Abstract
Context
The utility of fractional exhaled nitric oxide (FeNO) measurement in real-world asthma management requires investigation.
Objective
To determine whether FeNO-assisted care added to standard asthma management improves asthma control in a managed care organization.
Design
Prospective observational study in patients aged 12 years and older with uncontrolled persistent asthma identified during a scheduled visit to an Allergy Department that routinely used FeNO (FeNO-assisted care, n = 426) vs visits to 4 Allergy Departments that did not, but followed routine guideline-based care (standard care, n = 925). The FeNO-assisted care was based on FeNO level, asthma control status, and step-care level.
Main Outcome Measures
Composite primary outcome was 1 or more asthma exacerbations or 7 or more dispensed canisters containing short-acting β2-agonists in the follow-up year. Inverse probability of treatment weighting propensity scoring balanced covariates, and multivariable regression analyses compared outcomes between groups.
Results
Compared with standard care, FeNO-assisted care was not associated with reducing the primary composite outcome (adjusted risk ratio = 0.94, 95% confidence interval = 0.69–1.29, p = 0.71), nor with a reduction in asthma exacerbations or dispensing of 7 or more short-acting β2-agonist canisters as separate outcomes. In an atopic subgroup with aeroallergen sensitization, the composite outcome was similar between groups, but the rate of asthma exacerbations was lower with FeNO-assisted treatment (adjusted rate ratio = 0.67, 95% confidence interval = 0.49–0.91, p = 0.01).
Conclusion
These findings suggest future studies of FeNO-assisted care should be directed at the atopic phenotype.
Keywords: asthma control, asthma guidelines, asthma management, asthma medication, epidemiology, exacerbations, FeNO, fractional exhaled nitric oxide, outcomes, persistent asthma, short-acting β2-agonists
INTRODUCTION
Persistent asthma is a chronic disorder characterized by increased airflow obstruction, bronchial hyperresponsiveness, and, typically, eosinophilic inflammation. However, standard measures for its diagnosis and management rely on symptoms and lung function without monitoring inflammatory markers. Fractional exhaled nitric oxide (FeNO) is an established marker of airway inflammation associated with atopic or allergic asthma. This inflammation is stimulated by T-helper type 2 cells that secrete cytokines interleukin (IL)-4, IL-5, and IL-13 leading to type 2 immunity characterized by high immunoglobulin E (IgE) antibody levels and eosinophilia.1 Although FeNO assessment is conducted in some asthma specialty clinics, it is not routinely used to diagnose or to manage asthma.
Studies report that FeNO measurements might provide additional information for more complete asthma assessment.2 Elevated FeNO levels may help to 1) diagnose asthma,3,4 2) predict asthma exacerbations and loss of control,5–7 3) assess adherence to inhaled corticosteroid (ICS) treatment,8 and 4) guide management of chronic asthma.9–12 A prospective 1-year observational UK study reported that FeNO was useful to guide ICS therapy initiation and dosage, and to identify nonadherence.13 In the US, allergists incorrectly classified airway inflammation about half of the time in their patients compared with use of actual FeNO levels.14 A recent nonblinded, pragmatic, cluster-randomized 1-year trial among primary care practices compared care directed by both FeNO and the Asthma Control Questionnaire with care directed only by the Asthma Control Questionnaire in adult asthmatic patients.12 The study reported lower medication costs and the probability of cost-effectiveness with FeNO care.12 In other studies, higher FeNO levels were significantly associated with excess short-acting β2-agonist (SABA) use and asthma exacerbations in patients with persistent asthma.15,16 The American Thoracic Society recognized the potential utility of FeNO and published guidelines on its practical use.17 More recently, the Agency for Healthcare Research and Quality published a comparative effectiveness review on the clinical utility of FeNO in asthma management.18
Given these findings and the need for real-world studies to determine the utility of FeNO determinations in asthma specialist care, we conducted a prospective observational study in patients with persistent asthma. We determined whether FeNO-assisted care added to standard guideline-based care19 was associated with improved asthma outcomes. We hypothesized that knowledge of FeNO levels added to guideline-based care would improve asthma outcomes.
METHODS
Study Design
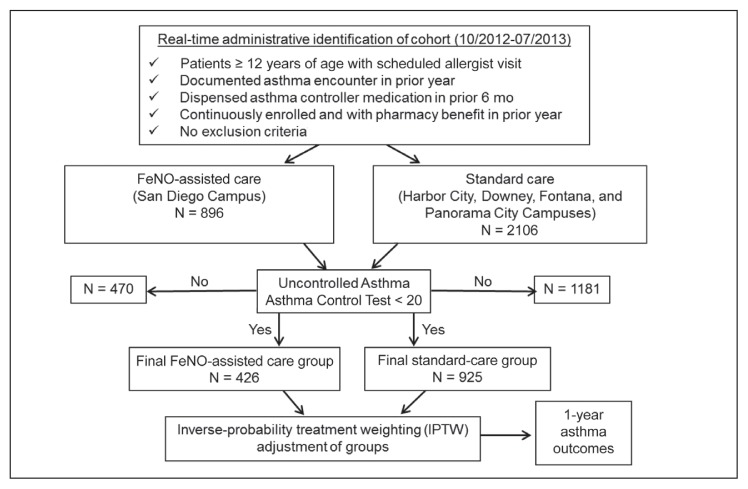
We conducted a prospective pragmatic observational study to evaluate the utility of FeNO assessment in asthma management of persistent asthma among allergists in a managed care setting. Eligible patients with persistent asthma scheduled for an allergy appointment between October 23, 2012, and July 31, 2013, at a participating Kaiser Permanente Southern California (KPSC) Allergy Department (San Diego, Harbor City, Downey, Fontana, and Panorama City Service Areas in California) were identified electronically using patient characteristics, medical utilization review, and pharmacy data from the KPSC Research Data Warehouse (Figure 1).20
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram and study design in Kaiser Permanente Southern California. CONSORT diagram depicts the process used to generate study subjects.
