This study uses Veterans Affairs Corporate Data Warehouse data to examine whether there is an association between prior hematologic malignant tumor and risk of head and neck cancer and assesses the overall survival among these patients.
Key Points
Question
Is there an association between increased risk and worse survival outcomes in patients with head and neck cancer and a history of hematologic malignant tumors?
Findings
In a study of 30 939 656 patients, prior hematologic malignant tumors were associated with the development of head and neck cancer at multiple subsites; 2-year and 5-year overall survival rates were also worse among patients with a history of hematologic malignant tumors for several head and neck cancer subsites.
Meaning
The findings suggest that patients with hematologic malignant tumors are at higher risk of developing head and neck cancers and that overall survival may be worse for patients with head and neck cancer and a history of hematologic malignant tumors.
Abstract
Importance
More than 1.3 million people in the United States have a hematologic malignant tumor currently or in remission. Previous studies have demonstrated an increased risk of secondary neoplasms in patients with hematologic malignant tumors, but research specifically on the risk of head and neck solid tumors in patients with prior hematologic malignant tumors is limited.
Objectives
To examine a possible association between prior hematologic malignant tumors and risk of head and neck cancer and to assess the overall survival (OS) among these patients.
Design, Setting, and Participants
This retrospective analysis used the Veterans Affairs (VA) Corporate Data Warehouse (CDW) to identify patients with diagnoses of hematologic malignant tumors and head and neck cancers. All patients in the VA CDW with a birthdate between January 1, 1910, and December 31, 1969, were included, for a cohort of 30 939 656 veterans. Data analysis was performed from August 15, 2018, to January 31, 2019.
Exposures
Outpatient problem lists were queried for diagnoses of hematologic malignant tumor and associated malignant tumors using International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes to categorize patients by history of hematologic malignant tumors.
Main Outcomes and Measures
Presence of head and neck cancer was determined from ICD-9 and ICD-10 codes of outpatient problem lists, and cancers were grouped by subsite. The OS was determined from date of death or last outpatient visit date.
Results
Of 30 939 656 patients (27 636 683 [89.3%] male; 13 971 259 [45.2%] white), 207 322 patients had a hematologic malignant tumor, of whom 1353 were later diagnosed with head and neck cancer. A history of hematologic malignant tumors was significantly associated with overall aerodigestive tract cancer, with a relative risk (RR) of 1.6 (95% CI, 1.5-1.7), as well as oral cavity (RR, 1.7; 95% CI, 1.5-1.9), oropharynx (RR, 1.7; 95% CI, 1.5-1.9), larynx (RR, 1.3; 95% CI, 1.2-1.5), nasopharynx (RR, 2.8; 95% CI, 2.1-3.9), sinonasal (RR, 3.0; 95% CI, 2.2-4.1), salivary gland (RR, 2.8; 95% CI, 2.4-3.3), and thyroid (RR, 2.1; 95% CI, 1.9-2.4) tumors on subsite analysis. A prior hematologic malignant tumor was also negatively associated with 2-year and 5-year OS for multiple subsites.
Conclusions and Relevance
A prior diagnosis of hematologic or associated malignant tumors was associated with an increased risk of solid head and neck cancers in a range of subsites. In addition, for several head and neck cancer subsites, patients with prior hematologic malignant tumors had worse 2-year and 5-year OS. These results indicate that a prior hematologic malignant tumor may be an adverse risk factor in the development and progression of head and neck cancer.
Introduction
Hematologic malignant tumors are among the most prevalent cancers in the United States. In 2019, the American Cancer Society estimates that there will be a combined 176 200 new diagnoses of leukemia, lymphoma, and myeloma in the country, accounting for 10% of all cancer diagnoses.1 In the past few decades, treatment of many hematologic malignant tumors has improved, and survival has correspondingly increased. The 5-year relative survival rate for chronic myelogenous leukemia, for example, has more than tripled since the 1970s.1 The combination of high incidence and enhanced survival has resulted in more than 1.3 million people in the United States having hematologic malignant tumor currently or in remission as of 2014.2
This large population of patients is unfortunately susceptible to future cancers. Studies3,4,5,6,7,8,9,10,11,12,13,14,15 of hematologic malignant tumors, such as chronic myelogenous leukemia, chronic lymphocytic leukemia or small lymphocytic lymphoma, Hodgkin lymphoma, non-Hodgkin lymphoma, and multiple myeloma, have demonstrated long-term elevated risk of developing secondary neoplasms. In studies16,17,18,19,20 of patients who have undergone hematopoietic stem cell transplant (HSCT), which is a common treatment for hematologic malignant tumors, there is also a significantly elevated risk of second cancers.
