Abstract
Background: The Global Initiative for Chronic Obstructive Lung Disease (GOLD) released an updated document in 2017 that excluded the spirometric parameter in the classification of patients. The validity of this new classification system in predicting mortality and respiratory hospitalization is still uncertain.
Methods: Outpatients (n=149) with chronic obstructive pulmonary disease (COPD) who underwent spirometry and six-minutes walking test from October 2011 to September 2013 were enrolled. The overall mortality and rate of respiratory hospitalization over a median of 61 months were analyzed. Kaplan-Meier survival analyses, receiver operaing curve analyses with areas under the curve (AUCs), and logistic regression analyses for GOLD 2007, GOLD 2011, GOLD 2017, and/or BODE index were performed to evaluate their abilities to predict mortality and respiratory hospitalization.
Results: Forty-two (53.2%) patients in 2011 GOLD C or D group were categorized into 2017 GOLD A or B group. The odds ratios of GOLD 2017 group C and group D relative to group A were 7.55 (95% CI, 1.25–45.8) and 25.0 (95% CI, 6.01–102.9) for respiratory hospitalization. Patients in GOLD 2017 group A and group B had significantly better survival (log-rank test, p<0.001) compared with patients in group D; however, survival among patients in GOLD 2007 groups and GOLD 2011 groups was comparable. The AUC values for GOLD 2007, GOLD 2011, GOLD 2017, and BODE index were 0.573, 0.624, 0.691, 0.692 for mortality (p=0.013) and 0.697, 0.707, 0.741, and 0.754 for respiratory hospitalization (p=0.296), respectively.
Conclusion: The new GOLD classification may perform better than the previous classifications in terms of predicting mortality and respiratory hospitalization.
Keywords: COPD, GOLD classification, mortality, respiratory hospitalization
Introduction
Chronic obstructive pulmonary disease (COPD) is the 4th leading cause of death globally; it caused 3.0 million deaths in 2016.1 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has been publishing documents for the diagnosis, classification, and treatment of patients with COPD since 2001, and the documents have been updated regularly. The recent major revision was in 2011 and 2017.2,3 Before the 2011 GOLD revision, patients with COPD were graded into GOLD stages 1 to 4 based on only one parameter, which was the percentage of the predicted value of forced expiratory volume in one second (FEV1% predicted) that was measured by spirometry. The spirometric grading system had some limitations because it did not take into account the patient’s symptoms and history of exacerbations.
Thus, the GOLD 2011 document combined symptoms, as assessed using the modified Medical Research Council (mMRC) dyspnea scale or COPD Assessment Test (CAT); spirometric severity of airflow limitation; and history of exacerbations to assess patients with COPD. Based on this combined assessment, patients with COPD were classified into category A, B, C, or D and were treated according to the recommendations for each category. Although the GOLD classification was not originally aimed to predict the survival of patients with COPD, the prognostic validity of GOLD 2011 was excellent; the mortality was obsreved to increase progressively from group A to group.4,5 However, some limitations of the GOLD 2011 classification were identified despite the new indexes that were introduced in it, and more complex assessments were implemented. Johannessen et al reported that the abilities GOLD 2007 and GOLD 2011 in predicting hospitalization and mortality were similar.6 Lange et al showed that mortality of patients in group B was higher than that in group C.7 Furthemore, the ability of GOLD 2011 to predict lung function decline was inferior to that of GOLD 2007 according to a report by Kim et al.8
Consequently, the GOLD 2017 classification, which classified patients with COPD into group A to D using 3 parameters – including mMRC dyspnea scale, CAT, and history of exacerbations – was announced recently.3 The use of the spirometric parameter with the purpose of classifying patients with COPD was excluded, and spirometry was recommended only as a diagnostic tool according to the GOLD 2017 document. Since the introduction of GOLD 2017, Lopez et al have reported that the GOLD 2011 model predicted survival better than the GOLD 2017 model does.9 With regard to the ability of predicting acute exacerbations, Marcoa et al reported that the GOLD 2017 and GOLD 2011 classification had a similar ability to predict future exacerbations.10 However, the evidence is still insufficient to conclude whether GOLD 2017 performs better than previous GOLD classifications.
