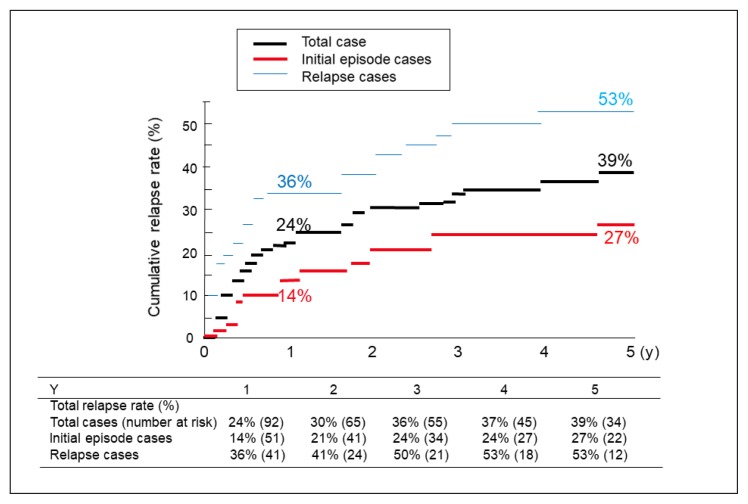
The overall cumulative relapse rates at 1, 2, 3, 4, and 5 years of follow-up were 24%, 30%, 36%, 37%, and 39%, respectively. The cumulative relapse rates were 14%, 21%, 24%, 24%, and 27%, for each of those follow-up years, for the initial episode cases and 36%, 41%, 50%, 53%, and 53%, respectively, for the relapse cases (Figure 2). The relapse cases showed significantly higher relapse rates than the initial episode cases (p = 0.0318). The mean time to relapse was 7 years 2 months for the initial episode cases and 4 years 3 months for the relapse cases.
Figure 2.
Cumulative relapse rates during follow-up after discharge for patients with ulcerative colitis. Log rank test between initial episode cases and relapse cases found p = 0.0318.
Secondary Endpoints
Four patients were mistakenly not asked to respond to the food-frequency questionnaire. Therefore, baseline PBDS was determined on the basis of 88 patients. The mean (SD) baseline PBDS+, PBDS−, and PBDS were 23.2 (8.1), 11.9 (6.2), and 10.9 (9.3), respectively (Table 2). For 41 patients, at the median follow-up period of 1 year, respective scores were 33.0 (5.6), 3.7 (3.8), and 29.3 (7.6). These 3 values were significantly better than those at baseline (p < 0.0001, Table 2). In the other 41 patients, at the median follow-up period of 6 years 5 months, the respective scores were 26.9 (7.6), 7.5 (4.9), and 19.4 (9.0). These 3 values were significantly better than those at baseline: p = 0.0171 for PBD score+ and p < 0.0001 for PBD score− and PBD score (Table 2).
Table 2.
Chronologic change in plant-based diet (PBD) score
| Timeframe | n | Follow-up (mo) | PBD score+ | p value | PBD score− | p value | PBD score (sum) | p value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |||||
| Baseline | 88 | — | — | 23.2 (8.1) | 24.0 (17.0–28.8) | 11.9 (6.2) | 12.0 (8.0–16.8) | 10.9 (9.3) | 12.0 (4.0–17.0) | |||
| Follow-upa | ||||||||||||
| Short term | 41 | 14.8 (7.6) | 12.0 (10.5–24.0) | 33.0 (5.6) | 33.0 (28.0–38.0) | < 0.0001b | 3.7 (3.8) | 3.0 (0.5–6.0) | < 0.0001b | 29.3 (7.6) | 31.0 (24.0–36.0) | < 0.0001b |
| Baseline | 41 | 23.9 (7.6) | 26.0 (17.5–29.0) | 11.9 (5.2) | 12.0 (9.0–16.5) | 12.0 (8.7) | 12.0 (0–15.0) | |||||
| Long term | 41 | 80.4 (36.6) | 76.5 (43.8–110.8) | 26.9 (7.6) | 26.0 (21.0–33.5) | 0.0171b | 7.5 (4.9) | 7.0 (3.5–10.0) | < 0.0001b | 19.4 (9.0) | 20.0 (12.5–25.5) | < 0.0001b |
| Baseline | 41 | 24.7 (7.0) | 25.0 (21.0–28.5) | 13.0 (5.2) | 12.0 (9.0–17.0) | 11.7 (8.8) | 13.0 (6.0–17.0) | |||||
Patients with short-term PBD score (n = 41) and those with long-term PBD score (n = 41) were different: 24 patients overlap.
p value obtained with paired t-test or Wilcoxon test.
