Key Points
Question
What are the facial nerve monitoring parameters associated with postoperative facial nerve weakness after parotid surgery?
Findings
In this case series that included 222 patients who underwent parotidectomy for benign disease, a postdissection threshold of greater than 0.25 milliamperes (mA) and having more than 8 mechanical events was associated with immediate postoperative nerve weakness, whereas a postdissection threshold of 0.25 mA or less and having 8 or fewer mechanical events was associated with normal postoperative facial nerve function.
Meaning
Accurate prediction of facial nerve function after parotid surgery may provide anticipatory guidance to patients and may provide surgeons with intraoperative feedback, which allows for adjustment in operative techniques and perioperative management.
This case series study analyzes intraoperative facial nerve monitoring data from adult patients who had a parotidectomy operation and identifies the monitoring parameters associated with postoperative nerve weakness.
Abstract
Importance
Prior studies suggest that the use of facial nerve monitoring decreases the rate of immediate postoperative facial nerve weakness in parotid surgery, but published data are lacking on normative values for these parameters or cutoff values to prognosticate facial nerve outcomes.
Objective
To identify intraoperative facial nerve monitoring parameters associated with postoperative weakness and to evaluate cutoff values for these parameters under which normal nerve function is more likely.
Design, Setting, and Participants
This retrospective case series of 222 adult patients undergoing parotid surgery for benign disease performed with intraoperative nerve monitoring was conducted at an academic medical institution from September 13, 2004, to October 30, 2014. The data analysis was conducted from May 2018 to January 2019.
Main Outcomes and Measures
The main outcome measure was facial nerve weakness. Receiver operating characteristic curves were generated to define optimal cut point to maximize the sensitivity and specificity of the stimulation threshold, mechanical events, and spasm events associated with facial nerve weakness.
Results
Of 222 participants, 121 were women and 101 were men, with a mean (SD) age of 51 (16) years. The rate of temporary facial nerve paresis of any nerve branch was 45%, and the rate of permanent paralysis was 1.3%. The mean predissection threshold was 0.22 milliamperes (mA) (range, 0.1-0.6 mA) and the mean postdissection threshold was 0.24 mA (range, 0.08-1.0 mA). The average number of mechanical events was 9 (range, 0-66), and mean number of spontaneous spasm events was 1 (range, 0-12). Both the postdissection threshold (area under the curve [AUC], 0.69; 95% CI, 0.62-0.77) and the number of mechanical events (AUC, 0.58; 95% CI, 0.50-0.66) were associated with early postoperative facial nerve outcome. The number of spasm events was not associated with facial nerve outcome. The optimal cutoff value for the threshold was 0.25 mA, and the optimal cutoff for number of mechanical events was 8. If a threshold of greater than 0.25 mA was paired with more than 8 mechanical events, there was a 77% chance of postoperative nerve weakness. Conversely, if a threshold was 0.25 mA or less and there were 8 mechanical events or less, there was 69% chance of normal postoperative nerve function. No parameters were associated with permanent facial nerve injury.
Conclusions and Relevance
Postdissection threshold and the number of mechanical events are associated with immediate postoperative facial nerve function. Accurate prediction of facial nerve function may provide anticipatory guidance to patients and may provide surgeons with intraoperative feedback allowing adjustment in operative techniques and perioperative management.
Introduction
Facial nerve injury is the most serious complication of parotid surgery. The rates of transient paresis in the literature range from 9% to 65%, while the rates of permanent paralysis range from 4% to 7%.1,2,3,4,5 Risk factors for facial nerve injury include revision surgery, advanced age, extent of surgery, inflammatory conditions, duration of surgery, and malignant disease.1,3,6,7 The consequences of facial nerve weakness include facial asymmetry, mastication difficulty, drooling, nasal obstruction, corneal abrasion, psychosocial distress, and possibly costly medical litigation.
The benefits of routine intraoperative facial nerve monitoring (FNM) have been clearly established in neurotologic surgery, but its utility in otolaryngologic procedures where the facial nerve is at risk remains controversial.2,8 Several prior studies have demonstrated that FNM decreases the risk of immediate postoperative facial nerve dysfunction but does not appear to influence final outcomes.6,9,10,11,12 Despite this, the use of intraoperative monitoring has become increasingly widespread. A survey performed in 2005 demonstrated that 60% of practicing otolaryngologists used intraoperative FNM for parotid surgery.13 Advocates suggest that monitoring may be a beneficial adjunct in patients with bulky tumors and in revision surgery.5 Others have demonstrated decreased operative times2,14 and increased patient satisfaction with the use of intraoperative monitoring.15
In an effort to use nerve monitoring for prognostication, several studies have attempted to define cutoff values for nerve monitoring parameters in FNM during neurotologic or skull base surgery16,17,18 and in recurrent laryngeal nerve monitoring during thyroid or parathyroid surgery.19,20,21,22,23 However, these cutoff values cannot be generalized to predict facial nerve outcomes in parotid surgery. There is a dearth of data reporting normative values for FNM parameters during parotidectomy, and there are no known cutoff values to prognosticate facial nerve outcomes. The objective of this study is to identify intraoperative nerve monitoring parameters that are associated with postparotidectomy facial nerve weakness and to evaluate clinically relevant cutoff values for these parameters associated with greater likelihood of normal postoperative nerve function.