The BMI, obstruction, dyspnea, exercise (BODE) index is a simple multidimensional grading system that predicts mortality of patients with COPD better than FEV1.11 Researches to compare predictive abilities between the GOLD 2017 classification and the BODE index regarding mortality and respiratory hospitalization are lacking.
Thus, the current study aimed to evaluate the abilities of the GOLD 2017 classification and compare those with the GOLD 2007 classification; GOLD 2011 classification; and the BODE index in terms of predicting mortality and respiratory hospitalization.
Materials and methods
Study patients
A total of 149 patients with COPD who were being followed up in the outpatient pulmonary clinic of a single tertiary referral hospital (Gyeongsang National University Hospital, Jinju, South Korea) were recruited from October 2011 to September 2013 in this non-interventional observational study. Patients were recruited if they had respiratory symptoms consistent with COPD, a smoking history of ≥10 pack years, and age of ≥40 years. The diagnosis of COPD was confirmed by spirometry if the post-bronchodilator FEV1/forced vital capacity (FVC) ratio was less than 0.7 according to the GOLD document.3 We included patients who underwent the six-minute walk test (6MWT) to calculate the BODE index. Patients were excluded if they had bronchial asthma, severe structural lung disease (including bronchiectasis and tuberculosis-destroyed lung), or uncontrolled malignant disease that affected survival. An exacerbation of COPD was defined as acute deterioration of respiratory symptoms – including coughing, sputum production, and/or dyspnea – that required systemic steroids or antibiotics. Respiratory hospitalization was defined as event of severe exacerbation that needed hospital admission or emergency room visit. All patients were followed up regularly at 3-month intervals in the outpatient department. This study was approved by the Institutional Ethics Committee of Gyeongsang National University Hospital. All patients provided written informed consent, in accordance with the Declaration of Helsinki.
Study design
Clinical information, including the CAT score, mMRC dyspnea score, and history of exacerbations, was recorded on the first day of the study after recruitment at the outpatient department. Based on the collected information and the results of spirometry, patients were assigned to 4 categories according to the GOLD 2007, GOLD 2011, and GOLD 2017 classifications. Other data on patients’ baseline demographic and clinical characteristics were collected by reviewing medical records retrospectively. The collected data included age, sex, body mass index (BMI), smoking status, 6MWT results, BODE index, and comorbid diseases. The diffusing capacity of carbon monoxide (DLco) and the residual volume (RV) of the lung were obtained if the patient performed each test adequately. Changes in the group assignments of the same patients between the GOLD 2011 and GOLD 2017 classifications were assessed. The date of death was identified on the first review of the medical record; if the identification was not possible, data from the Korean Death Registry on October 22, 2017 were used. Respiratory hospitalization was recorded when the patient was admitted to our hospital due to exacerbation, or it was identified by an interview during the regular outpatient visit if the patient had been admitted to another hospital. Patients who did not visit the outpatient clinic for more than 6 months were contacted by telephone, and the information on respiratory hospitalization was obtained. We classified patients into groups A to D based on the GOLD 2017 and GOLD 2011 classifications using the obtained information and assessed the change in their distribution among the groups. Finally, we compared the abilities of the BODE index, GOLD 2007, GOLD 2011, and GOLD 2017 classification in predicting mortality and respiratory hospitalization.