Although an increased risk of secondary malignant tumors after hematologic malignant tumors has been well established, limited research has examined the risk of head and neck solid tumors. A number of studies on the overall cancer risk among hematologic cancer survivors have included rates of head and neck cancers and often have found a significant increase in such tumors; for example, studies10,12,13,21,22 of patients with chronic lymphocytic leukemia and small lymphocytic lymphoma have found increased risks of oral cavity, laryngeal, pharyngeal, salivary gland, and thyroid tumors. In addition, studies17,18,19 of patients undergoing HSCT have found elevated risks of oral cavity and thyroid tumors. These studies are limited in that they only included a small number of head and neck cancer diagnoses; even the largest available studies, such as a 30-year study23 of 77 876 patients with non-Hodgkin lymphoma, include only 268 incidences of head and neck cancer. The rare studies3,17,18,24,25 that have specifically focused on head and neck cancer risk are similarly limited by few cases and short follow-up times.
Because the risk of secondary cancers in general after hematologic malignant tumors has been made clear, it is important to determine what potential risk there is for the development of head and neck cancers specifically. The limited number and small sample size of previous studies3,4,5,6,7,8,9,10,11,12,13,14,15,25 have precluded a full understanding of this potential risk. In particular, to our knowledge, there has been no robust assessment of risk at specific head and neck cancer subsites that would require higher-powered studies. In this study, we assessed a possible association between previously diagnosed hematologic malignant tumors and the development of head and neck cancer, the risk at specific subsites, and any association between prior hematologic malignant tumor and overall survival (OS).
Methods
Overview
The present study was a retrospective analysis using the Veterans Affairs (VA) Corporate Data Warehouse (CDW). The VA CDW is a national repository composed of data from the Veterans Health Administration’s information infrastructure, the Veterans Information Systems Technology Architecture, which began collecting data in 1985.26 The CDW is updated daily and incorporates clinical and other data information into relational databases. Permission to access the CDW was obtained from the institutional review board of the Veterans Affairs Portland Heath Care System, which determined that informed consent was not required. All data were deidentified.
Study Population and Clinical Data
The cohort included 30 939 656 patients identified from the VA CDW; patients were included if their birthdate was between January 1, 1910, and December 31, 1969. Basic demographic information, including sex and race, was included. Outpatient problem lists were queried for diagnoses of hematologic and associated malignant tumors and solid head and neck cancers using International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes (the eTable in the Supplement gives all ICD-9 and ICD-10 codes used). Diagnosis dates ranged from May 1, 1992, to August 31, 2018. Hematologic malignant tumors included all malignant neoplasms of lymphoid, hematologic, and related tissue. Only cases of hematologic malignant tumors that did not follow a prior diagnosis of head and neck cancer were considered. Head and neck cancers were grouped into subsites (Table 1). Because of the significant histopathologic differences in typical salivary gland and thyroid tumors compared with cancers of other head and neck sites, we grouped all cancers except those 2 subsites into an aerodigestive tract category as our metric of overall head and neck cancer. Limited data on current or past tobacco use and current or past alcohol use were obtained from outpatient problem list ICD-9 and ICD-10 codes that pertained to tobacco use or alcohol use and related disorders that the Centers for Disease Control and Prevention define as 100% attributable to alcohol use (eg, alcoholic cirrhosis).27 Survival time was assessed by comparing date of death, if available, with date of diagnosis. Date of death in the VA CDW is entered from official sources, such as Veterans Health Administration facilities and death certificates; it is relatively complete, with an estimated 70% of deaths recorded.28 For all patients with no date of death recorded, follow-up was censored at the date of the last outpatient visit. Data analysis was performed from August 15, 2018, to January 31, 2019.
Table 1. Patient Characteristics.
| Characteristic | All Patients (N = 30 939 656) |
|---|---|
| Cancer diagnoses, No. (per 100 000 patients) | |
| Hematologic malignant tumor not preceded by head and neck cancer | 207 322 (670) |
| Aerodigestive tract cancer | 87 238 (282) |
| Oral cavity | 27 921 (90) |
| Oropharynx | 20 127 (65) |
| Larynx | 32 047 (104) |
| Hypopharynx | 2870 (9) |
| Nasopharynx | 2127 (7) |
| Sinonasal | 2146 (7) |
| Salivary gland | 8703 (28) |
| Thyroid | 18 054 (58) |
| Patients, No. (%) | |
| Sex | |
| Male | 27 636 683 (89.3) |
| Female | 3 217 888 (10.4) |
| Race/ethnicity | |
| American Indian/Alaska Native | 156 070 (0.5) |
| Asian | 128 350 (0.4) |
| Black or African American | 2 837 703 (9.2) |
| Native Hawaiian/Pacific Islander | 156 869 (0.5) |
| White | 13 971 259 (45.2) |
| Unknown | 13 689 405 (44.2) |
| Known current or past alcohol use | 1 330 757 (4.3) |
| Known current or past tobacco use | 2 198 669 (7.1) |
Statistical Analysis
Statistical analysis was performed using SAS software, version 9.3 (SAS Institute Inc). The association of prior diagnosis of a hematologic malignant tumor, as well as demographic and substance use variables, with head and neck cancer subsites was assessed using a χ2 test on univariate analysis. Multivariate analysis using a binary logistic model with forward stepwise selection (criteria were 0.05 for entry and 0.1 for removal) was conducted separately for head and neck cancer subsites that were significantly associated with prior hematologic malignant tumors on univariate analysis. A nested cohort of all patients with head and neck cancer was used for survival analysis. Two-year and 5-year OS rates for all subsites significantly associated with history of hematologic malignant tumors were compared using χ2 tests. Kaplan-Meier survival curves and log-rank tests for 10-year OS were also performed: salivary gland and thyroid tumors were considered separately from aerodigestive tumors. All comparisons were 2-tailed with statistical significance set at P < .05. Effect size was calculated as relative risk (RR) or odds ratio with corresponding 95% CI.