Methods
Study Population
This study was approved by the institutional review board at the University of Michigan and written informed consent was obtained from all participants. We performed a retrospective analysis of a prospectively collected database of all patients who underwent primary parotidectomy for benign disease with intraoperative nerve monitoring between September 13, 2004, and October 30, 2014. Patients with malignant pathologic characteristics, preoperative facial nerve weakness, and patients with missing nerve monitoring data or postoperative nerve function data were excluded. Analysis took place from May 2018 to January 2019.
Other variables collected included patient demographics, tumor size, final tumor pathology, operative time, surgical procedure (superficial or deep/total parotidectomy), approach to the facial nerve (anterograde or retrograde), immune status, and intraoperative steroid dosing. Analysis of postoperative facial nerve function was based on documented postoperative notes, progress notes, and/or discharge summaries. Immediate facial nerve function was collected as a binary variable (weak vs not weak) and classified as any degree of asymmetry or paresis/paralysis in any facial nerve branch that developed within 14 days after surgery. Permanent nerve injury was defined as weakness in 1 or more branches of the facial nerve that lasted for 1 year or longer postoperatively.
Surgery and Intraoperative Monitoring
All procedures were performed under general anesthesia. Long-acting muscle paralysis was avoided owing to use of nerve monitoring. Surgical technique followed standard parotidectomy approaches, including both superficial and total parotidectomy. The approach taken to identify the nerve (anterograde vs retrograde) depended on surgeon preference and location of the tumor.
Facial nerve monitoring was performed by a trained audiologist using a 4-channel recording technique. Pairs of subdermal needle electrodes were positioned into the frontalis, orbicularis oculi, orbicularis oris, and mentalis muscles to monitor the temporal, zygomatic, buccal, and marginal mandibular branches of cranial nerve VII.24 The monitoring system was equipped to monitor nerve function with both continuous free-running electromyography (EMG) and by eliciting and recording compound muscle action potentials (CMAPs) evoked by direct nerve stimulation using a monopolar probe.25 Typical recording parameters were to use a differential amplification with 5000-dB gain and 10- to 1500-Hz band-pass filters. For free-running EMG, a 1-second sweep time duration was used; 50 milliseconds was used for stimulated EMG. Sensitivity was typically a 50-microvolt (μV) window (ie, 5 μV/division) for free run; stimulated EMG varied between 50 and 500 μV.
Prior to tumor extirpation, the facial nerve was identified, and the main trunk (in the case of anterograde nerve dissection) or a peripheral nerve branch (in the case of retrograde dissection) was stimulated to obtain a predissection threshold. After tumor extirpation, a stimulus threshold was established from the facial nerve proximal to the tumor bed. The final threshold was defined as the lowest stimulus that resulted in a CMAP in each peripheral nerve branch. For example, if all branches except the marginal mandibular branch responded when the main trunk was stimulated at 0.2 milliamperes (mA), and the marginal mandibular branch responded at 0.5 mA, the final threshold was recorded as 0.5 mA. If only 1 nerve branch was exposed and dissected during the surgery, the final threshold was the lowest stimulus resulting in a CMAP in that branch.
Neurophysiologic events were classified as mechanical events in which free-running EMG activity was elicited by surgical manipulation of the nerve, or as spasm events, in which free-running EMG was present from 1 or more channels associated with 1 or more facial muscles occurring seemingly spontaneously or owing to an unrecognized or unknown stimulus such as stretch, thermal stimulation, or irrigation.
Statistical Analysis
Normative FNM data was tabulated and included the mean predissection and postdissection thresholds, mean number of mechanical events, and the mean number of spasm events. Paired t tests were used to compare differences in mean postdissection threshold, mechanical events, and spasm events between patients with immediate postoperative nerve weakness and those with normal postoperative nerve function. Effect sizes and associated 95% confidence intervals (CIs) were calculated using Cohen d values. A Cohen d value of 0.2 was considered a small effect size; 0.5 was considered a medium effect size, and 0.8 a large effect size.26 Receiver operating characteristic (ROC) curves were generated to identify cutoff points in the FNM parameters associated with early and late postoperative nerve outcomes. From the ROC curves, we obtained area under the curve (AUC) of each monitoring parameter. Cutoff values for postdissection threshold, number of mechanical events, and number of spasm events were determined in the ROC curves to maximize the sensitivity and specificity. From these cutoff points, the positive and negative predictive values, sensitivity, specificity, and likelihood ratios of the FNM parameters were tabulated, and Fisher exact tests were performed. Positive and negative likelihood ratios for nerve monitoring parameters were also calculated.