Statistical analysis
Statistical analyses were performed using SPSS for Windows version 21.0 software (SPSS Inc., Chicago, IL, USA). The Pearson’s chi-squared test was used to analyze categorical variables. Comparison of continuous variables between GOLD group A, B, C, and D was performed using one-way analysis of variance with the Bonferroni post-hoc test. The independent-samples t-test was used to compare continuous variables between two groups. Multivariate binary regression analysis was used to assess the predictive ability of 2007, 2011, and 2017 GOLD classification for respiratory hospitalization after adjustment for age, gender, BMI, and smoking history. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. Kaplan–Meier survival curves were used to evaluate the survival of patients with COPD. The log-rank test was used to compare the mortalities within the subgroups in the 2007, 2011, and 2017 GOLD classifications. Receiver Operation Characteristic (ROC) curve analyses were used to test and compare the predictive accuracy for mortality and respiratory hospitalization among the BODE index, GOLD 2007, GOLD 2011, and GOLD 2017 classifications. MedCalc for Windows version 21.0 software (MedCalc Software, Ostend, Belgium) was used to compare areas under the curves (AUCs). A p-value <0.05 was considered to indicate statistical significance.
Results
Baseline characteristics and distribution of patients
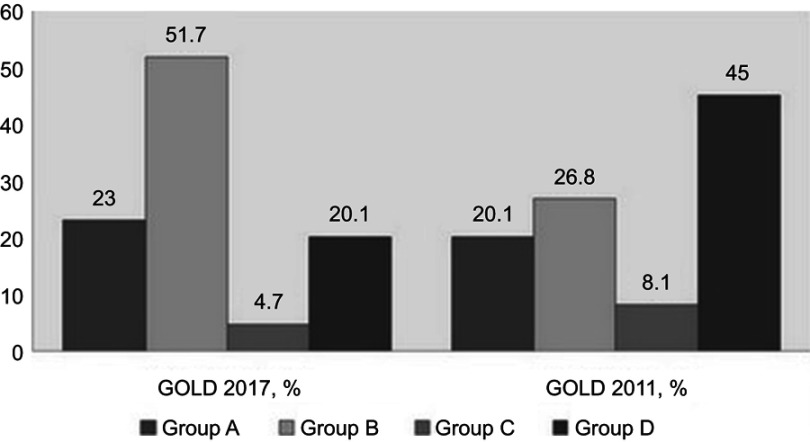
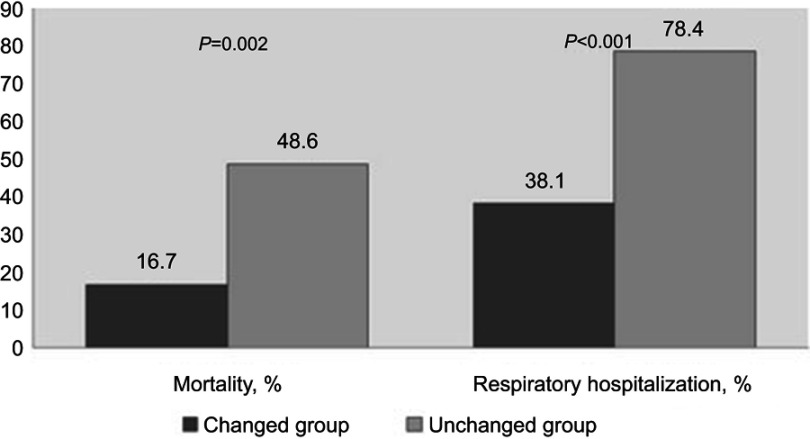
A total of 149 patients composed of 130 males (87.2%) and 19 females (12.8%) were enrolled. The mean age of the enrolled patients was 71.2±7.65 years, and the mean FEV1% predicted was 51.0±18.5. Over half of the patients were allocated to group B (n=77 [51.7%]), and 35 (23.5%), 30 (21.1%), and 7 (4.7%) patients were allocated to group A, D, and C, respectively, according to the GOLD 2017 classification. The BMI, number of exacerbations per one year, 6MWT results, and pulmonary function (including FEV1, DLco, and RV) were significantly different among GOLD groups A, B, C, and D. The BODE index was highest in group D followed by group B, group C, and group A (p<0.001) (Table 1). The prevalence of comorbid diseases – including hypertension, diabetes, and cardiovascular diseases – was comparable among the 4 groups. The allocation of 42 (53.2%) patients in 2011 GOLD group C or D was changed to 2017 GOLD group A or B according to the new classification. Thirty-seven (55.2%) patients in 2011 GOLD group D were categorized into 2017 GOLD group B, and 5 (41.7%) patients in 2011 GOLD group C were categorized into 2017 GOLD group A (Figure 1). Patients whose allocations were unchanged between GOLD 2011 and 2017 classifications had significantly higher rate of respiratory hospitalization and death compared with patients whose allocations were changed (Figure 2).