FeNO = fractional exhaled nitric oxide; 10/2012-07/2013 = October 2012 to July 2013.
Patients
Eligible patients were age 12 years and older, had a KPSC-specific encounter code for an asthma diagnosis, were dispensed an asthma controller in the prior 6 months, had continuous Health Plan enrollment (no gap longer than 45 days) and pharmacy benefit 1 year before the scheduled Allergy Department visit (index date), and manifested uncontrolled asthma determined by the Asthma Control Test (ACT) on the index date (see “Asthma Impairment Assessment” in the Study Measures section). Patients were excluded for the following conditions: 1) nonasthma lung disease in the prior 3 years: Chronic obstructive pulmonary disease, emphysema, cystic fibrosis, chronic bronchitis, bronchiectasis, pulmonary hypertension, ciliary dyskinesia, α1-antitrypsin disease, bronchiolitis obliterans, or hypereosinophilic syndromes (eosinophilic granulomatosis with polyangiitis); 2) autoimmune disorder in the prior 3 years; 3) immune deficiency, HIV, drug addiction, or active cancer requiring any therapy except for antihormonal therapy; 4) transplantation of a major organ or immunosuppressant therapy in past year or in the follow-up year; 5) omalizumab therapy within the prior 12 months; and 6) visits to both FeNO-assisted and standard-care Allergy Departments in the follow-up year. The main cohort included all eligible study patients, and the atopic cohort included only those patients from the main cohort with documented aeroallergen sensitization.
Study Sites
Five Allergy Departments in the KPSC Region where asthma care was routinely provided agreed to participate. The San Diego Allergy Department was the only one that had incorporated FeNO determination in the routine assessment of asthmatic patients, and as such was selected as the FeNO-assisted care study site. A management algorithm based on FeNO level, asthma control status, and step-care level was provided to the allergists at the FeNO-assisted care site (Table 1). The other 4 Allergy Departments, not using FeNO for asthma assessments, were selected for the standard-care sites. The standard-care sites followed their usual care practices, which were based on the National Asthma Education and Prevention Program (NAEPP) guideline.19
Table 1.
Algorithm for fractional exhaled nitric oxide (FeNO)-directed asthma management
| Asthma control statusa | FeNO level | ||
|---|---|---|---|
| Low FeNO (< 25 ppb) | Intermediate FeNO (25–50 ppb) | High FeNO (> 50 ppb) | |
| Controlled | Decrease ICS 1 step to low-dose ICS. Next step: Discontinue add-on medications sequentially. Next step: Go to “as-needed” ICS if patient is receiving low-dose monotherapy ICS. |
No change in ICS or Consider increase in ICS up to medium dose if FeNO is 35–50 ppb, particularly in adolescents or if FeNO trend has been increasing. |
Increase to medium-dose ICS if patient is receiving low-dose ICS. If patient is already receiving medium-dose ICS, consider adding LTRA, or increasing to high-dose ICS if patient is already receiving or failed to respond to LTRA. |
| Uncontrolled | Maintain ICS. Next step: Add sequentially LABA, LTRA, and in adults only, add tiotropium. |
Increase ICS to medium dose if patient is receiving low-dose ICS. Next step: Add LTRA. Next step: Go to high-dose ICS. Next step: Add LABA. Next step: In adults only, add tiotropium. |
Increase ICS up to maximal high-dose ICS. Next steps: Add sequentially LTRA, then LABA. Next step: In adults only, add tiotropium. |
Considerations related to the above algorithm:
- ICS should be first controller for controller-naïve patients.
- Individual patient characteristics should be considered in therapeutic choices.
- With a change in anti-inflammatory therapy, consider follow-up visit in 2–6 weeks for repeat FeNO.
- With change in bronchodilator therapy, consider phone contact in 2–6 weeks.
- With controlled asthma and no change in therapy, consider follow-up in 3-month intervals to evaluate the patient for a step down in therapy. Continue add-on therapy only if there is a documented improved clinical response.
Control status was based on results of Asthma Control Test and pulmonary function test, and on exacerbation history.
ICS = inhaled corticosteroid; LABA: long-acting β2-agonist; LTRA = leukotriene receptor antagonist.
Study Measures
Fractional Exhaled Nitric Oxide Determination
Before spirometry, FeNO was determined with the NIOX MINO handheld device21 (Circassia Pharmaceuticals AB [formerly Aerocrine AB], Oxford, UK) following the manufacturer’s recommended procedures for calibration and assessment.15 NIOX MINO adheres to the American Thoracic Society/European Respiratory Society 2005 equipment recommendations for measurement of FeNO.17,22
Asthma Risk Assessment
An asthma exacerbation was defined by a hospitalization or Emergency Department (ED) visit with a primary diagnosis of asthma (International Classification of Diseases, Ninth Revision Code 493.x), or an oral corticosteroid (OCS) dispensing within 5 days before or after an outpatient visit linked to a KPSC encounter coded for uncontrolled asthma (ie, acute exacerbation, status asthmaticus, acute asthma attack, uncontrolled asthma, or asthmatic bronchitis).20 Qualifying events had to be separated by more than 5 days from each other to be counted as distinct exacerbations.