Results
Patient and Tumor Characteristics
Data from 238 patients who underwent primary parotidectomy for benign disease were identified. Sixteen patients were excluded owing to insufficient FNM data or follow-up data (n = 12) or preexisting facial nerve weakness (n = 4). Demographic and clinical data from the remaining 222 patients (121 women, 101 men, mean [SD] age, 51 (16) years) are summarized in Table 1. Most tumors were pleomorphic adenomas (n = 175) followed by Warthin tumors (n = 47). The rate of immediate postoperative facial nerve weakness in any branch of the facial nerve (0-14 days after surgery) was 45% (n = 100). The rate of permanent facial nerve weakness (1 year after surgery) was 1.9% (n = 3). Thus, the vast majority of patients who developed immediate facial nerve weakness had full recovery of nerve function by 1 year postoperatively. Because so few patients had permanent facial nerve weakness, immediate facial nerve weakness was used as the primary outcome for the analyses. There were no differences in patient age, size of tumor, operating time, facial nerve dissection technique (anterograde vs retrograde vs no dissection), immune status, or intraoperative steroid dosing for patients who had normal postoperative facial nerve function compared with those with temporary facial nerve weakness.
Table 1. Demographic and Clinical Dataa.
| Characteristic | Not Weak (n = 122) | Weak (n = 100) |
|---|---|---|
| Age, mean (SD), y | 51.2 (17.6) | 50.9 (14.8) |
| Sex | ||
| Male | 51/122 (42) | 50/100 (50) |
| Female | 71/122 (58) | 50/100 (50) |
| Size of tumor, mean (SD), cm | 2.7 (1.7) | 2.9 (1.8) |
| Operating room time, mean (SD), min | 167 (65) | 182 (70) |
| Procedure | ||
| Superficial parotidectomy | 96/122 (79) | 68/100 (68) |
| Deep/total parotidectomy | 26/122 (21) | 32/100 (32) |
| Nerve dissection technique | ||
| Anterograde | 91/122 (75) | 84/100 (84) |
| Retrograde | 27/122 (22) | 15/100 (15) |
| Nerve not dissected | 4/122 (3) | 1/100 (1) |
| Immunocompromised | ||
| Yes | 6/122 (5) | 6/100 (6) |
| No | 116/122 (95) | 94/100 (94) |
| Intraoperative steroids | ||
| High dose (>4 mg) | 33/121 (27) | 26/98 (27) |
| Low dose (≤4 mg) | 77/121 (64) | 64/98 (65) |
| None | 11/121 (9) | 8/98 (8) |
Unless otherwise noted, all values reported as number (percentage) of study participants.
Normative Electrophysiologic Data
Normative data for our 3 FNM parameters of interest were established. The mean (SD) predissection threshold was 0.22 (0.1) mA, and the mean (SD) postdissection threshold was 0.24 (0.14) mA. Only 19% of patients (43 of 222) had predissection thresholds recorded, and thus threshold shift, or the difference between the predissection and postdissection thresholds was not analyzed. The average number of mechanical events was 9, but this ranged from 0 to 66 events. The average number of spasm events was 1, ranging from 0 to 12 events.