IQR = interquartile range; SD = standard deviation.
Safety
All patients ate the PBD, and none experienced a serious adverse effect that could be suspected of being caused by a PBD.
DISCUSSION
The cumulative relapse rates at 1 and 5 years in the initial episode cases after induction therapy incorporating PBD were 14% and 27%, respectively. The PBDS was significantly higher than baseline even at 6-year follow-up. The relapse rates were lower than those previously reported with conventional therapy in Europe. This difference is consistent with our hypothesis that this PBD prevents relapse of UC.
Relapsing and remitting are characteristic in the most patients with UC except for about 10% of patients with initial attack only.21 Approximately 20% of patients will undergo colectomy within 10 years, and 30% within 25 years. Relapse is most frequent in the first year of diagnosis, followed by the second year after diagnosis. Thereafter, disease activity decreases over time.17,21,22 The disease course in the preceding period is significantly correlated to the subsequent disease course.21 Therefore, the relapse rate is influenced by the period of remission and disease duration of the patients before entry to a study; a longer remission period translates to a lower relapse rate. Consequently, the relapse rate is best studied not by using patients with established UC that has been plaguing them for months to years but by studying patients who present with their first episode of UC.
Studies of relapse rates in inception UC cases are surprisingly scarce, but their available cumulative relapse rates are listed in Table 3.16,18,23–25 The dates (1970–2011) of these reports are from before the era of biologic use. The treatment guidelines for the induction and maintenance of remission of initial presentations of UC remained unchanged. The cumulative relapse rate at 1 year was reported to be around 50% (44%–51%) in Norway, Netherlands, and Denmark.16,23,24 The European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD) reported it to be 28%.18 Our 14% cumulative relapse rate is far better than those reported.
Table 3.
Cumulative relapse rate at 1 and 5 years for patients presenting with their first episode of ulcerative colitis
| Source | Country of study | Subjects | Cumulative relapse rate, % | |||
|---|---|---|---|---|---|---|
| Study period | Age, y | Current smoker, % (n/N) | 1 y | 5 y | ||
| Moum et al, 199716 | Norway | 1990–1993 | NA | 14 | 50 | NA |
| Höie et al, 200718 | 7 European countries, Israel | 1991–1993 | NA | 12.6 (97/771) | 28a | 58a |
| Romberg-Camps et al, 200923 | Netherlands | 1991–2003 | Mean = 42.0 | NA | 44 | NA |
| Vester-Andersen et al, 201424 | Denmark | 2003–2004 | Median = 37.3 (IQR = 1.5–93.5) | 15 (45/300) | 51 | 75 |
| Kitano et al, 201125 | Japan | 1970–2011 | Mean = 28.5 (SD = 38.5) | NA | 68.1 | NA |
| Chiba et al, 2018b10 | Japan | 2003–2016 | Median = 34 (IQR = 22–51) | NA | 4 | 17 |
| Present study | Japan | 2003–2017 | Median = 35 (IQR = 22–56) | 18 (9/51) | 14 | 27 |
Estimated from a Kaplan-Meier plot.
Cases of educational hospitalization provided a plant-based diet.
IQR = interquartile range; NA = not available; SD = standard deviation.
If the number of severe cases decreases after colectomy during the induction phase, it might explain the low relapse rate in the follow-up period. Our colectomy rate during the induction phase was 1.9% (1/52). It was 0.6% (4/630) in a report from Romberg-Camps et al23 and 1.1% (8/710) in a report from Burisch et al.26 Therefore, it is unlikely that the colectomy rate influenced the relapse rate in the present study.
Some clinical and environmental factors are known to be associated with relapse; young age at diagnosis is associated and smoking is inversely associated with relapse.27 These factors are shown in Table 3, and it is unlikely that they explain our favorable outcomes.