Asthma Impairment Assessment
The validated ACT assessed asthma control.23 A score below 20 indicates uncontrolled asthma, and a score less than 16 indicates very poorly controlled asthma.23–25 Seven or more SABA canisters dispensed in a year (equivalent to ≥ 4 puffs/d), a validated administrative marker for uncontrolled asthma, was used to define excessive SABA use.26 In December 2012, KPSC initiated a Pharmacy Therapeutic Substitution program in which individual physicians signed an albuterol therapeutic substitution form that allowed KPSC pharmacies to convert multicanister albuterol inhaler dispensations to 1 canister only. By June 2013, approximately 90% of physicians signed off on this change. This program led to a marked reduction in the dispensing of albuterol and, as such, reduced the proportion of patients dispensed 7 or more SABA canisters during the study.
Other Assessments
Allergen sensitization was determined by specific IgE or immediate skin tests to aeroallergens. Specifically, aeroallergen sensitization was determined by Phadia ImmunoCAP (Thermo Fisher Scientific Inc, Carlsbad, CA) to 7 perennial and 6 pollen allergens (a level of specific IgE ≥ 0.35 IU/mL was a positive test) or a panel of immediate skin test aeroallergens specific to each Allergy Department as previously reported (a wheal ≥ 3 mm larger than saline control indicated a positive test result).15 Spirometry using electronic pneumotach spirometers by American Thoracic Society standards was determined at the index visit and follow-up visits at each study site.15
Levels of education and median household income were determined by geocoding address information to the census-block level and linking to census-based block group-level socioeconomic information.20 The Charlson Comorbidity Index,27 other comorbidities, and controller medication use were captured electronically from the KPSC Research Data Warehouse.20
The Asthma Risk Scale was determined by previously validated criteria that included ED visits or hospitalizations and dispensing of excessive SABA canisters or OCS.28 The Asthma Medication Ratio, a Healthcare Effectiveness Data and Information Set quality measure predictive of future asthma ED visits or hospitalizations, was assessed as the number of dispensed asthma controller units (inhaled controller medication canisters or 30-day supplies of oral controller medications) divided by the total number of controller units and SABA canisters dispensed. A ratio of 0.5 or greater is the minimum value for determining appropriate asthma controller medication management.29 The NAEPP step-care level was based on controller dispensing as previously reported.30 Dispensing of 9 or more units of controllers per year, equivalent to at least 75% adherence, was used as a proxy for adherence to treatment.31
Outcomes
The composite outcome was an asthma exacerbation or dispensing of 7 or more SABA canisters in the outcome year. Other outcomes were asthma exacerbations, asthma-related ED visits, hospitalizations, OCS dispensed, and 7 or more SABA canisters dispensed.
Statistical Analyses
The FeNO-assisted care was hypothesized to reduce the composite outcome by 25%. The estimate of sample size was based on unpublished data from a prior study,15 in which 116 (38.2%) of 304 patients with persistent asthma aged 12 to 56 years receiving ICS therapy had uncontrolled asthma as determined by an ACT score below 20. Of these 116 patients, 56 (48.3%) had 1 or more asthma exacerbations or 7 or more SABA canisters dispensed in the follow-up year. The present study had 80% power at the 0.05 level to detect a 25% reduction in the composite primary outcome with a total of 776 patients (388 per group). Given the needed time to identify about 400 eligible patients for the FeNO-assisted group and the need to identify both groups during the same period, we estimated that the standard-care group would be about 2-fold larger. The sample size was inflated to account for the atopic subgroup analysis.
Baseline covariates between the 2 study groups in the main cohort were balanced by using inverse probability of treatment weighting (IPTW).32,33 For each study subject, the probability of receiving FeNO-assisted care was first estimated by using logistic regression models based on the following covariates at baseline: 1) demographics (age, sex, race/ethnicity, geocoded median household income, state insurance program, Medicare, duration of Health Plan membership of ≥ 15 years, and current smoking); 2) comorbidities (obesity, Charlson Comorbidity Index, rhinitis, sinusitis, acute upper respiratory tract infection, infectious diseases, endocrine/immune disorders, dermatologic diseases, musculoskeletal disorders, and anxiety); 3) medication dispensing (≥ 7 SABA canisters, ICS, ICS/long-acting β2 agonist combination, leukotriene modifiers, 9 or more units of controllers, NAEPP step-care level, and Asthma Medication Ratio ≥ 0.5); 4) aeroallergen sensitization; and 5) indicators of uncontrolled asthma (OCS cumulative dose, abnormal result of forced expiratory volume in the first second of expiration/forced vital capacity [FEV1/FVC] for age, asthma exacerbations, ACT score below 16, and Asthma Risk Scale). A weight for each subject was calculated. For patients in the FeNO-assisted care group, the weight was the inverse of the propensity score calculated above; for patients in the standard-care group, the weight was the inverse of 1 minus the propensity score. The weights were then applied in regression models described in the next paragraph to estimate the group effects on the outcomes.32–34
To compare baseline covariates, a χ2 test was applied. Standardized differences were calculated to compare the patient baseline characteristics before and after the weights were applied.35 A standardized difference of variables between treatment groups less than 0.1 indicates good balance between comparison groups.34 Cox proportional hazards regression models were used to estimate hazard ratios of developing the outcomes and their 95% confidence intervals (CI). Poisson regression models with a robust standard error were used to estimate risk and rate ratios and their 95% CI.36,37 When risk ratios (RRs) were estimated, the analyses were limited to subjects who had complete follow-up in the 12-month follow-up year. The analyses were performed in all study subjects and in atopic patients with aeroallergen sensitization (prespecified). SAS (Version 9.3 for Windows, SAS Institute Inc, Cary, NC) was applied to analyze data. Tests were 2-tailed with significance set at p < 0.05.
RESULTS
Patient Characteristics
Eight hundred ninety-six patients were initially identified for the FeNO-assisted group and 2106 patients were identified for the standard-care group. After exclusion of patients with controlled asthma (ACT score ≥ 20), the final study groups with uncontrolled asthma consisted of 426 in the FeNO-assisted care group and 925 in the standard-care group (Figure 1).