Nerve Monitoring Parameters Associated With Postoperative Nerve Outcome
The mean (SD) postdissection threshold was higher in patients with immediate postoperative nerve weakness (0.29 [0.17] mA) compared with those with normal postoperative nerve function (0.20 [0.10] mA; Cohen, d = 0.65; 95% CI, 0.38-0.92). There were no clinically meaningful differences in the mean number of mechanical events or spasm events between groups.
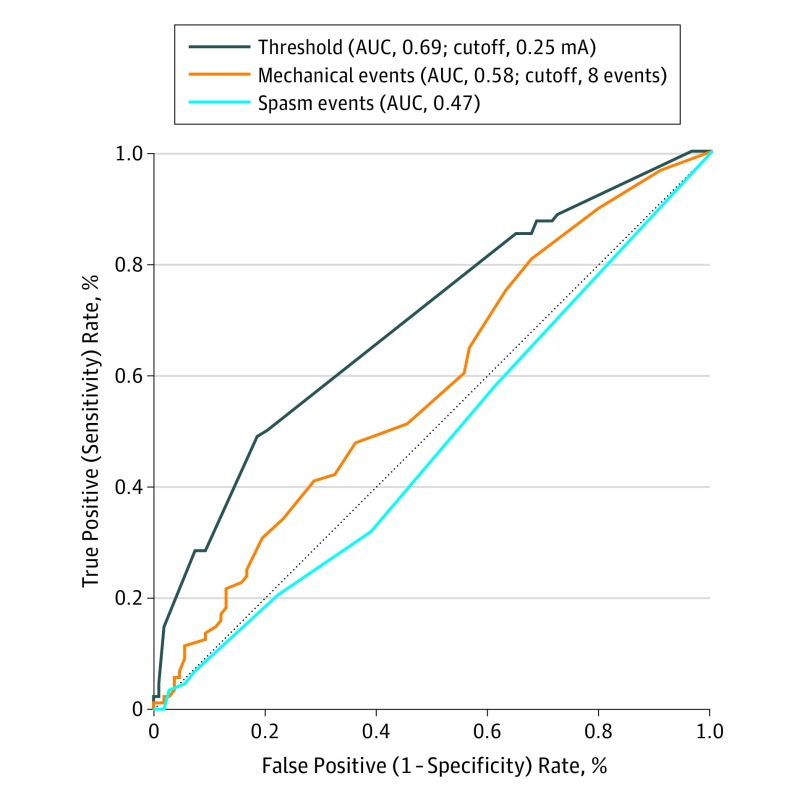
The ROC curves are presented in Figure 1. The area under the ROC curves demonstrates that the postdissection threshold and the number of mechanical events were associated with early postoperative nerve outcomes, while the number of spasm events were not. The AUC for postdissection threshold was 0.69 (95% CI, 0.62-0.77); for mechanical events, 0.58 (95% CI, 0.50-0.66); and for spasm events, 0.47 (95% CI, 0.39-0.55). The optimal cutoff value, defined as the point on the ROC curve that maximized sensitivity and specificity for facial nerve outcome, for postdissection threshold was 0.25 mA with a sensitivity of 47% (95% CI, 37%-57%), specificity of 82% (95% CI, 74%-88%), positive likelihood ratio of 2.70, and negative likelihood ratio of 0.64. The optimal cutoff value for mechanical events was 8 events, with a sensitivity of 42% (95% CI, 32%-53%), specificity of 69% (95% CI, 59%-77%), positive likelihood ratio of 1.34, and negative likelihood ratio of 0.84. Cutoff values for spasm events were not determined because this FNM parameter was not shown to be significantly associated with facial nerve outcome. ROC curves were also created for late or permanent facial nerve outcome and showed no statistically significant association with any FNM parameter, potentially owing to an underpowered analysis. Figure 2 presents scatter plots demonstrating how the study population’s threshold values, number of intraoperative mechanical events, and number of spasm events relate to screening cutoff values.
Figure 1. Receiver Operating Characteristic (ROC) Curves for Facial Nerve Monitoring Parameters.
The ROC curves demonstrate the association of threshold, mechanical events, and spasm events with immediate facial nerve outcome. The diagonal reference line represents a test that randomly guesses the outcome and thus is a worthless test. AUC indicates area under the receiver operating curve.
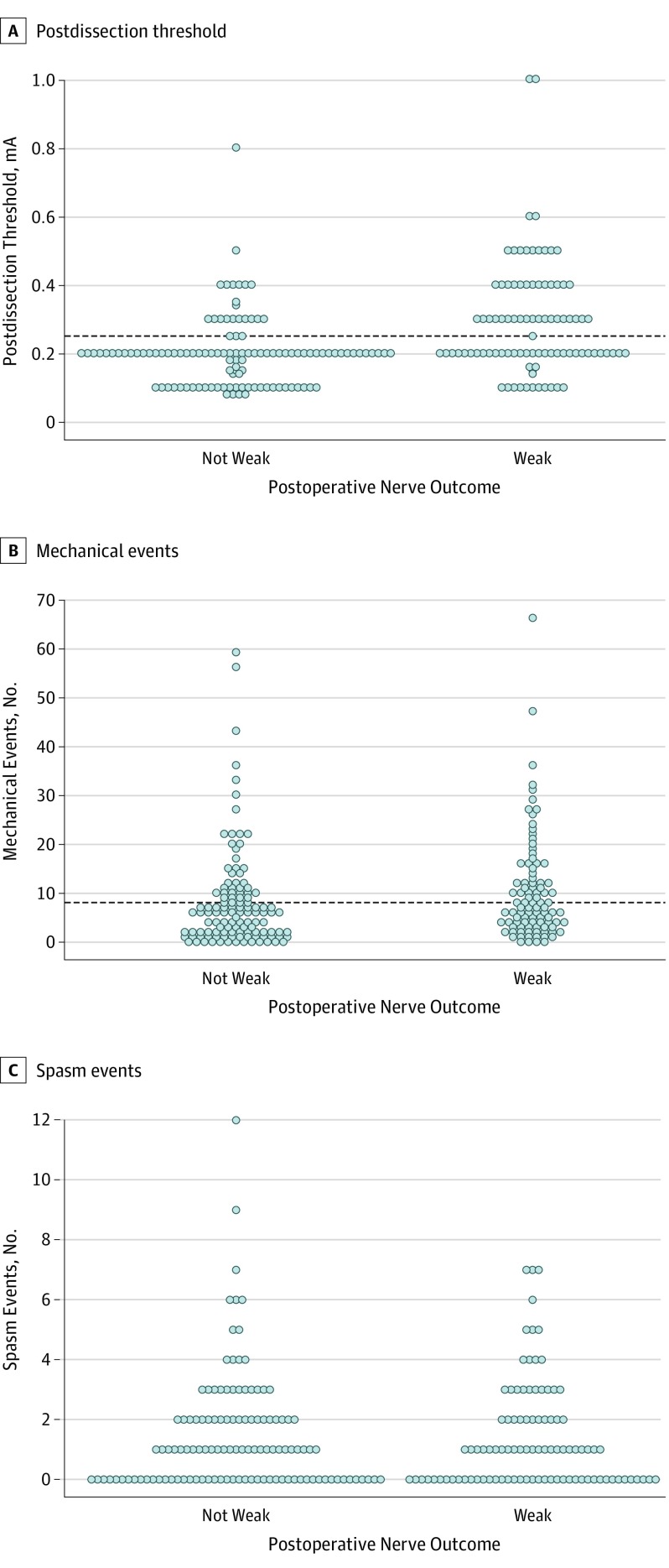
Figure 2. Postdissection Thresholds, Mechanical Events, and Spasm Events.