Results
Patient Characteristics
Patient characteristics are given in Table 1. Of the 30 939 656 patients included in the study (27 636 683 [89.3%] male; 13 971 259 [45.2%] white), a total of 207 322 patients (670 per 100 000 patients) had hematologic malignant tumors that did not follow a prior diagnosis of head and neck cancer, and 87 238 (282 per 100 000 patients) had aerodigestive tract cancer. A total of 2 198 669 patients (7.1%) reported current or past tobacco use, and 1 330 757 (4.3%) reported current or past alcohol use in our limited assessment.
Hematologic Malignant Tumor and Subsequent Head and Neck Cancer
Of the 207 322 patients with hematologic malignant tumors, 1353 (652 per 100 000 patients) received a subsequent diagnosis of head and neck cancer. The mean (SD) time from hematologic malignant tumor diagnosis to head and neck cancer diagnosis was 45.1 (45) months (median, 31 months; range, 0-234 months). On univariate analysis (Table 2), a prior hematologic malignant tumor was significantly associated with aerodigestive tract cancer diagnosis, with an RR of 1.61 (95% CI, 1.51-1.71). All head and neck cancer subsites other than the hypopharynx were also significantly associated with prior hematologic malignant tumors, with RRs ranging from 1.31 (95% CI, 1.17-1.48) for laryngeal tumors to 3.03 (95% CI, 2.24-3.29) for sinonasal tumors.
Table 2. Univariate Analysis of Head and Neck Cancer and Prior Hematologic Malignant Tumor.
| Variable | All Patients (N = 30 939 656) | Prior Hematologic Malignant Tumor | Relative Risk (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| No (n = 30 732 334) | Yes (n = 207 322) | ||||||
| Patients, No. | Cancer Diagnoses, per 100 000 Patients | Patients, No. | Cancer Diagnoses, per 100 000 Patients | Patients, No. | Cancer Diagnoses, per 100 000 Patients | ||
| Aerodigestive tract cancer | 87 238 | 282 | 86 301 | 281 | 937 | 452 | 1.61 (1.51-1.71) |
| Oral cavity | 27 921 | 90 | 27 602 | 90 | 319 | 154 | 1.71 (1.54-1.92) |
| Oropharynx | 20 127 | 65 | 19 898 | 65 | 229 | 110 | 1.71 (1.50-1.94) |
| Larynx | 32 047 | 104 | 31 766 | 103 | 281 | 136 | 1.31 (1.17-1.48) |
| Hypopharynx | 2870 | 9 | 2845 | 9 | 25 | 12 | 1.30 (0.88-1.93) |
| Nasopharynx | 2127 | 7 | 2087 | 7 | 40 | 19 | 2.84 (2.08-3.88) |
| Sinonasal | 2146 | 7 | 2103 | 7 | 43 | 21 | 3.03 (2.24-4.10) |
| Salivary gland | 8703 | 28 | 8541 | 28 | 162 | 78 | 2.81 (2.41-3.29) |
| Thyroid | 18 054 | 58 | 17 800 | 58 | 254 | 123 | 2.1 (1.87-2.40) |
A multivariate analysis of hematologic malignant tumor and patient characteristics (in which all demographic variables were entered) revealed that tobacco use, alcohol use, race/ethnicity, sex, and prior hematologic malignant tumor were significantly associated with incidence of cancer at most subsites (Table 3). The only variable not significant on any of the subsite multivariate analyses and thus not included in the final model was history of hematologic malignant tumor in the larynx tumor analysis.
Table 3. Multivariate Analysis of Head and Neck Cancer and Prior Hematologic Malignant Tumor.