Table 1.
Baseline characteristics of enrolled subjects by GOLD 2017 classification
| Characteristics | A (n=35) | B (n=77) | C (n=7) | D (n=30) | p-value |
|---|---|---|---|---|---|
| Age, yr | 70.3±7.51 | 71.2±7.62 | 70.7±9.38 | 72.6±7.70 | 0.661 |
| Male gender, % | 100 | 83.1 | 100 | 80 | 0.033 |
| BMI, kg/m2 | 23.2±2.93 | 21.4±2.90 | 23.2±2.76 | 20.2±3.66 | 0.001 |
| Smoking status, n (%) Current smoker Former smoker |
12 (34.3) 23 (65.7) |
33 (42.9) 44 (57.1) |
2 (28.6) 5 (71.4) |
6 (20.0) 24 (80.0) |
0.162 |
| FEV1, % predicted | 62.8±13.4 | 51.6±19.1 | 54.5±12.4 | 34.9±10.4 | <0.001 |
| DLco, % predicted (n=110) | 87.6±21.7 | 71.3±26.8 | 88.1±12.1 | 53.9±20.6 | <0.001 |
| Residual volume, % predicted (n=116) | 171.2±58.4 | 179.6±58.2 | 151.9±19.6 | 213.5±76.3 | 0.096 |
| Number of exacerbations per one year | 0.14±0.36 | 0.29±0.46 | 2.00±0.00 | 2.83±1.09 | <0.001 |
| 6MWT | 393.1±74.8 | 325.0±88.2 | 372.9±39.9 | 286.0±84.2 | <0.001 |
| BODE index | 1.83±0.89 | 3.61±2.08 | 2.00±0.82 | 5.57±1.63 | <0.001 |
| Comorbid diseases, n (%) Hypertension Diabetes Cardiovascular disease |
7 (20) 3 (8.6) 8 (22.9) |
14 (18.2) 6 (7.8) 9 (11.7) |
3 (42.9) 2 (28.6) 1 (14.3) |
12 (40.0) 5 (16.7) 5 (16.7) |
0.064 0.232 0.504 |
| Respiratory hospitalization, n (%) | 6 (17.1) | 25 (32.5) | 4 (57.1) | 25 (83.3) | <0.001 |
| Mortality, n (%) | 4 (11.4) | 15 (19.5) | 2 (28.6) | 16 (53.3) | 0.001 |
Note: Values are presented as mean ± SD unless otherwise noted.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; BMI, body mass index; FEV1, forced expiratory volume in 1 second; DLco, diffusing capacity for carbon monoxide; 6MWT, 6 min walk test; BODE, body-mass index, airway obstruction, dyspnea, and exercise.
Figure 1.
Distribution (%) of patients with COPD by GOLD 2017 and GOLD 2011 classifications.
Figure 2.
Comparison of mortality and respiratory hospitalization between the unchanged group C/D and group A/B that was from 2011 GOLD group C/D.