Patients were predominately middle-aged, female, nonwhite, middle-income, nonsmokers, and long-term Health Plan enrollees (see Supplement Table S1, available at: www.thepermanentejournal.org/files/2019/18-109-Suppl.pdf). Comorbidities were frequent (46.6% obese, 66.3% with rhinitis, and 30.7% with sinusitis), and asthma burden in the prior year was high (56.0% had ≥ 1 asthma exacerbation, 19.2% had ≥ 7 SABA canisters dispensed, 65.3% were medium to high risk on the Asthma Risk Scale, and 30.9% required NAEPP step-care levels 5 to 6 [see Supplement Table S1, available at: www.thepermanentejournal.org/files/2019/18-109-Suppl.pdf]). At the initial visit, 58.6% of the patients were very poorly controlled with an ACT score less than 16, and 26.9% had an abnormal FEV1/FVC ratio for age. Aeroallergen sensitization, the phenotype most likely to benefit from FeNO-assisted care, was noted in 78.5% of the patients, with the majority (52.3%) sensitized to both perennial and pollen allergens (see Supplement Table S1, available at: www.thepermanentejournal.org/files/2019/18-109-Suppl.pdf).
In the FeNO-assisted group, the mean FeNO level (standard deviation) was 35.6 (33.5) ppb, with 79 patients (18.5%) at a high level (> 50 ppb), 100 (23.5%) at an intermediate level (25–50 ppb), 219 (51.4%) at a low level (< 25 ppb), and 28 (6.6%) without a test (testing not done or unacceptable at baseline). Of these latter 28 patients, 9 (32.1%) had a FeNO level determined during follow-up.
Study groups differed in many baseline features before IPTW adjustment and became balanced after IPTW adjustments (see Supplement Table S1, available at: www.thepermanentejournal.org/files/2019/18-109-Suppl.pdf).
Of the enrolled patients, 404 (94.8%) of 426 in the FeNO-assisted care group and 875 (94.6%) of 925 in the standard-care group had complete 1-year follow-up.
Primary Outcome
The primary outcome was 1 or more asthma exacerbations or 7 or more SABA dispensed canisters in the outcome year.
Main Study Cohort
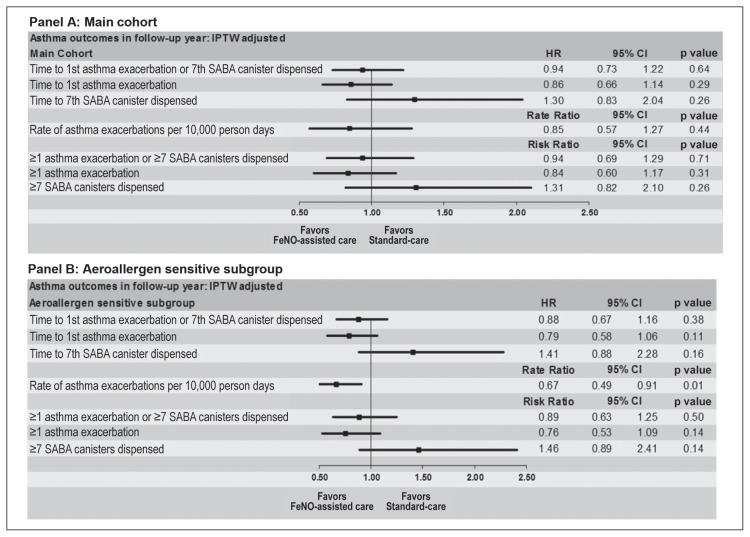
A similar frequency of the combined primary outcome (35.9% vs 37.2%, p = 0.71) occurred in FeNO-assisted care compared with the standard-care group (Table 2). Compared with standard care, FeNO-assisted care was associated with a similar risk (adjusted RR = 0.94, 95% CI = 0.69–1.29, p = 0.71) and hazard rate (adjusted hazard ratio = 0.94, 95% CI = 0.73–1.22, p = 0.64) of developing the composite outcome (Figure 2, Panel A).
Table 2.
Comparison of primary and secondary asthma outcomes and process factors in FeNO-assisted care and standard-care groups adjusted by Inverse-probability of treatment weighting (IPTW) in the main study cohorta
| Outcomes and Treatments | FeNO-assisted care (n = 401.5) | Standard-care (n = 877.7) | p valueb |
|---|---|---|---|
| Primary outcomes | |||
| ≥1 asthma exacerbation or ≥7 SABA dispensed canisters, no. (%) | 143.9 (35.9) | 326.6 (37.2) | 0.71 |
| Secondary outcomes | |||
| Asthma exacerbations | |||
| Rate 10,000 person-days (95% CI) | 15.6 (13.7–17.7) | 18.8 (17.0–20.0) | 0.44 |
| ≥1, N (%) | 124.9 (31.1) | 306.4 (34.9) | 0.31 |
| Any asthma hospitalizations, N (%) | 10.0 (2.5) | 9.2 (1.1) | 0.28 |
| Any asthma ED visits, N (%) | 17.4 (4.3) | 33.7 (3.8) | 0.80 |
| Any asthma hospitalizations or ED visits, N (%) | 18.7 (4.7) | 38.2 (4.4) | 0.87 |
| OCS cumulative dose, N (%) | 0.48 | ||
| None | 203.6 (50.7) | 458.1 (52.2) | |
| 0 – < 1.8 g | 172.1 (42.9) | 379.8 (43.3) | |
| 1.8 – < 3.6 g | 21.4 (5.3) | 31.5 (3.6) | |
| ≥ 3.6 g | 4.4 (1.1) | 8.3 (0.9) | |
| ≥7 SABA canisters dispensed | 34.5 (8.6) | 58.8 (6.7) | 0.26 |
| Treatments, no. (%) | |||
| Asthma Medication Ratio, ≥0.5, N (%) | 306.7 (76.4) | 711.5 (81.1) | 0.12 |
| Leukotriene modifiers, N (%) | 170.5 (42.5) | 404.0 (46.0) | 0.35 |
| Tiotropium bromide, N (%) | 37.7 (9.4) | 80.5 (9.2) | 0.92 |
| Asthma controllers | |||
| ≥1 | 358.9 (89.4) | 780.5 (88.9) | 0.83 |
| ≥9 | 204.3 (50.9) | 387.8 (44.2) | 0.08 |
| NAEPP step-care level, N (%) | 0.31 | ||
| 1 | 20.0 (5.0) | 34.9 (4.0) | |
| 2 | 44.4 (11.1) | 105.0 (12.0) | |
| 3 | 57.7 (14.4) | 119.1 (13.6) | |
| 4 | 128.0 (31.9) | 219.5 (25.0) | |
| 5 | 112.2 (27.9) | 315.4 (35.9) | |
| 6 | 16.5 (4.1) | 25.9 (3.0) | |
| unclassified | 22.6 (5.6) | 58.0 (6.6) | |
Number of patients may not be whole numbers because of weighting by IPTW. Frequency data includes patients with complete 12-month data and rate data includes entire main cohort of 1351 patients
p values were based on robust Poisson regression analyses adjusted by IPTW.