| Variable | Odds Ratio (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Aerodigestive Tract Cancer | Oral Cavity Cancer | Oropharynx Cancer | Larynx Cancer | Nasopharynx Cancer | Sinonasal Cancer | Salivary Gland Cancer | Thyroid Cancer | ||
| Tobacco use | |||||||||
| Unknown | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| Known use | 3.56 (3.50-3.62) | 3.61 (3.51-3.72) | 3.01 (2.91-3.12) | 3.88 (3.76-3.97) | 3.32 (2.98-3.66) | 3.30 (2.97-3.69) | 3.26 (3.10-3.44) | 1.89 (1.81-1.97) | |
| Alcohol use | |||||||||
| Unknown | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| Known use | 2.40 (2.35-2.44) | 2.71 (2.62-2.80) | 2.53 (2.43-2.63) | 2.05 (1.98-2.11) | 1.36 (1.18-1.57) | 1.28 (1.11-1.48) | 1.11 (1.03-1.19) | 1.07 (1.00-1.13) | |
| Race/ethnicity | |||||||||
| White | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| American Indian/Alaska Native | 0.61 (0.55-0.67) | 0.50 (0.41-0.60) | 0.68 (0.56-0.82) | 0.64 (0.55-0.76) | 1.31 (0.82-2.09) | 0.61 (0.31-1.17) | 0.70 (0.52-0.93) | 0.69 (0.57-0.84) | |
| Asian | 0.51 (0.45-0.58) | 0.42 (0.33-0.55) | 0.32 (0.23-0.45) | 0.37 (0.28-0.48) | 5.88 (4.52-7.66) | 0.93 (0.50-1.73) | 1.13 (0.87-1.47) | 1.14 (0.96-1.35) | |
| Black/African American | 0.74 (0.72-0.76) | 0.50 (0.47-0.52) | 0.69 (0.66-0.72) | 0.94 (0.91-0.97) | 1.25 (1.11-1.42) | 0.90 (0.79-1.04) | 0.72 (0.68-0.78) | 0.61 (0.58-0.65) | |
| Native Hawaiian/Pacific Islander | 0.81 (0.74-0.88) | 0.69 (0.58-0.81) | 0.84 (0.71-0.99) | 0.77 (0.66-0.90) | 2.11 (1.46-3.05) | 1.14 (0.71-1.84) | 0.87 (0.67-1.13) | 1.14 (0.98-1.32) | |
| Unknown | 0.48 (0.47-0.49) | 0.53 (0.51-0.54) | 0.30 (0.29-0.32) | 0.55 (0.54-0.56) | 0.47 (0.42-0.53) | 0.48 (0.43-0.54) | 0.30 (0.29-0.32) | 0.21 (0.20-0.22) | |
| Sex | |||||||||
| Male | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| Female | 0.15 (0.14-0.16) | 0.20 (0.19-0.22) | 0.17 (0.15-0.19) | 0.08 (0.07-0.09) | 0.30 (0.23-0.40) | 0.22 (0.16-0.30) | 0.46 (0.41-0.52) | 1.87 (1.79-1.95) | |
| Hematologic malignant tumor | |||||||||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| Yes | 1.15 (1.08-1.25) | 1.24 (1.10-1.38) | 1.19 (1.04-1.35) | 0.94 (0.84-1.06) | 2.11 (1.54-2.89) | 2.22 (1.64-3.00) | 1.98 (1.70-2.32) | 1.63 (1.44-1.84) | |
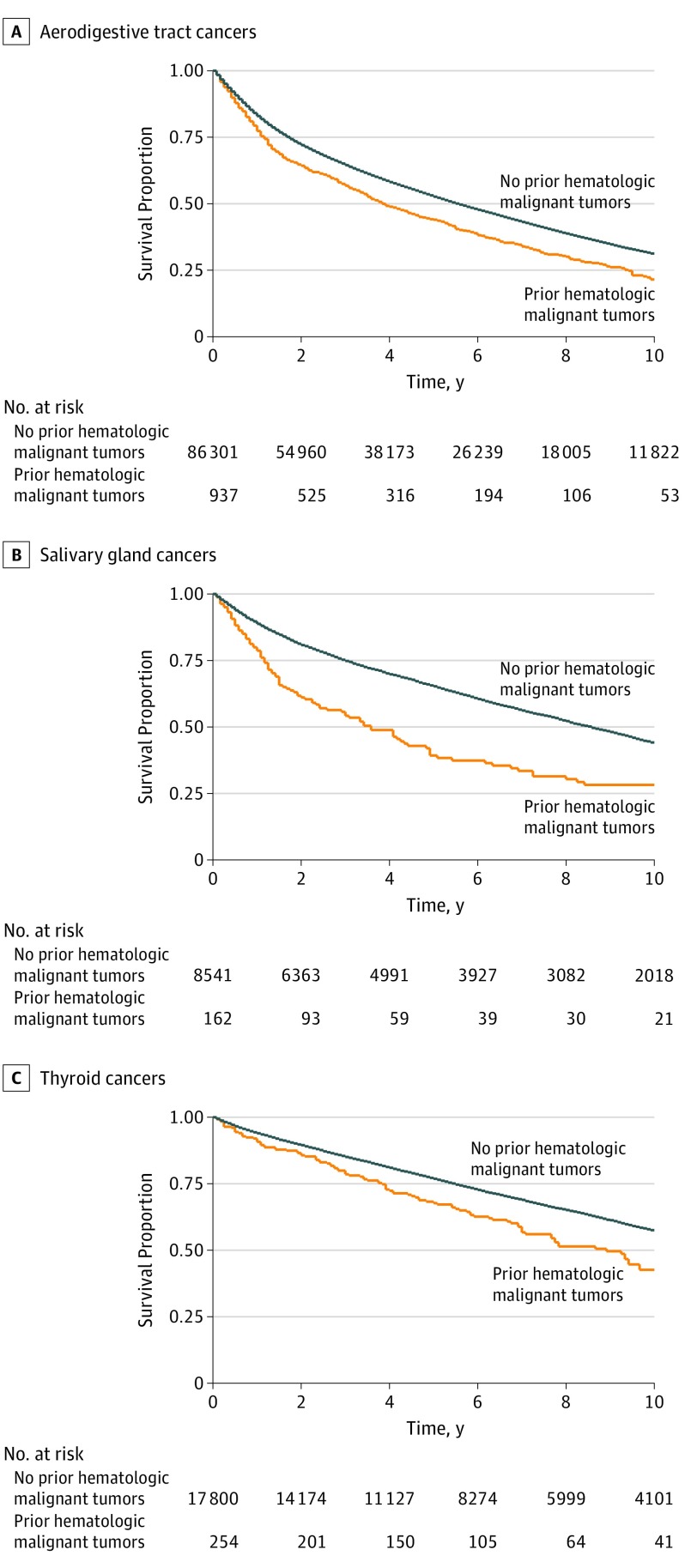
Median follow-up time for all patients with head and neck cancer was 51 months (43 months for those with a prior hematologic malignant tumor and 51 without; range, 0-308 months). Both 2-year and 5-year OS for aerodigestive tract cancers was significantly lower among patients with prior hematologic malignant tumors compared with those without (Table 4), with a 2-year OS difference of 61% vs 69% (RR, 0.88; 95% CI, 0.83-0.93) and a 5-year OS difference of 34% vs 45% (RR, 0.76; 95% CI, 