Mucosal healing has been recently recommended as the goal of IBD treatment.28 Patients with mucosal healing have better prognosis than those without mucosal healing in regard to relapse rate, admission rate, and colectomy rate. However, mucosal healing is achieved in only about one-third (88 of 298) of treated patients with UC.29 In addition, the mucosal healing rate increases along with a longer clinical remission: 30%, 45.9%, and 62.9% for clinical remission less than 1 year, 1 to 3 years, and more than 3 years, respectively.30 Mucosal healing was not discussed in other reports of inceptive cases.16,18,23–25
The large difference between most practices worldwide and our practice lies in our greater propensity to hospitalize initial cases of UC. The current international guideline recommends hospitalization only for severe cases.11 Mild or moderate cases are treated on an outpatient basis. Our cases, even mild cases, were treated on an inpatient basis. In most of the patients, there was no hesitation about admission because they were aware of the abnormality and wanted to be treated. Hospitalization provides an excellent opportunity to implement and teach PBD. This difference in practice could explain the difference in relapse rates between our cohort and those of other authors (Table 3).
Meat and alcohol were reported to be associated with relapse of UC in a follow-up study,31 and legumes and potatoes were inversely associated.32 Our PBD is consistent with these observations: Meat and alcohol are limited, and legumes and potatoes are recommended. In this study, PBDS+ and PBDS− in the short-term (median = 1 year) were significantly higher and lower than baseline, respectively. Consequently, PBDS was significantly higher than baseline. Similarly, significantly higher PBDS than baseline was observed even in the long-term (median = 6 years 5 months, Table 2). On the basis of median PBDS 31.0 and 20.0 in the short term and long term respectively, it seems that patients tend to lose their determination to stay on the PBD over time. This illustrates the difficulty of maintaining PBD lifelong. On the other hand, patients experienced normalization of diarrhea and/or bloody stool while on the PBD in the hospital setting, leading to appreciation of the PBD and its benefits. It was frequently heard that adherence to the PBD improved when patients feared relapse. Hospitalization seemed to enhance their self-management skills. We speculate that experience and appreciation of PBD during hospitalization contributed greatly to prevention of relapse.
The relapse rate for initial episode cases at 1 year (14%) in this study was higher than that for cases of educational hospitalization (4%) in a prior study.10 The study participants were different. Participants who underwent educational hospitalization were patients with mild UC or in remission who did not need immediate treatment.10 In the present study, participants were in the active phase with severity ranging from mild to severe. Although there are reports that the severity at diagnosis is irrelevant to the relapse rate,33 our data indicate that severe cases tend to relapse more than do mild cases. Significantly higher relapse rates in relapse cases (at 1 year: 36%) than in initial episode cases (14%, Figure 2) could be also explained by the difference in severity between subjects (Table 1).
Of note, medication was not provided in 11 cases in the present study. Two of the 11 cases were moderate in severity. Adverse events caused by 5-aminosalicylic acid-exacerbated diarrhea, and withdrawal of the drug resulted in remission. The other 9 patients not receiving medication were mild in severity, similar to cases of educational hospitalization.10 They achieved remission in about 2 weeks. We further confirmed a previous observation: A subset of patients with mild UC achieve remission by replacing an omnivorous diet with PBD. The proportion was about one-third of mild cases (9/31).
Our study had some limitations. There was no control group, and the sample size was small. Although PBDS is available for each patient at baseline, the PBDS was developed at the late stage of the study.8 Therefore, short− and long-term chronologic changes in PBDS were not obtained from the same patients. Patients with short-term PBDS (n = 41) and those with long-term PBDS (n = 41) were different: 24 patients overlap (Table 2). Comparison of PBDS at 3 time points—baseline, short-term, and long-term—from the same patients would have been more appropriate.
Many other modern diseases are chronic and caused by an unhealthy lifestyle,34,35 of which diet is one component. Therefore, treating these diseases only with medication and surgery will not be able to prevent or fully resolve them unless we also address the problematic lifestyle. Bodai et al35 described the dramatic effect and the need for lifestyle medicine in a variety of chronic diseases. Our modality is indeed lifestyle medicine.36
Changes in lifestyle including dietary habits are not easy.37 Considering that maximum weight loss typically is greatest 6 to 12 months after initiation of a weight loss diet, with steady regaining of weight subsequently,38,39 significant adherence to PBD compared with the baseline even 6 years later in this study was remarkable. We believe that hospitalization plays a critical role in replacement of an omnivorous diet with PBD. Hospitalization helps limit risk factors for IBD and our health such as smoking, alcohol, sweets, and animal foods, and patients benefit from preventive factors every day such as intake of vegetables and fruits. Although most of the developed world restricts hospitalization to severe cases and surgical intervention, mostly treating mild cases and educational interventions on an outpatient basis, hospitalization can be an effective approach for dietary replacement in lifestyle medicine.