CI = confidence interval; ED = Emergency Department; FeNO = fractional exhaled nitric oxide; IPTW = inverse probability of treatment weighting; NAEPP = National Asthma Education Prevention Program; OCS = oral corticosteroid; SABA: short-acting β2-agonist.
Figure 2.
Adjusted hazard ratio (HR) (95% confidence interval [CI]), risk ratio (95% CI), or rate ratio (95% CI) of developing the asthma outcomes in the follow-up year in the main cohort. Panel A. All 1361 enrolled patients (426 FeNO-assisted care vs 925 standard-care patients) were included for estimating hazard and rate ratios, and 1279 patients (404 FeNO-assisted care vs 875 standard-care patients) with 12-month data were included for estimating risk ratio. Panel B: Atopic subgroup with aeroallergen sensitization. A total of 1061 patients (337 FeNO-assisted care vs 724 standard-care patients) were included for estimating hazard and rate ratio, and 1002 patients (317 FeNO-assisted care vs 685 standard-care patients) with 12-month data were included for estimating risk ratio.
FeNO = fractional exhaled nitric oxide; IPTW = inverse probability of treatment weighting; SABA = short-acting β2-agonist.
Atopic Subgroup with Aeroallergen Sensitization
In the prespecified subgroup analyses limited to only patients with aeroallergen sensitization (79.8% of the cohort), the FeNO-assisted care group exhibited a similar composite outcome compared with standard care (adjusted RR = 0.89, 95% CI = 0.63–1.25, p = 0.50 and adjusted hazard ratio = 0.88, 95% CI = 0.67–1.16, p = 0.38, Figure 2, Panel B).
Secondary Outcomes
Main Study Cohort
Compared with the standard-care group, the FeNO-assisted group evidenced a similar rate of asthma exacerbations (adjusted rate ratio = 0.85, 95% CI = 0.57–1.27) and risk of an asthma exacerbation (adjusted RR = 0.84, 95% CI = 0.60–1.17; Figure 2, Panel A).
Atopic Subgroup with Aeroallergen Sensitivity
The rate of asthma exacerbations (adjusted rate ratio 0.67, 95% CI 0.49–0.91, p = 0.01, Figure 2, Panel B) was less in the FeNO-assisted care compared with standard-care group. There were no significant differences between study groups in the frequency of or time to the first asthma exacerbation (Figure 2, Panel B).
DISCUSSION
We studied whether knowledge of FeNO levels added to guideline-based care would improve asthma outcomes in patients aged 12 years and older with persistent asthma who were receiving care in an Allergy Department within a managed care setting. We found that compared with standard care, FeNO-assisted care was not associated in the main cohort with reducing the primary composite outcome of dispensing of 7 or more SABA canisters or an asthma exacerbation, nor with these variables as separate outcomes. However, in an atopic subgroup with aeroallergen sensitization, the rate of asthma exacerbations was lower with FeNO-assisted treatment (adjusted rate ratio = 0.67, 95% CI = 0.49–0.91, p = 0.01). The significantly lower asthma exacerbation rate in the atopic subgroup with FeNO-assisted care was consistent with findings found in other atopic cohorts,18 particularly associated with varying asthma phenotypes.38–42
The results of the present study were generally consistent with findings from several meta-analyses of randomized controlled clinical trials carried out with varying designs on the added utility of FeNO measurements to standard guideline care in adults alone,43,44 children alone,45,46 or both children and adults.2,11,47 The recent Agency for Healthcare Research and Quality comparative evidence report on FeNO utility noted that the use of asthma management algorithms that incorporate FeNO testing in adults and children age 5 years and older reduced the risk of overall asthma exacerbations (strength of evidence: High), and possibly the risk of severe asthma exacerbations requiring oral steroids (strength of evidence: Moderate), but “did not affect other outcomes such as hospitalization, quality of life, asthma control, or FEV1% predicted.”18 The most recent meta-analysis of 7 randomized studies included a study in pregnant asthmatic patients48 and in primary care patients49 and compared tailoring asthma medications on the basis of FeNO levels vs primarily clinical symptoms. The meta-analysis found that the frequency of moderate asthma exacerbations decreased (odds ratio = 0.60, 95% CI = 0.43–0.84). However, the meta-analysis did not find a difference between FeNO intervention and controls in exacerbations requiring rescue OCS (odds ratio = 0.86, 95% CI = 0.50–1.48), hospitalizations, or daily clinical symptoms, end-of-study FeNO levels, or ICS dose. The meta-analysis concluded that “the universal use of FeNO to help guide therapy in adults with asthma cannot be advocated,” but because the main benefit was a reduction in asthma exacerbations, FeNO-tailored intervention “may be most useful in adults who have frequent exacerbations.”44 The randomized controlled study in primary care by Syk et al49 demonstrated that FeNO-guided care did not significantly improve the primary outcome of change in the Mini Asthma Quality of Life Questionnaire score but did improve significantly, but not to a clinically meaningful level, the score on the Asthma Control Questionnaire. Moreover, as in the present study, FeNO-guided care did not reduce severe exacerbations requiring systemic corticosteroids in the main cohort49; however, evidence for reduction in asthma exacerbations was noted in the atopic subgroup. Moderate exacerbations, defined as “deterioration in symptoms with the need to step up controller treatment for at least 2 days, with or without a concomitant clinic visit or contact” were reduced by FeNO-guided care,49 but were not measured in the present study. In the present study, there was some evidence that in an atopic subgroup, the rate of asthma exacerbations was reduced with FeNO-assisted care.