Each dot represents a single patient’s postdissection threshold value (A), number of intraoperative mechanical events (B), and number of intraoperative spasm events (C). The horizontal line represents the optimal cutoff value associated with postoperative nerve outcome. A value above the horizontal line suggests early postoperative nerve weakness. No cutoff value is provided for spasm events (C) because it was not statistically significant. mA Indicates milliamperes.
Sensitivity, specificity, positive predictive value (PPV), negative predictive values (NPV), positive likelihood, and negative likelihood ratios for the selected cutoff values are detailed in Tables 2 and 3. If the postdissection threshold was greater than 0.25 mA, then there was a 69% chance that the patient had immediate postoperative nerve weakness (95% CI, 57%-79%) vs 34% chance of postoperative nerve weakness (95% CI, 26%-43%) when the threshold was 0.25 mA or less (Table 2). Similarly, for mechanical events, if the patient experienced greater than 8 mechanical events, there was a 52% chance of immediate postoperative nerve weakness (95% CI, 40%-60%) compared with 40% (95% CI, 32%-49%) when there were 8 or fewer mechanical events (Table 2). If a postdissection threshold of greater than 0.25 mA was paired with more than 8 mechanical events, the PPV increased to 77% (95% CI, 57%-90%), the specificity increased to 96% (95% CI, 91%-99%), and the positive likelihood ratio increased to 4.28. If a postdissection threshold of 0.25 mA or less was paired with 8 or fewer mechanical events, the NPV increased to 69% (95% CI, 61%-77%), the sensitivity increased to 71% (95% CI, 60%-81%), and the negative likelihood ratio decreased to 0.54 (Table 3).
Table 2. Postdissection Threshold and Mechanical Events Associated With Postoperative Nerve Outcome.
| Weak | Not Weak | Total | Probability, % (95% CI) | |
|---|---|---|---|---|
| PPV, threshold >0.25 mA | 44 | 20 | 64 | 69 (58-79) |
| NPV, threshold ≤0.25 mA | 49 | 94 | 143 | 65 (61-70) |
| Total | 93 | 114 | 207 | NA |
| Sensitivity of positive likelihood ratio, 2.70 | NA | NA | NA | 47 (37-57) |
| Specificity of negative likelihood ratio, 0.64 | NA | NA | NA | 82 (74-88) |
| PPV, mechanical events >8 | 40 | 37 | 77 | 52 (43-61) |
| NPV, mechanical events ≤8 | 55 | 81 | 136 | 60 (54-64) |
| Total | 95 | 118 | 213 | NA |
| Sensitivity of positive likelihood ratio, 1.34 | NA | NA | NA | 42 (32-53) |
| Specificity of negative likelihood ratio, 0.84 | NA | NA | NA | 69 (59-77) |
Abbreviations: NA, not applicable; NPV, negative predictive value; PPV, positive predictive value.
Table 3. Paired Postdissection Threshold and Mechanical Events Associated With Postoperative Nerve Outcome.
| Weak | Not Weak | Total | Probability, % (95% CI) | |
|---|---|---|---|---|
| PPV, threshold >0.25 mA and mechanical >8 | 17 | 5 | 22 | 77 (57-90) |
| NPV, threshold ≤0.25 mA or mechanical ≤8 | 72 | 107 | 179 | 58 (57-62) |
| Total | 89 | 112 | 201 | NA |
| Sensitivity of positive likelihood ratio, 4.28 | NA | NA | NA | 19 (12-29) |
| Specificity of negative likelihood ratio, 0.85 | NA | NA | NA | 96 (90-99) |
| PPV, threshold >0.25 mA or mechanical >8 | 63 | 50 | 113 | 56 (50-62) |
| NPV, threshold ≤0.25 mA and mechanical ≤8 | 26 | 59 | 85 | 69 (61-77) |
| Total | 89 | 109 | 198 | NA |
| Sensitivity of positive likelihood ratio, 1.54 | NA | NA | NA | 71 (60-81) |
| Specificity of negative likelihood ratio, 0.54 | NA | NA | NA | 54 (44-64) |
Abbreviations: NA, not applicable; NPV, negative predictive value; PPV positive predictive value.