0.66-0.84). Subsites that were significantly associated with prior hematologic malignant tumors on univariate analysis were assessed, and several also were associated with significantly lower 2-year and 5-year OS among patients with prior hematologic malignant tumors. In particular, 5-year OS for salivary gland tumors was 61% among patients with no history of hematologic malignant tumors compared with 32% among patients with prior hematologic cancer (RR, 0.52; 95% CI, 0.41-0.67). Log-rank tests of 10-year OS among patients with aerodigestive tract, salivary gland, or thyroid tumors also revealed significantly decreased OS among patients with a history of hematologic malignant tumors at that time (Figure).
Table 4. Association of Prior Hematologic Malignant Tumor With 2-Year and 5-Year Overall Survival Among Patients With Head and Neck Cancer .
| Variable | 2-Year Overall Survival | 5-Year Overall Survival | ||||
|---|---|---|---|---|---|---|
| Hematologic Malignant Tumor, No. (%) | Relative Risk (95% CI) | Hematologic Malignant Tumor, No. (%) | Relative Risk (95% CI) | |||
| No | Yes | No | Yes | |||
| Aerodigestive tract cancer | 54 960 (69) | 525 (61) | 0.88 (0.83-0.93) | 31 572 (45) | 252 (34) | 0.76 (0.66-0.84) |
| Oral cavity | 17 795 (68) | 191 (63) | 0.93 (0.85-1.01) | 10 508 (45) | 98 (37) | 0.83 (0.70-0.97) |
| Oropharynx | 11 946 (69) | 139 (68) | 1.00 (0.91-1.09) | 6191 (44) | 65 (41) | 0.94 (0.78-1.13) |
| Larynx | 21 307 (72) | 141 (54) | 0.75 (0.67-0.84) | 12 646 (47) | 65 (28) | 0.59 (0.48-0.73) |
| Nasopharynx | 1270 (66) | 21 (62) | 0.94 (0.72-1.22) | 771 (46) | 8 (31) | 0.67 (0.38-1.20) |
| Sinonasal | 1331 (70) | 23 (55) | 0.78 (0.60-1.04) | 835 (50) | 13 (34) | 0.69 (0.44-1.07) |
| Salivary gland | 6363 (80) | 93 (60) | 0.75 (0.66-0.86) | 4416 (61) | 43 (32) | 0.52 (0.41-0.67) |
| Thyroid | 14 174 (88) | 201 (85) | 0.96 (0.91-1.02) | 9638 (72) | 128 (63) | 0.88 (0.79-0.97) |
Figure. Overall Survival Among Patients With a History of Hematologic Malignant Tumors.
Discussion
Although survivors of hematologic malignant tumors consistently have an elevated risk of secondary neoplasms, it remains unclear what risk might exist for head and neck cancers specifically. A few small studies have found a significantly increased risk, but there has been no consensus on the association with particular head and neck subsites and survival. Ultimately, the current study aimed to address this gap in the literature by providing an evaluation of incidence of head and neck cancer after hematologic malignant tumors by using a robust national database. With more than 30 million patients and 1353 cases of head and neck cancer after hematologic malignant tumor, this study may be the highest-powered study to date to examine this association.