Differences in findings between the present study and those previously reported may in part be because of lack of randomization of the present study, infrequent follow-up visits given the real-world study design, and capturing more severe exacerbations requiring OCS in the present study. Other possible reasons for the differences include disparities between the utility of FeNO-assisted care in atopic and nonatopic patient phenotypes, treatment-directed decisions based on different FeNO cutoff points, different pharmacologic intervention algorithms, differences in asthma control status at baseline, and the presence of many asthma management programs at KPSC.
Several limitations of the current study deserve comment. IPTW statistically balanced the baseline differences between study groups; however, potential incomplete adjustment may still exist because administrative data did not allow for collection of all factors that may be related to future asthma exacerbation. As such, differences between nonrandomized groups may still exist, with potential bias remaining that could have affected the study findings. Because the study was designed to be real-world, follow-up visits were not prespecified, but determined by physician preference. As observed, only about 60% of patients in both study groups had a return Allergy Department visit. The infrequency of return visits limits the potential effect of FeNO assessments on management and asthma outcomes. In retrospect, the inclusion of nonatopic patients in the present study, who comprised more than 20% of the cohort, might have limited the effect seen with FeNO-assisted care. Although a recommended guideline of asthma management was given to FeNO-assisted group physicians, the dispensing of controllers and the NAEPP step-care levels were similar between groups during the outcome year. It was hypothesized that FeNO assessment would alter controller dispensing patterns on the basis of FeNO level. This was not observed. The institution of a systemwide KPSC program that changed ordering practices for albuterol prescriptions from multiple to only 1 canister per dispensing during the study was unanticipated and could have affected the primary composite outcome of albuterol use and exacerbations. It cannot be determined whether this change affected the 2 groups differentially, but the FeNO-assisted care group did evidence a higher proportion of patients at baseline with 7 or more dispensed SABA canisters. Another potential limitation was that exacerbations required physician coding of specific events, which could be inconsistent among physicians.
It should be highlighted that a managed care organization like KPSC delivers health care in an organized system that manages cost, utilization, and quality. Managed care members of KPSC may have different characteristics from patients in nonmanaged care organizations, and, as such, the present findings may not be generalizable to all patients with asthma. It also should be noted that FeNO determination has associated costs, both tangible (equipment and disposable materials) and intangible (medical assistant and patient’s time), with the latter potentially interfering with usual patient flow in a busy practice. As such, cost-benefit analyses, not done in the present study, are needed. As noted, 1 study provided evidence suggestive of an economic benefit.12
CONCLUSION
This observational study used IPTW adjustment to balance differing baseline covariates between study groups and compared FeNO-assisted care with standard care among allergists. The study did not observe an improvement in overall asthma control assessed by a composite primary outcome of excess SABA use or an asthma exacerbation with FeNO-assisted care compared with standard care for either the primary composite outcome or secondary outcomes in the main cohort composed of patients with both atopic and nonatopic asthma. However, in the atopic subgroup a lower asthma exacerbation rate was found with FeNO-assisted care, which agreed with findings from real-world randomized studies. Given the lack of randomization in the current study and the need to statistically balance baseline differences between study groups, cautious interpretation of the study findings is needed. The lessons learned from the present study could be the impetus for more definitive real-world randomized studies, particularly in determining the utility of FeNO-assisted care in patients with evidence of atopic type 2 asthma.50
Acknowledgments
Circassia Pharmaceuticals AB (formerly Aerocrine AB), Oxford, UK, funded this study by a grant to Kaiser Permanente Southern California Research and Evaluation Department. Kaiser Permanente Southern California developed the protocol and performed data collection, extraction, analysis, and manuscript preparation. Aerocrine AB approved the protocol and manuscript.
We thank and received approval from William W Crawford, MD; Noah J Friedman, MD; Eve H Gordon, MD; Margaret L Kurohara, MD; and Luis F Saca, MD; Chiefs of Service of the KPSC Allergy Departments to acknowledge their and their staffs’ assistance in the conduct of the study.
Kathleen Louden, ELS, of Louden Health Communications performed a primary copy edit.