CONCLUSION
The relapse rate for initial episode cases of UC after treatment incorporating PBD on an inpatient basis during the induction phase was 14% at 1 year and 27% at 5 years, which were far lower than those reported with conventional treatment. Significantly higher PBDS was observed compared with baseline even after 6 years of follow-up. A PBD was found to be effective in prevention of UC relapse, and hospitalization seemed to be effective at inducing habitual dietary changes.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications performed a primary copy edit.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: Current state of the art. Nat Rev Gastroenterol Hepatol. 2016 Jan;13(1):13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017 Feb;152(2):313–21. doi: 10.1053/j.gastro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Shanahan F. Disorders of a modern lifestyle: Reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008 Sep;57(9):1185–91. doi: 10.1136/gut.2007.122143. [DOI] [PubMed] [Google Scholar]
- 4.Hold GL. Western lifestyle: A ‘master’ manipulator of the intestinal microbiota? Gut. 2014 Jan;63(1):5–6. doi: 10.1136/gutjnl-2013-304969. [DOI] [PubMed] [Google Scholar]
- 5.Mowat C, Cole A, Windsor A, et al. IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011 May;60(5):571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 6.Chiba M, Nakane K, Komatsu M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm J. 2019;23:18–107. doi: 10.7812/TPP/18-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010 May;16(20):2484–95. doi: 10.3748/wjg.v16.i20.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiba M, Nakane K, Takayama Y, et al. Development and application of a plant-based diet scoring system for Japanese patients with inflammatory bowel disease. Perm J. 2016 Fall;20(4):62–8. doi: 10.7812/TPP/16-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba M, Tsuji T, Nakane K, et al. Induction with infliximab and plant-based diet as first-line (IPF) therapy in Crohn disease: Single-group trial. Perm J. 2017;21:17–9. doi: 10.7812/TPP/17-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba M, Nakane K, Tsuji T, et al. Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: A single-group trial. Perm J. 2018;22:17–167. doi: 10.7812/TPP/17-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010 Mar;105(3):501–23. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 12.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: A population-based study. Gastroenterology. 2001 Aug;121(2):255–60. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 13.Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. International Programme to Develop New Indexes for Crohn’s Disease (IPNIC) group. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012 Feb;61(2):241–7. doi: 10.1136/gutjnl-2011-300049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955 Oct;2(4947):1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Prev Med. 1980 Jul;9(4):469–83. doi: 10.1016/0091-7435(80)90042-0. [DOI] [PubMed] [Google Scholar]
- 16.Moum B, Ekbom A, Vatn MH, et al. Clinical course during the 1st year after diagnosis in ulcerative colitis and Crohn’s disease. Results of a large, prospective population-based study in southeastern Norway, 1990–93. Scand J Gastroenterol. 1997 Oct;32(10):1005–12. doi: 10.3109/00365529709011217. [DOI] [PubMed] [Google Scholar]
- 17.Henriksen M, Jahnsen J, Lygren I, et al. IBSEN Study Group. Ulcerative colitis and clinical course: Results of a 5-year population-based follow-up study (the IBSEN study) Inflamm Bowel Dis. 2006 Jul;12(7):543–50. doi: 10.1097/01.MIB.0000225339.91484.fc. [DOI] [PubMed] [Google Scholar]
- 18.Höie O, Wolters F, Riis L, et al. European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD) Ulcerative colitis: Patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007 Aug;102(8):1692–701. doi: 10.1111/j.1572-0241.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 19.Solberg IC, Lygren I, Jahnsen J, et al. IBSEN Study Group. Clinical course during the first 10 years of ulcerative colitis: Results from a population-based inception cohort (IBSEN Study) Scand J Gastroenterol. 2009;44(4):431–40. doi: 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 20.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and complications. Gut. 2006 Jun;55(6):749–53. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: Analysis of changes in disease activity over years. Gastroenterology. 1994 Jul;107(1):3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 22.Magro F, Rodrigues A, Vieira AL, et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012 Mar;18(3):573–83. doi: 10.1002/ibd.21815. [DOI] [PubMed] [Google Scholar]