Risk of Head and Neck Cancer After Hematologic Malignant Tumor
Our study found that, in a sample of 30.9 million veterans, prior diagnosis of a hematologic malignant tumor was significantly associated with a higher risk of subsequent head and neck cancer diagnosis. Of the patients with a hematologic malignant tumor, 452 per 100 000 patients (937 patients) were later diagnosed with aerodigestive tract cancer, compared with 281 per 100 000 patients (86 301 patients) in the group with no prior hematologic malignant tumors, which is a 1.6-fold increase. This finding is in keeping with prior studies5,6,8,10,12,13,17,18,19,21,22,23,24,29 that found an overall increased risk of head and neck cancers after various hematologic malignant tumors or HSCT. The present study was a higher-powered analysis than previous research, with more than 5 times the number of cases of head and neck cancer after a hematologic malignant tumor than in any prior study that we were able to identify.23 The mean time from hematologic malignant tumor diagnosis to head and neck cancer diagnosis was nearly 4 years, although diagnoses were as many as 19 years apart; previous studies3,4,5,6,7,8,9,10,11,12,13,14,15 have suggested that an elevated risk of head and neck cancer remains many years after hematologic malignant tumor diagnosis or HSCT. One study30 found solid malignant tumors in 15% of patients at 15 years after HSCT; the risk of solid tumor development appeared to accelerate with time after HSCT, from 1.3 to 1.6 times the rate expected at 5-year follow-up to more than 3 times the rate at 15 years.
In addition to an association with overall increased incidence of aerodigestive tract cancers, prior hematologic malignant tumors were associated with neoplasms at nearly all head and neck cancer subsites. We found a significantly increased risk of oral cavity, oropharynx, salivary gland, nasopharynx, sinonasal, larynx, and thyroid tumors on univariate analysis. This association remained statistically significant on multivariate analysis for all subsites except the larynx. Of note, on multivariate analysis, tobacco use had the strongest association with subsequent head and neck cancer, although hematologic malignant tumor history was almost as closely associated with alcohol use.
To our knowledge, an association between hematologic malignant tumor and subsequent nasopharynx or sinonasal tumors has never been previously reported, likely because of the rarity of cancers at these subsites, which necessitates a large study to identify enough cases for adequate analytical power. In the current study, the elevated risk was substantial; with RRs of 2.8 for nasopharynx and 3.0 for sinonasal tumors in patients with hematologic malignant tumors compared with patients with no prior hematologic malignant tumors, these sites were at the highest increased risk of any head and neck subsite. It is possible that this elevated rate in the nasopharynx was associated with increased susceptibility to Epstein-Barr virus (EBV). Patients undergoing HSCT are at increased risk of EBV infections, which are a major cause of morbidity and mortality in this patient population, particularly because EBV can lead to secondary lymphoproliferative disorders.31 A similar although less substantial increase in risk of oropharyngeal tumors, which are often related to human papillomavirus (HPV), was seen as well.32 Unfortunately, EBV and HPV involvement could not be assessed in this data set.
The origin of the general increase in aerodigestive tract cancers, as well as salivary gland and thyroid tumors, for patients with hematologic malignant tumors is likely multifactorial. Previous studies3,4,5,6,7,8,9,10,11,12,13,14,15 of increased secondary malignant tumor risk after a hematologic malignant tumor have proposed a number of mechanisms. Some likely factors are inherent to the hematologic malignant tumor. For example, in B-cell chronic myelogenous leukemia, cellular and humoral immunity becomes defective, rendering the patient susceptible to infectious agents (which may be carcinogenic, like EBV and HPV) and altering immune surveillance.33 In addition, hematologic malignant tumors in general are managed with some combination of radiotherapy, chemotherapy, and HSCT, all of which can affect immune function and may result in higher risk of subsequent cancer development.25,34 However, research has been inconclusive; radiation therapy, for example, has been found to increase secondary malignant tumor risk in some studies but not others.24,35,36
Differences in OS
Although a history of hematologic malignant tumors may be a relatively minor risk factor for the development of head and neck cancers compared with other known factors, such as tobacco and alcohol use, it may be a more substantial risk factor for patient outcomes. Specifically, OS may be markedly worse among patients with prior hematologic malignant tumors.
For patients with aerodigestive tract cancers, the 5-year OS rate was 34% among those with prior hematologic malignant tumors vs 45% among those without, which is a nearly 25% decrease. Patients with salivary gland and laryngeal tumors with a history of hematologic malignant tumors had nearly half the 5-year OS rates of those without. Patients with thyroid and oral cavity cancer also had a reduced 5-year OS associated with prior hematologic malignant tumors. Similarly, 10-year OS analysis found significant reductions in survival among patients with hematologic malignant tumors with aerodigestive tract, salivary gland, and thyroid tumors. These findings are similar to those of a 2015 study24 of survival among patients with head and neck squamous cell carcinoma after Hodgkin lymphoma, which found that a history of Hodgkin lymphoma was negatively associated with OS among patients with head and neck squamous cell carcinoma. It is impossible to know from our data what the cause of this decreased OS was. Decreased survival may be associated with the hematologic malignant tumor itself, a more aggressive head and neck cancer, the presence of other secondary tumors, decreased immune surveillance, or other factors that we were unable to measure.
Limitations
Although the VA CDW database proved to be useful for the current study, it has some limitations. Analyzing a veteran population raises a question of generalizability because the patients included in this study do not reflect the overall population. In addition, VA medical centers do not necessarily represent head and neck surgical oncology practices in the general population. There is the potential for missing data, and not all variables of interest are available in the database. Our assessments of tobacco and alcohol use were limited because those variables are not readily available. However, we were able to include all diagnoses of tobacco or alcohol use disorder as well as all diagnoses of disorders considered by the Centers for Disease Control and Prevention to be 100% attributable to alcohol use that were present in outpatient problem lists. We were likely able to identify the highest tobacco and alcohol users, who would be more likely to have such diagnoses present. Another missing piece of data is the treatment regimen for patients with hematologic malignant tumors, which might affect our results. We also did not have clear vital status data available in the VA CDW and thus were not able to ideally assess survival; although we had death dates for many patients, there were likely a number of deceased patients without those data available who were instead censored by last outpatient follow-up date.