False positive and negative rates are also detailed in Tables 2 and 3. When the threshold was greater than 0.25 mA, there was a 31% chance (95% CI, 20%-44%) that the nerve was not weak postoperatively (false positive rate); and when the threshold was 0.25 mA or less, there was a 35% chance (95% CI, 27%-43%) that the nerve was weak postoperatively (false negative rate) (Table 2). When there were more than 8 mechanical events, there was a 47% chance (95% CI, 35%-58%) that the nerve was not weak postoperatively (false positive rate); and when there were 8 or fewer mechanical events, there was a 40% chance (95% CI, 32%-49%) that the nerve was weak postoperatively (false negative rate) (Table 2). When the threshold of greater than 0.25 mA was paired with more than 8 mechanical events, the false positive rate decreased to 22% (95% CI, 55%-92%). When a threshold of 0.25 mA or less was paired with fewer than 8 mechanical events, the false negative rate decreased to 31% (95% CI, 21%-42%) (Table 3).
Discussion
In this study, we report normative values for FNM parameters during benign parotid surgery and determine cutoff values associated with facial nerve outcomes. Postdissection threshold and the number of intraoperative mechanical events were both shown to be associated with immediate facial nerve outcomes. The number of intraoperative spasm events was not shown to be associated with facial nerve outcomes. None of the investigated FNM parameters were found to be associated with permanent facial nerve outcomes, potentially owing to the limited number of permanent paralysis cases in the data set.
The optimal cutoff value associated with early facial nerve outcome for postdissection threshold was 0.25 mA, and the optimal cutoff value for intraoperative mechanical events was 8. In other words, when postdissection threshold is greater than 0.25 mA and/or there have been more than 8 mechanical events, then the surgeon may anticipate a temporary facial nerve weakness. Conversely, normal postoperative facial nerve function is more likely when the postdissection threshold is 0.25 mA or less and when the number of intraoperative mechanical events is fewer than 8 events. In our study, the PPV was 77%, and the specificity was 96% when the threshold of greater than 0.25 mA was paired with more than 8 mechanical events. The NPV was 69% when a threshold of 0.25 mA or less was paired with 8 mechanical events or fewer. The sensitivity was 71% if either the threshold was greater than 0.25 mA or there were more than 8 mechanical events.
Studies in the neurotologic and skull base literature have similarly demonstrated the predictive ability of FNM parameters, and have published the sensitivity, specificity, NPV, and PPV rates of these tests.16,17,18 A study by Neff et al16 demonstrated that a threshold of greater than 0.05 mA combined with a maximum response amplitude of less than 240 μV predicted postoperative nerve weakness 1 year postoperatively in 88% of patients who underwent vestibular schwannoma surgery. A threshold of 0.05 mA or less combined with an amplitude of 240 μV or greater predicted normal postoperative nerve function in 85% of patients in this population. A study by Isaacson et al17 also used threshold stimulus and amplitude to predict facial nerve outcomes after vestibular schwannoma surgery, and applying their proposed formula resulted in 89% sensitivity, 83% specificity, 94% PPV, and 71% NPV. Similarly, several studies have investigated the predictive values for recurrent laryngeal nerve monitoring in parathyroid and thyroid surgery.19,20,21,22,23 A study by Faden et al20 used ROC curves to identify the single threshold level to optimize the sensitivity and specificity of predicting postoperative recurrent laryngeal nerve function, which was found to be 0.5 mA (sensitivity, 68%; specificity, 84%).20 Normative facial nerve monitoring data obtained from skull-base surgery cannot be generalized to facial nerve monitoring in parotid surgery, because stimulation in parotid surgery occurs at a distal segment of the nerve outside of the skull base where its electrophysiologic properties have changed. Similarly, data from recurrent laryngeal nerve monitoring cannot be generalized to the facial nerve because recurrent laryngeal nerve monitoring uses surface electrodes that are less sensitive than needle electrodes embedded in the end organ.27
This study’s reported sensitivity, specificity, PPV, and NPV rates for these cutoff values are similar to those published for FNM in skull base surgery as well as recurrent laryngeal nerve monitoring in thyroid or parathyroid surgery.26 This information is useful for the surgeon to provide prognostic information and anticipatory guidance to the patient and family. If the postdissection threshold is greater than 0.25 mA and the patient experiences more than 8 mechanical events intraoperatively, the surgeon may tell the patient to expect temporary facial nerve weakness and may counsel or intervene appropriately. Anticipated upper division paresis may prompt aggressive eye care to prevent against corneal abrasions. Additionally, optimal cutoff values can be used by the surgeon intraoperatively to alter operative technique. For example, if the nerve is experiencing frequent mechanical stimulations, then the surgeon may change his or her approach to the nerve dissection and retraction or may use the operating microscope to assist with a difficult dissection.
There are clinically important false positive and false negative rates with intraoperative nerve monitoring, and event optimal cutoffs are not perfect. When the postdissection threshold is greater than 0.25 mA and there are greater than 8 mechanical events, there is a 22% chance that the nerve will not be weak postoperatively (false positive rate). False positives can occur when there is blood or fluid in the surgical field, fascia covering the nerve, incorrect use of the stimulator, accidental use of paralytic agent, or misplaced recording electrodes. When the postdissection threshold is 0.25 mA or less and there are 8 mechanical events or fewer, there is a 31% chance that the nerve will be weak postoperatively (false negative rate). False negatives can occur when the nerve is being stimulated distal to the site of the injury, if injury occurs subsequent to the last testing stimulation, or in cases of delayed neurapraxia. Prior studies have similarly demonstrated the false negative and false positive potential of intraoperative facial nerve monitoring.9,15 Thus, while intraoperative facial nerve monitoring can be used to predict facial nerve outcomes, it cannot substitute for excellent anatomic knowledge and surgical judgement.