Footnotes
Disclosure Statement
R S Zeiger has received research support from Aerocrine AB, Oxford, UK; AstraZeneca/MedImmune, Gaithersburg, MD; Genentech, South San Francisco, CA; GlaxoSmithKline Corp, Brentford, Middlesex, UK; Merck & Co, Kenilworth, NJ; and the US National Heart, Lung, and Blood Institute, Bethesda, MD. R S Zeiger also has received consultancy fees from Astra Zeneca, London, UK; F. Hoffman-La Roche AG, Basel, Switzerland; GlaxoSmithKline; Novartis Corp, Basel, Switzerland; Merck & Co; Patara Pharma, San Diego, CA; Regeneron Pharmaceuticals, Tarrytown, NY; Sanofi SA, Paris, France; Teva Pharmaceutical Industries Ltd, Peta Tikvah, Israel; and Theravance Biopharma, South San Francisco, CA. M Schatz has received research support from Aerocrine AB, GlaxoSmithKline, AstraZeneca/MedImmune, and Merck & Co. S-J Yang and W Chen received research support from Kaiser Permanente Southern California.
Authors’ Contributions
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors and were entirely responsible for the conduct of the study and writing of the manuscript.
References
- 1.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6(9):1368–70. [PubMed] [Google Scholar]
- 2.Harnan S, Essat M, Gomersall T, et al. Exhaled nitric oxide for the diagnosis of asthma in adults and children: A systematic review. Value Health. 2015 Nov;18(7):A345. doi: 10.1016/j.jval2015.09.607. [DOI] [Google Scholar]
- 3.Nordvall SL, Janson C, Kalm-Stephens P, Foucard T, Toren K, Alving K. Exhaled nitric oxide in a population-based study of asthma and allergy in school children. Allergy. 2005 Apr;60(4):469–75. doi: 10.1111/j.1398-9995.2005.00735.x. [DOI] [PubMed] [Google Scholar]
- 4.Pedrosa M, Cancelliere N, Barranco P, López-Carrasco V, Quirce S. Usefulness of exhaled nitric oxide for diagnosing asthma. J Asthma. 2010;47(7):817–21. doi: 10.3109/02770903.2010.491147. [DOI] [PubMed] [Google Scholar]
- 5.Pijnenburg MW, Hofhuis W, Hop WC, de Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005 Mar;60(3):215–8. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harkins MS, Fiato KL, Iwamoto GK. Exhaled nitric oxide predicts asthma exacerbation. J Asthma. 2004 Jun;41(4):471–6. doi: 10.1081/jas-120033990. [DOI] [PubMed] [Google Scholar]
- 7.Langley EW, Gebretsadik T, Hartert TV, Peebles RS, Jr, Arnold DH. Exhaled nitric oxide is associated with severity of pediatric acute asthma exacerbations. J Allergy Clin Immunol Pract. 2014 Sep-Oct;2(5):618–20. doi: 10.1016/j.jaip.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsara M, Donnelly D, Iqbal S, Elliott T, Everard ML. Relationship between exhaled nitric oxide levels and compliance with inhaled corticosteroids in asthmatic children. Respir Med. 2006 Sep;100(9):1512–7. doi: 10.1016/j.rmed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005 Mar 26;352(21):2163–73. doi: 10.1056/nejmoa043596. [DOI] [PubMed] [Google Scholar]
- 10.Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: A randomised controlled trial. Lancet. 2008 Sep;372(9643):1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petsky HL, Cates CJ, Lasserson TJ, et al. A systematic review and meta-analysis: Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils) Thorax. 2012 Mar;67(3):199–209. doi: 10.1136/thx.2010.135574. [DOI] [PubMed] [Google Scholar]
- 12.Honkoop PJ, Loijmans RJ, Termeer EH, et al. Asthma Control Cost-Utility Randomizer Trial Evalution (ACCURATE) Study Group. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: A cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015 Mar;135(3):682–8.e11. doi: 10.1016/j.jaci.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Price D, Ryan D, Burden A, et al. Using fractional exhaled nitric oxide (FeNO) to diagnose steroid-responsive disease and guide asthma management in routine care. Clin Transl Allergy. 2013 Nov 7;3(1):37. doi: 10.1186/2045-7022-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaForce C, Brooks E, Herje N, Dorinsky P, Rickard K. Impact of exhaled nitric oxide measurements on treatment decisions in an asthma specialty clinic. Ann Allergy Asthma Immunol. 2014 Dec;113(6):619–23. doi: 10.1016/j.anai.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Zeiger RS, Schatz M, Zhang F, et al. Association of exhaled nitric oxide to asthma burden in asthmatics on inhaled corticosteroids. J Asthma. 2011 Feb;48(1):8–17. doi: 10.3109/02770903.2010.539295. [DOI] [PubMed] [Google Scholar]
- 16.Zeiger RS, Schatz M, Zhang F, et al. Elevated exhaled nitric oxide is a clinical indicator of future uncontrolled asthma in asthmatic patients on inhaled corticosteroids. J Allergy Clin Immunol. 2011 Aug;128(2):412–4. doi: 10.1016/j.jaci.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Dweik RA, Boggs PB, Erzurum SC, et al. American Thoracic Society Committee on Inerpretatin of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide Levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011 Sep 1;184(5):602–15. doi: 10.1164/rccm.9120-11st. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Pianosi P, Keogh K, et al. Comparative Effectiveness Review no. 197 [Internet] Rockville, MD: Agency for Healthcare Research and Quality; 2017. The clinical utility of fractional exhaled nitric oxide (FeNO) in asthma management. [cited 17 Sept 2018]. AHRQ Publication no. 17(18)-EHC030-EF. Available from: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/cer-197-fractional-exhaled-nitric-oxide.pdf. [PubMed] [Google Scholar]
- 19.Guidelines for the diagnosis and management of asthma (EPR-3) [Internet] Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. Aug, [cited 2018 Dec 27]. Available from: www.nhlbi.nih.gov/guidelines/asthma/epr3/index.htm. [Google Scholar]