There are also limitations to this study’s conclusions that are inherent to the population of patients with hematologic malignant tumors. Patients with a diagnosed malignant tumor are likely to have closer follow-up than other patients; there may be an effect of increased surveillance on the higher rate of subsequent head and neck cancer diagnoses in patients with hematologic malignant tumors. In addition, patients with multiple malignant tumors may have some factor associated with the formation of both cancers that we were not able to assess in this study. However, that may not be a limitation as much as a point of observation; for example, genetic polymorphisms are linked to hematologic malignant tumors and head and neck cancers, which may have been present in patients in this study population.37,38
Conclusions
This study provided evidence of a positive association between a history of hematologic malignant tumor and subsequent development of head and neck cancer in a VA population. Multiple head and neck cancer subsites were significantly associated with prior hematologic malignant tumor, and an elevated risk for tumors of nasopharyngeal and sinonasal subsites was found. Cancers in those sites, as well as the oral cavity, salivary gland, oropharynx, and thyroid, remained significantly associated with a history of hematologic malignant tumor on multivariate analysis that included tobacco and alcohol use. Prior hematologic malignant tumor was also negatively associated with OS. Ultimately, this study enriches our understanding of the risk of head and neck cancer for patients with prior hematologic malignant tumors.
eTable. ICD Codes for Cancer Subsites
References
- 1.American Cancer Society Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- 2.Howlader NNA, Krapcho M, Miller D, Cronin KA, eds. SEER Cancer Statistics Review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017. [Google Scholar]
- 3.Budrukkar A, Muttagi S, Shahid T, et al. Second primary head and neck squamous cell cancers with aggressive behavior in patients with chronic myeloid leukaemia. Br J Oral Maxillofac Surg. 2012;50(6):504-507. doi: 10.1016/j.bjoms.2011.08.012 [DOI] [PubMed] [Google Scholar]
- 4.Eichenauer DA, Becker I, Monsef I, et al. Secondary malignant neoplasms, progression-free survival and overall survival in patients treated for Hodgkin lymphoma: a systematic review and meta-analysis of randomized clinical trials. Haematologica. 2017;102(10):1748-1757. doi: 10.3324/haematol.2017.167478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giri S, Bhatt VR, Verma V, et al. Risk of second primary malignancies in patients with follicular lymphoma: a United States population-based study. Clin Lymphoma Myeloma Leuk. 2017;17(9):569-574. doi: 10.1016/j.clml.2017.06.028 [DOI] [PubMed] [Google Scholar]
- 6.Hisada M, Chen BE, Jaffe ES, Travis LB. Second cancer incidence and cause-specific mortality among 3104 patients with hairy cell leukemia: a population-based study. J Natl Cancer Inst. 2007;99(3):215-222. doi: 10.1093/jnci/djk030 [DOI] [PubMed] [Google Scholar]
- 7.Mudie NY, Swerdlow AJ, Higgins CD, et al. Risk of second malignancy after non-Hodgkin’s lymphoma: a British cohort study. J Clin Oncol. 2006;24(10):1568-1574. doi: 10.1200/JCO.2005.04.2200 [DOI] [PubMed] [Google Scholar]
- 8.Razavi P, Rand KA, Cozen W, Chanan-Khan A, Usmani S, Ailawadhi S. Patterns of second primary malignancy risk in multiple myeloma patients before and after the introduction of novel therapeutics. Blood Cancer J. 2013;3(6):e121. doi: 10.1038/bcj.2013.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373(26):2499-2511. doi: 10.1056/NEJMoa1505949 [DOI] [PubMed] [Google Scholar]
- 10.Hisada M, Biggar RJ, Greene MH, Fraumeni JF Jr, Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98(6):1979-1981. doi: 10.1182/blood.V98.6.1979 [DOI] [PubMed] [Google Scholar]
- 11.Mellemgaard A, Geisler CH, Storm HH. Risk of kidney cancer and other second solid malignancies in patients with chronic lymphocytic leukemia. Eur J Haematol. 1994;53(4):218-222. doi: 10.1111/j.1600-0609.1994.tb00192.x [DOI] [PubMed] [Google Scholar]
- 12.Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol. 2010;28(33):4935-4944. doi: 10.1200/JCO.2010.29.1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schöllkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;121(1):151-156. doi: 10.1002/ijc.22672 [DOI] [PubMed] [Google Scholar]
- 14.Travis LB, Curtis RE, Hankey BF, Fraumeni JF Jr. Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst. 1992;84(18):1422-1427. doi: 10.1093/jnci/84.18.1422 [DOI] [PubMed] [Google Scholar]