Respiratory hospitalization
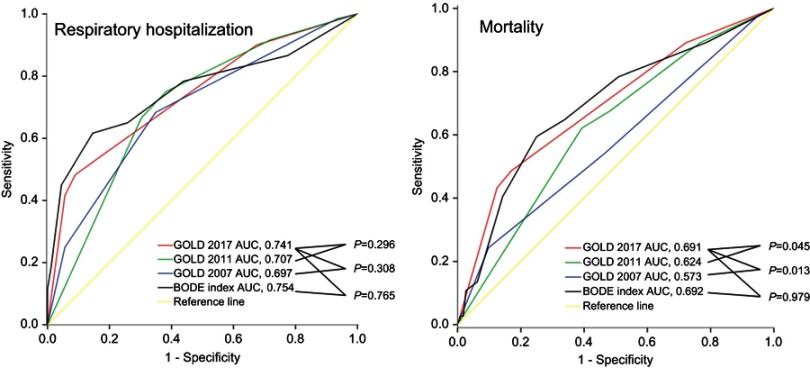
Respiratory hospitalization occurred in 60 (40.3%) patients during the median follow-up of 61 months. The events of respiratory hospitalization most commonly occurred in group D followed by group C, group B, and group A (p<0.001) (Table 1). Multivariate regression analysis showed that patients in 2007 GOLD group 4 (OR, 31.5; 95% CI, 2.52–394.4; relative to group 1), in 2011 GOLD group D (OR, 6.75; 95% CI, 2.07–22.0; relative to group A), and in 2017 GOLD group C (OR, 7.55; 95% CI, 1.25–45.8) and group D (OR, 25.0; 95% CI, 6.01–102.9) (both relative to group A) were more likely to undergo respiratory hospitalization after adjusting for age, BMI, and smoking history (Table 2). ROC curve analyses showed that the AUCs of GOLD 2007, GOLD 2011, GOLD 2017, and BODE index in predicting respiratory hospitalization were 0.697, 0.707, 0.741, and 0.754, which were not statistically different (Figure 3-A and Table 3).
Table 2.
Predictive factors for respiratory hospitalization based on the univariate and multivariate* logistic regression analyses
| GOLD | Respiratory hospitalization | |
|---|---|---|
| OR (95% CI) | Adjusted OR (95% CI) * | |
| 2007 GOLD 1 | 1 | 1 |
| 2007 GOLD 2 | 2.08 (0.23–18.4) | 2.83 (0.31–26.1) |
| 2007 GOLD 3 | 6.00 (0.67–53.4) | 8.24 (0.87–78.2) |
| 2007 GOLD 4 | 18.0 (1.72–188.1) | 31.5 (2.52–394.4) |
| 2011 GOLD A | 1 | 1 |
| 2011 GOLD B | 1.67 (0.50–5.52) | 1.40 (0.40–4.85) |
| 2011 GOLD C | 3.57 (0.80–15.9) | 3.67 (0.79–17.1) |
| 2011 GOLD D | 7.41 (2.52–21.8) | 6.75 (2.07–22.0) |
| 2017 GOLD A | 1 | 1 |
| 2017 GOLD B | 2.32 (0.86–6.32) | 1.93 (0.67–5.60) |
| 2017 GOLD C | 6.44 (1.14–36.6) | 7.55 (1.25–45.8) |
| 2017 GOLD D | 24.2 (6.57–88.8) | 25.0 (6.01–102.9) |
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; OR, odds ratio; CI, confident interval.
Note: *Multivariate models adjusted for gender, age, body mass index, and smoking history.
Figure 3.
Receiver operator characteristics curves of 2007 GOLD, 2011 GOLD, 2017 GOLD, and BODE index to predict respiratory hospitalization (3-A) and mortality (3-B).
Table 3.
p-values in the comparisons of the predictive accuracy of the BODE index and the GOLD 2007, GOLD 2011, and GOLD 2017 classifications using ROC curve analyses
| p-values for mortality | p-values for respiratory hospitalization | |
|---|---|---|
| BODE vs GOLD 2007 | 0.002 | 0.009 |
| BODE vs GOLD 2011 | 0.088 | 0.175 |
| BODE vs GOLD 2017 | 0.979 | 0.765 |
| GOLD 2007 vs GOLD 2011 | 0.150 | 0.715 |
| GOLD 2007 vs GOLD 2017 | 0.013 | 0.308 |
| GOLD 2011 vs GOLD 2017 | 0.045 | 0.296 |
Abbreviations: ROC, Receiver Operation Characteristic; BODE, BMI, Obstruction, Dyspnea, Exercise; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Mortality
Thirty-three (24.8%) out of the 149 patients with COPD died during the study period. The mortality rate in the GOLD 2017 groups A, B, C, and D were 11.4%, 19.5%, 28.6%, and 53.3%, respectively (p=0.001) (Table 1). The AUCs of GOLD 2007, GOLD 2011, GOLD 2017, and BODE index in predicting death were 0.573, 0.624, 0.691, and 0.692. The differences in the AUCs were statistically significant between GOLD 2017 and GOLD 2011 (p=0.045) (Table 3) and between GOLD 2017 and GOLD 2007 (p=0.013) (Table 3). The predictive abilities of GOLD 2017 and BODE index for mortality were similar according to the ROC curve analysis (p=0.979) (Figure 3B). A comparison of the overall survival using the Kaplan–Meier curve revealed that survival was not significantly different among the 2007 GOLD groups 1 to 4 and among 2011 GOLD groups A to D. However, survival was significantly different among the GOLD groups A to D classified according to the GOLD 2017 classification (log-rank test, p<0.001) (Figure 4).