- 20.Zeiger RS, Schatz M, Dalal AA, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016 Jan-Feb;4(1):120–9. doi: 10.1016/j.jaip.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Silkoff PE, Wakita S, Chatkin J, et al. Exhaled nitric oxide after beta2-agonist inhalation and spirometry in asthma. Am J Respir Crit Care Med. 1999;159(3):940–4. doi: 10.1164/ajrccm.159.3.9805044. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society (ATS); European Respiratory Society (ERS) ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005 Apr 15;171(8):912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 23.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004 Jan;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719–23. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 25.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–56. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Schatz M, Zeiger RS, Vollmer WM, et al. Validation of a beta-agonist long-term asthma control scale derived from computerized pharmacy data. J Allergy Clin Immunol. 2006;117(5):995–1000. doi: 10.1016/j.jaci.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Schatz M, Nakahiro R, Jones CH, Roth RM, Joshua A, Petitti D. Asthma population management: Development and validation of a practical 3-level risk stratification scheme. Am J Manag Care. 2004 Jan;10(1):25–32. [PubMed] [Google Scholar]
- 29.Schatz M, Zeiger RS, Vollmer WM, et al. The controller-to-total asthma medication ratio is associated with patient-centered as well as utilization outcomes. Chest. 2006 Jul;130(1):43–50. doi: 10.1378/chest.130.1.43. [DOI] [PubMed] [Google Scholar]
- 30.Zeiger RS, Schatz M, Li Q, Zhang F, Purdum AS, Chen W. Step-up care improves impairment in uncontrolled asthma: An administrative data study. Am J Manag Care. 2010;16(12):897–906. [PubMed] [Google Scholar]
- 31.Guidelines and measures National Quality Measures Clearinghouse [Internet] Rockville, MD: Agency for Healthcare Research and Quality; 2017. [cited 2016 Aug 19]. Available from: www.qualitymeasures.ahrq.gov/content.aspx?id=48617. [DOI] [PubMed] [Google Scholar]
- 32.Rosenbaum P. Model-based direct adjustment. J Am Statist Assoc. 1987;82(398):387–94. doi: 10.2307/2289440. [DOI] [Google Scholar]
- 33.Hirano K, Imbens G. Estimation of causal effects using propensity score weighting: An application to data on right heart catheterization. Health Services and Outcome Research Methodology. 2001;2(3–4):259–78. [Google Scholar]
- 34.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J Clin Epidemiol. 2001 Apr;54(4):387–98. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 35.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Shi J, Qian L, Azen SP. Comparison of robustness to outliers between robust Poisson models and log-binomial models when estimating relative risks for common binary outcomes: A simulation study. BMC Med Res Methodol. 2014 Jun 26;14:82. doi: 10.1186/1471-2288-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denlinger LC, Phillips BR, Ramratnam S, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program-3 Investigators. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–13. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loymans RJ, Sterk PJ. Exacerbation-prone asthma: A separate bioclinical phenotype? Am J Respir Crit Care Med. 2017 Feb 1;195(3):275–7. doi: 10.1164/rccm.201609-1819ED. [DOI] [PubMed] [Google Scholar]
- 40.Zeiger RS, Schatz M, Li Q, et al. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2(6):741–50. doi: 10.1016/j.jaip.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Qiu R, Xie J, Chung KF, et al. Asthma phenotypes defined from parameters obtained during recovery from a hospital-treated exacerbation. J Allergy Clin Immunol Pract. 2018 Nov-Dec;6(6):1960–7. doi: 10.1016/j.jaip.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Fitzpatrick AM, Moore WC. Severe asthma phenotypes—how should they guide evaluation and treatment? J Allergy Clin Immunol Pract. 2017 Jul-Aug;5(4):901–8. doi: 10.1016/j.jaip.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Essat M, Harnan S, Gomersall T, et al. Fractional exhaled nitric oxide for the management of asthma in adults: A systematic review. Eur Respir J. 2016 Mar;47(3):751–68. doi: 10.1183/13993003.01882-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petsky HL, Kew KM, Turner C, Chang AB. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst Rev. 2016 Sep 1;9:CD011440. doi: 10.1002/14651858.CD011440.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jartti T, Wendelin-Saarenhovi M, Heinonen I, Hartiala J, Vanto T. Childhood asthma management guided by repeated FeNO measurements: A meta-analysis. Paediatr Respir Rev. 2012;13(3):178–83. doi: 10.1016/j.prrv.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Lu M, Wu B, Che D, Qiao R, Gu H. FeNO and asthma treatment in children: A systematic review and meta-analysis. Medicine (Baltimore) 2015 Jan;94(4):e347. doi: 10.1097/MD.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehtimaki L, Csonka P, Makinen E, Isojärvi J, Hovi SL, Ahovuo-Saloranta A. Predictive value of exhaled nitric oxide in the management of asthma: A systematic review. Eur Respir J. 2016 Sep;48(3):706–16. doi: 10.1183/13993003.00699-2016. [DOI] [PubMed] [Google Scholar]
- 48.Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: A double-blind, randomised controlled trial. Lancet. 2011 Sep 10;378(9795):983–90. doi: 10.1016/S0140-6736(11)60971-9. [DOI] [PubMed] [Google Scholar]
- 49.Syk J, Malinovschi A, Johansson G, et al. Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: A randomized, controlled trial. J Allergy Clin Immunol Pract. 2013 Nov-Dec;1(6):639–48.e8. doi: 10.1016/j.jaip.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Murphy VE, Jensen ME, Mattes J, et al. The Breathing for Life Trial: A randomised controlled trial of fractional exhaled nitric oxide (FENO)-based management of asthma during pregnancy and its impact on perinatal outcomes and infant and childhood respiratory health. BMC Pregnancy Childbirth. 2016 May;17:111. doi: 10.1186/s12884-016-0890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]