Limitations
A limitation of the present study is that patients with permanent facial nerve weakness represent a small sample size (n = 3), and thus we are unable to fully evaluate whether FNM parameters were associated with permanent or late facial nerve outcomes. Predissection thresholds were not reliably collected in this patient population, and thus we were unable to analyze threshold shifts, the difference in predissection and post dissection thresholds, or how these correlate with postoperative nerve outcomes. Additionally, all patients in this study had FNM performed by an audiologist trained in continuous FNM. Alternative methods of FNM include using the Medtronic NIM 3.0 unattended system, which automatically recognizes electric and mechanical stimuli and converts these into sounds to alert the surgeon. No studies to our knowledge have compared facial nerve outcomes with trained personnel performing continuous monitoring vs the NIM monitor. In addition, how the normative data in this study apply to other monitoring systems requires further study. We cannot determine whether our results would be reliable in patients undergoing FNM with the use of an unattended NIM machine. One could assume that threshold data would be consistent, but the number of mechanical events is not captured by the NIM system. The data presented herein support the use of a more sophisticated facial nerve monitoring system that captures multiple nerve monitoring parameters. In the present study, pairing postdissection threshold with intraoperative mechanical events was associated with increased sensitivity, specificity, PPV, NPV, and positive and negative likelihood ratios.
Conclusions
Normative values of FNM parameters in parotid surgery and optimal cutoff values provide surgeons with a baseline for the interpretation of data and prognostication of postoperative nerve function. The postdissection threshold and the number of intraoperative mechanical events were associated with immediate facial nerve outcome. A postdissection threshold of less than 0.25 mA and having fewer than 8 mechanical events was associated with normal facial nerve function postoperatively. Prognostication of facial nerve function could provide anticipatory guidance to the patient and may also be used to adjust operative technique and perioperative management.
References
- 1.Meier JD, Wenig BL, Manders EC, Nenonene EK. Continuous intraoperative facial nerve monitoring in predicting postoperative injury during parotidectomy. Laryngoscope. 2006;116(9):1569-1572. doi: 10.1097/01.mlg.0000231266.84401.55 [DOI] [PubMed] [Google Scholar]
- 2.Grosheva M, Klussmann JP, Grimminger C, et al. Electromyographic facial nerve monitoring during parotidectomy for benign lesions does not improve the outcome of postoperative facial nerve function: a prospective two-center trial. Laryngoscope. 2009;119(12):2299-2305. doi: 10.1002/lary.20637 [DOI] [PubMed] [Google Scholar]
- 3.Dulguerov P, Marchal F, Lehmann W. Postparotidectomy facial nerve paralysis: possible etiologic factors and results with routine facial nerve monitoring. Laryngoscope. 1999;109(5):754-762. doi: 10.1097/00005537-199905000-00014 [DOI] [PubMed] [Google Scholar]
- 4.Laccourreye H, Laccourreye O, Cauchois R, Jouffre V, Ménard M, Brasnu D. Total conservative parotidectomy for primary benign pleomorphic adenoma of the parotid gland: a 25-year experience with 229 patients. Laryngoscope. 1994;104(12):1487-1494. doi: 10.1288/00005537-199412000-00011 [DOI] [PubMed] [Google Scholar]
- 5.Upton DC, McNamar JP, Connor NP, Harari PM, Hartig GK. Parotidectomy: 10-year review of 237 cases at a single institution. Otolaryngol Head Neck Surg. 2007;136(5):788-792. doi: 10.1016/j.otohns.2006.11.037 [DOI] [PubMed] [Google Scholar]
- 6.Terrell JE, Kileny PR, Yian C, et al. Clinical outcome of continuous facial nerve monitoring during primary parotidectomy. Arch Otolaryngol Head Neck Surg. 1997;123(10):1081-1087. doi: 10.1001/archotol.1997.01900100055008 [DOI] [PubMed] [Google Scholar]
- 7.Mra Z, Komisar A, Blaugrund SM. Functional facial nerve weakness after surgery for benign parotid tumors: a multivariate statistical analysis. Head Neck. 1993;15(2):147-152. doi: 10.1002/hed.2880150210 [DOI] [PubMed] [Google Scholar]
- 8.Harper CM, Daube JR. Facial nerve electromyography and other cranial nerve monitoring. J Clin Neurophysiol. 1998;15(3):206-216. doi: 10.1097/00004691-199805000-00004 [DOI] [PubMed] [Google Scholar]