- 23.Romberg-Camps MJ, Dagnelie PC, Kester AD, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009 Feb;104(2):371–83. doi: 10.1038/ajg.2008.38. [DOI] [PubMed] [Google Scholar]
- 24.Vester-Andersen MK, Vind I, Prosberg MV, et al. Hospitalization, surgical and medical recurrence rates in inflammatory bowel disease 2003–2011—A Danish population-based cohort study. J Crohns Colitis. 2014 Dec;8(12):1675–83. doi: 10.1016/j.crohns.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Kitano A, Okawa K, Nakamura S, Komeda Y, Ochiai K, Matsumoto T. The long-term assessment of the patients with ulcerative colitis (> 10 years follow-up, mean follow-up 21.7 years) [in Japanese] J New Remedies Clin. 2011 Jul;60(7):1347–55. [Google Scholar]
- 26.Burisch J, Pedersen N, Cukovic-Cavka S, et al. EpiCom Group. Initial disease course and treatment in an inflammatory bowel disease inception cohort in Europe: The EECO-EpiCom cohort. Inflamm Bowel Dis. 2014 Jan;20(1):36–46. doi: 10.1097/01.MIB.0000436277.13917.c4. [DOI] [PubMed] [Google Scholar]
- 27.Liverani E, Scaioli E, Digby RJ, Bellanova M, Belluzzi A. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol. 2016 Jan;22(3):1017–33. doi: 10.3748/wjg.v22.i3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinink AR, Lee TC, Higgins PD. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: A meta-analysis. Inflamm Bowel Dis. 2016;22:1859–69. doi: 10.1097/MIB.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 29.Yoshino T, Yamakawa K, Nishimura S, Watanabe K, Yazumi S. The predictive variable regarding relapse in patients with ulcerative colitis after achieving endoscopic mucosal healing. Intest Res. 2016 Jan;14(1):37–42. doi: 10.5217/ir.2016.14.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi HY, Chan FK, Tsang SW, et al. Factors associated with mucosal healing in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2015 Apr;21(4):840–6. doi: 10.1097/MIB.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 31.Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut. 2004 Oct;53(10):1479–84. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasson L, Canova C, Vettorato MG, Savarino E, Zanotti R. Influence of diet on the course of inflammatory bowel disease. Dig Dis Sci. 2017 Aug;62(8):2087–94. doi: 10.1007/s10620-017-4620-0. [DOI] [PubMed] [Google Scholar]
- 33.Monstad I, Hovde Ø, Solberg IC, Moum BA. Clinical course and prognosis in ulcerative colitis: Results from population-based and observational studies. Ann Gastroenterol. 2014;27(2):95–104. [PMC free article] [PubMed] [Google Scholar]
- 34.Hyman MA, Ornish D, Roizen M. Lifestyle medicine: Treating causes of disease. Altern Ther Health Med. 2009 Nov-Dec;15(6):12–4. [PubMed] [Google Scholar]
- 35.Bodai BI, Nakata TE, Wong WT, et al. Lifestyle medicine: A brief review of its dramatic impact on health and survival. Perm J. 2018;22:17–025. doi: 10.7812/TPP/17-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiba M, Nakane K, Komatsu M. Lifestyle medicine in inflammatory bowel disease [letter] Perm J. 2018;22:18–062. doi: 10.7812/TPP/18-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desroches S, Lapointe A, Ratte S, Gravel K, Legatee F, Turcotte S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev. 2013 Feb;28(2):CD008722. doi: 10.1002/14651858.CD008722.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shai I, Schwarzfuchs D, Henkin Y, et al. Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008 Jul;359(3):229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 39.Atallah R, Filion KB, Wakil SM, et al. Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: A systematic review of randomized controlled trials. Circ Cardiovasc Qual Outcomes. 2014 Nov;7(6):815–27. doi: 10.1161/CIRCOUTCOMES.113.000723. [DOI] [PubMed] [Google Scholar]