- 15.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27(6):904-910. doi: 10.1200/JCO.2008.17.5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adhikari J, Sharma P, Bhatt VR. Risk of secondary solid malignancies after allogeneic hematopoietic stem cell transplantation and preventive strategies. Future Oncol. 2015;11(23):3175-3185. doi: 10.2217/fon.15.252 [DOI] [PubMed] [Google Scholar]
- 17.Chen MH, Chang PM, Li WY, et al. High incidence of oral squamous cell carcinoma independent of HPV infection after allogeneic hematopoietic SCT in Taiwan. Bone Marrow Transplant. 2011;46(4):567-572. doi: 10.1038/bmt.2010.163 [DOI] [PubMed] [Google Scholar]
- 18.Cohen A, Rovelli A, Merlo DF, et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT Late Effects Working Party Study. J Clin Oncol. 2007;25(17):2449-2454. doi: 10.1200/JCO.2006.08.9276 [DOI] [PubMed] [Google Scholar]
- 19.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336(13):897-904. doi: 10.1056/NEJM199703273361301 [DOI] [PubMed] [Google Scholar]
- 20.Danylesko I, Shimoni A. Second malignancies after hematopoietic stem cell transplantation. Curr Treat Options Oncol. 2018;19(2):9. doi: 10.1007/s11864-018-0528-y [DOI] [PubMed] [Google Scholar]
- 21.Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105(7):1076-1081. doi: 10.1038/bjc.2011.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiernik PH. Second neoplasms in patients with chronic lymphocytic leukemia. Curr Treat Options Oncol. 2004;5(3):215-223. doi: 10.1007/s11864-004-0013-7 [DOI] [PubMed] [Google Scholar]
- 23.Tward JD, Wendland MM, Shrieve DC, Szabo A, Gaffney DK. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107(1):108-115. doi: 10.1002/cncr.21971 [DOI] [PubMed] [Google Scholar]
- 24.Chowdhry AK, McHugh C, Fung C, Dhakal S, Constine LS, Milano MT. Second primary head and neck cancer after Hodgkin lymphoma: a population-based study of 44,879 survivors of Hodgkin lymphoma. Cancer. 2015;121(9):1436-1445. doi: 10.1002/cncr.29231 [DOI] [PubMed] [Google Scholar]
- 25.Chung JC, Tsang RK, To VS, et al. Secondary head and neck cancer in patients with history of hematological malignancy. Head Neck. 2013;35(5):729-732. doi: 10.1002/hed.23026 [DOI] [PubMed] [Google Scholar]
- 26.Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi: 10.1377/hlthaff.2014.0054 [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Alcohol Related Disease Impact (ARDI) Application. 2013. https://nccd.cdc.gov/DPH_ARDI/default/default.aspx. Accessed November 28, 2018.
- 28.Maynard C. Ascertaining Veterans’ Vital Status: VA Data Sources for Mortality Ascertainment and Cause of Death. Database & Methods Cyberseminar Series. Hines, IL: VA Information Resource Center; 2017. [Google Scholar]
- 29.Maule M, Scélo G, Pastore G, et al. Risk of second malignant neoplasms after childhood leukemia and lymphoma: an international study. J Natl Cancer Inst. 2007;99(10):790-800. doi: 10.1093/jnci/djk180 [DOI] [PubMed] [Google Scholar]
- 30.Rizzo JD, Curtis RE, Socié G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175-1183. doi: 10.1182/blood-2008-05-158782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roddie C, Peggs KS. Immunotherapy for transplantation-associated viral infections. J Clin Invest. 2017;127(7):2513-2522. doi: 10.1172/JCI90599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944-1956. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- 33.Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. MedGenMed. 2007;9(4):35. [PMC free article] [PubMed] [Google Scholar]
- 34.Castillo JJ, Gertz MA. Secondary malignancies in patients with multiple myeloma, Waldenström macroglobulinemia and monoclonal gammopathy of undetermined significance. Leuk Lymphoma. 2017;58(4):773-780. doi: 10.1080/10428194.2016.1217527 [DOI] [PubMed] [Google Scholar]
- 35.Boukheris H, Ron E, Dores GM, Stovall M, Smith SA, Curtis RE. Risk of radiation-related salivary gland carcinomas among survivors of Hodgkin lymphoma: a population-based analysis. Cancer. 2008;113(11):3153-3159. doi: 10.1002/cncr.23918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronckers CM, Sigurdson AJ, Stovall M, et al. Thyroid cancer in childhood cancer survivors: a detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166(4):618-628. doi: 10.1667/RR3605.1 [DOI] [PubMed] [Google Scholar]
- 37.Flores-Obando RE, Gollin SM, Ragin CC. Polymorphisms in DNA damage response genes and head and neck cancer risk. Biomarkers. 2010;15(5):379-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seedhouse C, Bainton R, Lewis M, Harding A, Russell N, Das-Gupta E. The genotype distribution of the XRCC1 gene indicates a role for base excision repair in the development of therapy-related acute myeloblastic leukemia. Blood. 2002;100(10):3761-3766. doi: 10.1182/blood-2002-04-1152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. ICD Codes for Cancer Subsites