- 9.Sood AJ, Houlton JJ, Nguyen SA, Gillespie MB. Facial nerve monitoring during parotidectomy: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;152(4):631-637. doi: 10.1177/0194599814568779 [DOI] [PubMed] [Google Scholar]
- 10.Yuan W, Sun JJ, Li JR, Guo HG. Intraoperative facial nerve monitoring in parotid gland surgery [in Chinese]. Zhonghua Yi Xue Za Zhi. 2010;90(6):397-399. [PubMed] [Google Scholar]
- 11.López M, Quer M, León X, Orús C, Recher K, Vergés J. Usefulness of facial nerve monitoring during parotidectomy [in Spanish]. Acta Otorrinolaringol Esp. 2001;52(5):418-421. [DOI] [PubMed] [Google Scholar]
- 12.Savvas E, Hillmann S, Weiss D, Koopmann M, Rudack C, Alberty J. Association between facial nerve monitoring with postoperative facial paralysis in parotidectomy. JAMA Otolaryngol Head Neck Surg. 2016;142(9):828-833. doi: 10.1001/jamaoto.2016.1192 [DOI] [PubMed] [Google Scholar]
- 13.Lowry TR, Gal TJ, Brennan JA. Patterns of use of facial nerve monitoring during parotid gland surgery. Otolaryngol Head Neck Surg. 2005;133(3):313-318. doi: 10.1016/j.otohns.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 14.Wolf SR, Schneider W, Suchy B, Eichhorn B. Intraoperative facial nerve monitoring in parotid surgery [in German]. HNO. 1995;43(5):294-298. [PubMed] [Google Scholar]
- 15.Eisele DW, Wang SJ, Orloff LA. Electrophysiologic facial nerve monitoring during parotidectomy. Head Neck. 2010;32(3):399-405. doi: 10.1002/hed.21190 [DOI] [PubMed] [Google Scholar]
- 16.Neff BA, Ting J, Dickinson SL, Welling DB. Facial nerve monitoring parameters as a predictor of postoperative facial nerve outcomes after vestibular schwannoma resection. Otol Neurotol. 2005;26(4):728-732. doi: 10.1097/01.mao.0000178137.81729.35 [DOI] [PubMed] [Google Scholar]
- 17.Isaacson B, Kileny PR, El-Kashlan H, Gadre AK. Intraoperative monitoring and facial nerve outcomes after vestibular schwannoma resection. Otol Neurotol. 2003;24(5):812-817. doi: 10.1097/00129492-200309000-00020 [DOI] [PubMed] [Google Scholar]
- 18.Mandpe AH, Mikulec A, Jackler RK, Pitts LH, Yingling CD. Comparison of response amplitude versus stimulation threshold in predicting early postoperative facial nerve function after acoustic neuroma resection. Am J Otol. 1998;19(1):112-117. [PubMed] [Google Scholar]
- 19.Randolph GW, Kamani D. Intraoperative electrophysiologic monitoring of the recurrent laryngeal nerve during thyroid and parathyroid surgery: experience with 1,381 nerves at risk. Laryngoscope. 2017;127(1):280-286. doi: 10.1002/lary.26166 [DOI] [PubMed] [Google Scholar]
- 20.Faden DL, Orloff LA, Ayeni T, Fink DS, Yung K. Stimulation threshold greatly affects the predictive value of intraoperative nerve monitoring. Laryngoscope. 2015;125(5):1265-1270. doi: 10.1002/lary.24960 [DOI] [PubMed] [Google Scholar]
- 21.Genther DJ, Kandil EH, Noureldine SI, Tufano RP. Correlation of final evoked potential amplitudes on intraoperative electromyography of the recurrent laryngeal nerve with immediate postoperative vocal fold function after thyroid and parathyroid surgery. JAMA Otolaryngol Head Neck Surg. 2014;140(2):124-128. doi: 10.1001/jamaoto.2013.6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eid I, Miller FR, Rowan S, Otto RA. The role of nerve monitoring to predict postoperative recurrent laryngeal nerve function in thyroid and parathyroid surgery. Laryngoscope. 2013;123(10):2583-2586. doi: 10.1002/lary.23946 [DOI] [PubMed] [Google Scholar]
- 23.Chan W-F, Lo C-Y. Pitfalls of intraoperative neuromonitoring for predicting postoperative recurrent laryngeal nerve function during thyroidectomy. World J Surg. 2006;30(5):806-812. doi: 10.1007/s00268-005-0355-8 [DOI] [PubMed] [Google Scholar]
- 24.Kileny PR. Intraoperative monitoring for parotid surgery In: Stach BA, ed. The Audiologist’s Handbook of Intraoperative Neurophysiological Monitoring. San Diego, CA: Plural Publishing Inc; 2019:89-97. [Google Scholar]
- 25.Mannarelli G, Griffin GR, Kileny P, Edwards B. Electrophysiological measures in facial paresis and paralysis. Oper Tech OtolaryngolHead Neck Surg. 2012;23(4):236-247. doi: 10.1016/j.otot.2012.08.003 [DOI] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed London, England: Routeledge; 1988. [Google Scholar]
- 27.Randolph GW, Dralle H, Abdullah H, et al. ; International Intraoperative Monitoring Study Group . Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011;121(suppl 1):1-16. doi: 10.1002/lary.21119 [DOI] [PubMed] [Google Scholar]