Figure 4.

Kaplan-Meier curves of the overall survival according to the 2007, 2011, and 2017 GOLD classifications.
Discussion
The current study was conducted to verify the prognostic validity of the new GOLD 2017 classification to predict respiratory hospitalization and mortality in patients with stable COPD. We found that the odds ratios of respiratory hospitalization in 2017 GOLD group C and D were higher than those in 2011 GOLD group C and D, and being in 2017 GOLD group C and D was a significant predictor of respiratory hospitalization. With respect to mortality, the Kapan-Meier survival curves of the 2017 GOLD ABCD groups were statistically different, and the 2017 GOLD classification had a discriminatory power similar to that of the BODE index and a significantly better discriminatory power than those of the 2011 GOLD and 2007 GOLD classifications.
The proportion of patients in 2017 GOLD groups in this study was similar to that in the studies by Tudoric et al and Marcoa et al10,12 in which approximately 50% of the patients were categorized into group B. Furthermore, the proportion of patients in group A, C and D was similar in those studies. However, Lopez et al and Menezes et al reported that over half of the patients in their COPD cohorts consisted of patients in 2017 GOLD group .9,13 It is uncertain how theses differences in the patients composition impact the study results.
The current study demonstrated that the use of the GOLD 2017 classification shifted patients with COPD to groups of milder severity compared with the GOLD 2011 classification. This is the opposite of the phenomenon observed with the use of the revised GOLD 2011 classification, which shifted the patients with COPD to more severe categories compared with the GOLD 2007 classification.14 Recent studies evaluating performance and validity of the new GOLD 2017 classification showed that a considerable number of patients were shifted from 2011 GOLD group C to 2017 GOLD group A and from 2011 GOLD group D to 2017 GOLD group .9,10,15 Tan et al have reported that 65.5% of patients were reassigned to 2017 GOLD group A or B from 2011 GOLD group C or D. Likewise, 53.2% patients in 2011 GOLD groups C or D were shifted to 2017 GOLD groups A or B in this study. These changes may contribute in increasing the prognostic performance of the GOLD 2017 classification. The mortality and rate of respiratory hospitalization in the unchanged group were higher than those in the group whose group allocations were shifted in this study.
Some researchers have recently compared the predictive accuracy of the GOLD 2017, GOLD 2011, and GOLD 2007 classifications for mortality following the release of the GOLD 2017 documents. Menezes et al and Gedebjerg et al showed that the predictive accuracy of the new GOLD 2017 classification was not higher than those of the previous classification systems.13,16 Interestingly, the old GOLD 2011 classification predicted mortality better than the new GOLD 2017 classification did according to a study by Lopez et al.9 The results of this study with regard to mortality prediction were somewhat different from results of previous studies. This study showed that the AUCs of GOLD 2007, GOLD 2011, and GOLD 2017 classifications for mortality prediction increased in that sequence. Furthermore, discriminatory power of the GOLD 2017 classification was significantly better than those of the GOLD 2007 and GOLD 2011 classifications.
Scarce data exist regarding the accuracy of the GOLD 2017 classification in the prediction of acute exacerbations and respiratory hospitalizations. Marcoa et al have reported that exacerbation were most common in GOLD 2017 group D (67.3%) followed by group C (55.6%), B (42%), and A (28.6%); these results were similar with the results of this study. The authors also showed that the GOLD 2017 and GOLD 2011 classifications identified a comparable number of patients with exacerbations after adjusting for confounding variables.10 This study showed that the GOLD 2017 classification identified more patients with respiratory hospitalization than the GOLD 2011 classification did. In addition, the odds ratios of being in not only GOLD 2017 group D but also group C relative to group A were statistically significant for respiratory hospitalization; however, the odds ratio of being in GOLD 2011 group C relative to group A was not significant in this study.
The BODE index is a multidimensional grading system that predicts the risk of death better than GOLD 2007 classification does in patients with COPD.11 Torres et al have reported that the BODE index predicts survival better than GOLD 2011 classification does.17 In the current study, the BODE index had a better predictive accuracy for mortality than the GOLD 2007 classification had; however, the predictive abilities of the BODE index were comparable to those of the GOLD 2011 and GOLD 2017 classifications. The BODE index has also been evaluated as a multidimensional system of COPD severity with regard to its prediction of exacerbations and hospitalizations. Martin et al and Ong et al have reported that the BODE index predicted future exacerbations and hospitalizations better than the GOLD 2007 classification did.18,19 Studies comparing the predictive performance of the BODE index and the 2011 GOLD and 2017 GOLD classifications for exacerbations and hospitalizations are lacking. Our data showed that the GOLD 2007 classification had a lesser discriminatory power than the BODE index had. however, the GOLD 2017 classification had a discriminatory power comparable to that of the BODE index.
Some limitations of the current study need to be acknowledged. First, the number of enrolled patients was quite small; this is because we excluded patients without a CAT score and a BODE index. Second, few patients in 2017 GOLD group C were included in this study. It is a common observation that the number of GOLD group C patients is smaller than that of other groups; less than 5% of all enrolled patients belonged to GOLD group C in this study. It is possible that the skewed patient enrollment may have lead to biased study results. Third, the causes of death could not be identified. Thus, the respiratory mortality could not be analyzed. Nevertheless, this study has several advantages. First, this was prospectively designed observational study. Second, the median follow-up time was over 5 years; this relatively long follow-up facilitated analysis of survival as well as analysis of acute exacerbations. Third, the CAT score was applied in all enrolled patients according to the original GOLD document. Some previous studies assigned patients to GOLD ABCD groups without using the CAT score, which may impact the study accuracy. Fourth, we calculated the BODE index in all enrolled patients. The BODE index is a well-established scoring system with regard assesssing the prognosis of patients with COPD; therefore, it was very important to compare the predictive accuracy of the BODE index and the new GOLD classification.
Conclusions
The revised GOLD 2017 classification has an accuracy similar to that of the BODE index and a better accuracy than those of the GOLD 2007 and GOLD 2011 classifications in terms of predicting mortality and respiratory hospitalization according to the results of this study. Further studies with a large number of patients with COPD are needed.
Acknowledgments
The authors thank all residents of Gyeongsang National University Hospital who participated in collecting data in this study.
Abbreviation list
GOLD, Global Initiative for Chronic Obstructive Lung Disease; COPD, Chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; mMRC, modified Medical Research Council; CAT, COPD Assessment Test; BODE, the BMI, obstruction, dyspnea, exercise; FVC, forced vital capacity; 6MWT, six-minute walk test; DLco, diffusing capacity of carbon monoxide; RV, residual volume; ORs, Odds ratios; CIs, confidence intervals; ROC, Receiver Operation Characteristic;AUCs, areas under the curves.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors certify that there is no conflicts of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Zhou W, Heist RS, Liu G, et al. Smoking cessation before diagnosis and survival in early stage non-small cell lung cancer patients. Lung Cancer. 2006;53(3):375–380. doi: 10.1016/j.lungcan.2006.05.017 [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 3.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0150WS [DOI] [PubMed] [Google Scholar]
- 4.Soriano JB, Alfageme I, Almagro P, et al. Distribution and prognostic validity of the new global initiative for chronic obstructive lung disease grading classification. Chest. 2013;143(3):694–702. doi: 10.1378/chest.12-1053 [DOI] [PubMed] [Google Scholar]
- 5.Nishimura K, Oga T, Tsukino M, Hajiro T, Ikeda A, Jones PW. Reanalysis of the Japanese experience using the combined COPD assessment of the 2011 GOLD classification. Respir Investig. 2014;52(2):129–135. doi: 10.1016/j.resinv.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 6.Johannessen A, Nilsen RM, Storebo M, Gulsvik A, Eagan T, Bakke P. Comparison of 2011 and 2007 global initiative for chronic obstructive lung disease guidelines for predicting mortality and hospitalization. Am J Respir Crit Care Med. 2013;188(1):51–59. doi: 10.1164/rccm.201212-2276OC [DOI] [PubMed] [Google Scholar]
- 7.Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–981. doi: 10.1164/rccm.201207-1299OC [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Yoon HI, Oh YM, et al. Lung function decline rates according to GOLD group in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1819–1827. doi: 10.2147/COPD.S87766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera Lopez C, Casanova Macario C, Marin Trigo JM, et al. Comparison of 2017 and 2015 global initiative for obstructive lung disease: impact on grouping and outcomes. Am J Respir Crit Care Med. 2017;15;197(4):463–469. [DOI] [PubMed] [Google Scholar]
- 10.Marcoa R, Rodrigues DM, Dias M, et al. Classification of Chronic Obstructive Pulmonary Disease (COPD) according to the new Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017: comparison with GOLD 2011. COPD. 201. 8;15(1):21–26. [DOI] [PubMed] [Google Scholar]
- 11.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 12.Tudoric N, Koblizek V, Miravitlles M, et al. GOLD 2017 on the way to a phenotypic approach? Analysis from the phenotypes of COPD in Central and Eastern Europe (POPE) Cohort. Eur Respir J. 2017;49(4):1602518. doi: 10.1183/13993003.02518-2016 [DOI] [PubMed] [Google Scholar]
- 13.Menezes AM, Wehrmeister FC, Perez-Padilla R, et al. The PLATINO study: description of the distribution, stability, and mortality according to the global initiative for chronic obstructive lung disease classification from 2007 to 2017. Int J Chron Obstruct Pulmon Dis. 2017;12:1491–1501. doi: 10.2147/COPD.S150887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano JB, Lamprecht B, Ramirez AS, et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient data. Lancet Respir Med. 2015;3(6):443–450. doi: 10.1016/S2213-2600(15)00157-5 [DOI] [PubMed] [Google Scholar]
- 15.Tan WC, Bourbeau J, Aaron SD, et al. GOLD 2017 classification and lung function decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 201. 8;1;197(5):670–673. [DOI] [PubMed] [Google Scholar]
- 16.Gedebjerg A, Szepligeti SK, Wackerhausen LH, et al. Prediction of mortality in patients with chronic obstructive pulmonary disease with the new global initiative for chronic obstructive lung disease 2017 classification: a cohort study. Lancet Respir Med. 2018;6(3):204–212. doi: 10.1016/S2213-2600(18)30002-X [DOI] [PubMed] [Google Scholar]
- 17.de Torres JP, Casanova C, Marin JM, et al. Prognostic evaluation of COPD patients: GOLD 2011 versus BODE and the COPD comorbidity index COTE. Thorax. 2014;69(9):799–804. doi: 10.1136/thoraxjnl-2013-203884 [DOI] [PubMed] [Google Scholar]
- 18.Marin JM, Carrizo SJ, Casanova C, et al. Prediction of risk of COPD exacerbations by the BODE index. Respir Med. 2009;103(3):373–378. doi: 10.1016/j.rmed.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 19.Ong KC, Earnest A, Lu SJ. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest. 2005;128(6):3810–3816. doi: 10.1378/chest.128.6.3810 [DOI] [PubMed] [Google Scholar]